Cognitive Impairment in ANCA-Associated Vasculitis: A Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Diagnostic Criteria
2.2. Ethics
2.3. Cognitive Evaluation
2.4. Neuropsychiatric Evaluation
2.5. Brain MRI
2.6. Statistical Analysis
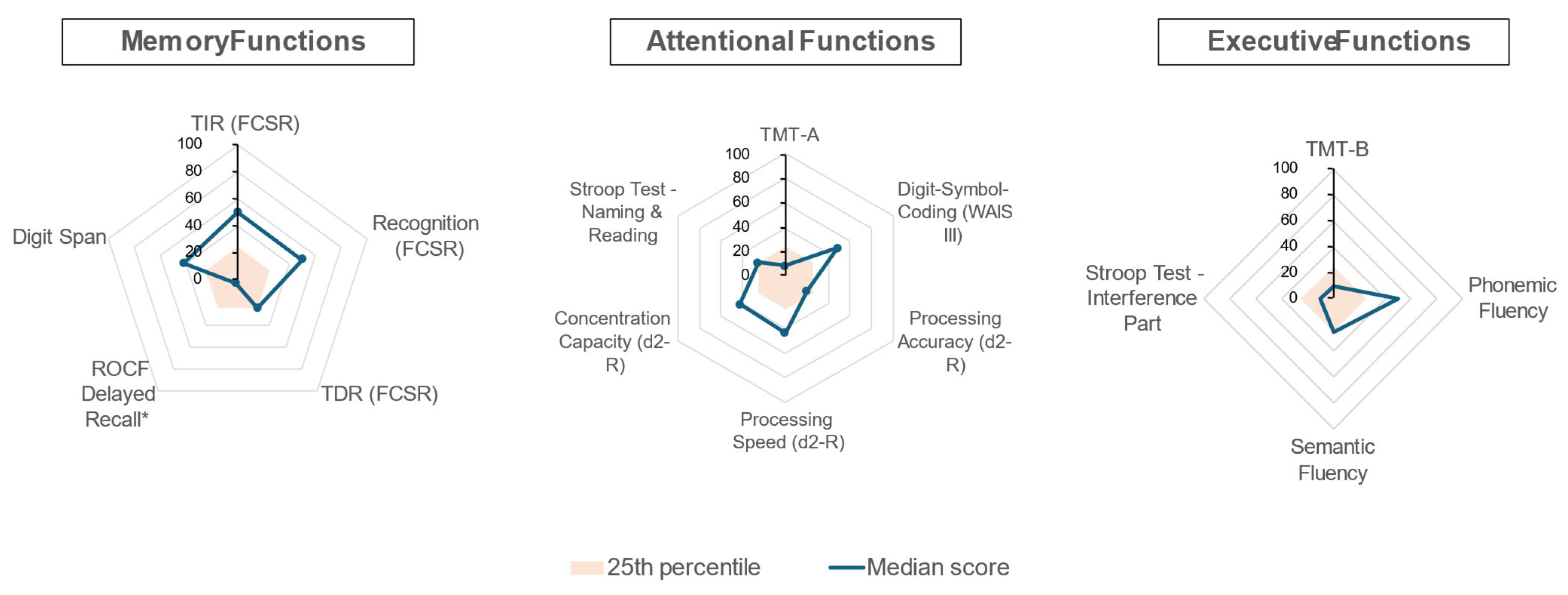
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Capra, R.; Rovaris, M.; Chiari, S.; Codella, M.; Miozzo, A.; Gregorini, G.; Filippi, M. Frequency and patterns of subclinical cognitive impairment in patients with ANCA-associated small vessel vasculitides. J. Neurol. Sci. 2002, 195, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Castillejos, N.; Jiménez-González, A.; Hinojosa-Azaola, A. No Difference in Cognitive Dysfunction Among Patients with ANCA-Associated Vasculitis, Rheumatoid Arthritis or Chronic Kidney Disease. J. Int. Neuropsychol. Soc. 2019, 25, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.E.; Baptista, T.S.A.; Molina, J.K.; Motta, J.G.; Prado, A.D.; Piovesan, D.M.; de Nardi, T.; Viola, T.W.; Vieira, É.L.M.; Teixeira, A.L.; et al. Cognitive impairment in rheumatoid arthritis: Role of lymphocyte subsets, cytokines and neurotrophic factors. Clin. Rheumatol. 2018, 37, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, M.; Anderson, E.; Carroll, K.R.; Tehrani, N.; Volpe, B.T.; Diamond, B. Cognitive dysfunction in SLE: An understudied clinical manifestation. J. Autoimmun. 2022, 132, 102911. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; McClean, A.; Harper, L.; Amft, E.N.; Dhaun, N.; A Luqmani, R.; A Little, M.; Jayne, D.R.; Flossmann, O.; McLaren, J.; et al. The characterisation and determinants of quality of life in ANCA associated vasculitis. Ann. Rheum. Dis. 2014, 73, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.A.; Luqmani, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Levi, L.; Alagaratnam, J.; Van Bremen, K.; Mastrangelo, A.; Waalewijn, H.; Molina, J.; Guaraldi, G.; Winston, A.; Boesecke, C.; et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med. 2023, 24, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Lange, M.; Rigal, O.; Correia, H.; Giffard, B.; Beaumont, J.L.; Clisant, S.; Wagner, L. French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 3. Support. Care Cancer 2012, 20, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Poitrenaud, J.; Israël, L.; Barrère, H.; Le Roch, K. Version française de l’échelle de difficultés cognitives de McNair et Khan. In De la Plainte Mnésique à la Maladie d’Alzheimer; Michel, B.-F., Derouesné, C., Gély-Nargeot, M.-C., Eds.; Solal: Shinagawa City, Japan, 1997; pp. 159–168. [Google Scholar]
- Roussel, M.; Godefroy, O. La batterie GRECOGVASC; De Boeck Supérieur: Paris, France, 2016. [Google Scholar]
- González-Suárez, I.; Arpa, J.; Ríos-Blanco, J.J. Brain Microvasculature Involvement in ANCA Positive Vasculitis. Cerebrovasc. Dis. 2016, 41, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.A.; Dehghan, N.; Chen, W.; Xie, H.; Esdaile, J.M.; Avina-Zubieta, J.A. Mortality in ANCA-associated vasculitis: Ameta-analysis of observational studies. Ann. Rheum. Dis. 2017, 76, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Pittam, B.; Gupta, S.; Ahmed, A.E.; Hughes, D.M.; Zhao, S.S. The prevalence and impact of depression in primary systemic vasculitis: A systematic review and meta-analysis. Rheumatol. Int. 2020, 40, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B.; Limphaibool, N.; Puszczewicz, M. Cytokine secretion and the risk of depression development in patients with connective tissue diseases. Psychiatry Clin. Neurosci. 2019, 73, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Suzzi, S.; Tsitsou-Kampeli, A.; Schwartz, M. The type I interferon antiviral response in the choroid plexus and the cognitive risk in COVID-19. Nat. Immunol. 2023, 24, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Murray, A.D.; Jones, G.T.; Reid, D.M.; Macfarlane, G.J.; Waiter, G.D. Neural correlates of fatigue in granulomatosis with polyangiitis: A functional magnetic resonance imaging study. Rheumatology 2014, 53, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Hewitt, C.A.; Litchfield, I.; Morgan, M.D.; Chanouzas, D.; Caulfield, H.K.; Coughlan, L.; Dean, C.; Fletcher, K.; Cramp, F.; et al. Management of fatigue with physical activity and behavioural change support in vasculitis: A feasibility study. Rheumatology 2021, 60, 4130–4140. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.C.; Dawson, J.; Doll, H.; Cronholm, P.F.; Milman, N.; Kellom, K.; Ashdown, S.; Easley, E.; Gebhart, D.; Lanier, G.; et al. Validation of the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire. Ann. Rheum. Dis. 2018, 77, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
| All Patients n = 12 | ||
|---|---|---|
| Female sex, n (%) | 5 (42) | |
| Age (years) [IQR] | 68 [59–71] | |
| Body Mass Index, (kg/m2) [IQR] | 24.75 [4–39] | |
| Substance use | Active smoking | 0 (0) |
| Alcohol | 0 (0) | |
| Type of AAV vasculitis, n (%) | ||
| GPA | 8 (67) | |
| MPA | 1 (8) | |
| EGPA | 3 (25) | |
| ANCA positivity, n (%) | ||
| Anti-PR3 | 5 (42) | |
| Anti-MPO | 6 (50) | |
| Non-specific | 1 (8) | |
| Organ involvement at diagnosis, n (%) | ||
| Peripheral nervous system | 7 (58) | |
| Renal failure with proteinuria | 4 (33) | |
| Pulmonary hemorrhage | 2 (17) | |
| BVAS score at diagnosis, median [IQR] | 10 [10–18.5] | |
| Vasculitis relapse, n (%) | 7 (58) | |
| Treatments | ||
| Induction treatment receiveda, n (%) | ||
| Plasma exchanges | 1 (8) | |
| Cyclophosphamide | 6 (50) | |
| Rituximab | 4 (33) | |
| Received cumulative corticosteroid dose, grams, median [IQR] | 22.3 [15.7–26.6] | |
| 92 [55–127] | ||
| Current status | Corticosteroids | 5 (42) |
| Post-diagnosis delay, (months) [IQR] | Immunosuppressive treatments | 4 (33) |
| Current treatments, n (%) | Psychotropic treatments | 5 (42) |
| Sequelae of neuropathy | 7 (58) | |
| Vasculitis activity | 0 | 10 (83) |
| ≥1 | 2 (17) | |
| 2 [1–2.5] | ||
| BVAS score, n (%) | 1 (8) | |
| 0 (0) | ||
| VDI score [IQR] | ||
| ANCA positivity, n (%) | No sign of vasculitis activity | 12 (100) |
| CRP > 5 mg/L, n (%) | Fazekas grade 2 vascular leukoencephalopathy | 2 (17) |
| Brain MRI results, n (%) | Hippocampal atrophy (Scheltens grade 2 or 3) | 3 (25) |
| Vascular sequelae (deep lacunes and junctional sequelae) | 0 (0) | |
| Microbleeds | 3 (25) | |
| Cortico-subcortical sequelae | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camard, M.; Moises, A.; Bourdic, K.; Venditti, L.; Denier, C.; Henry, J.; Sterpu, R.; David, P.; De Menthon, M.; Lambotte, O.; et al. Cognitive Impairment in ANCA-Associated Vasculitis: A Cross-Sectional Pilot Study. J. Clin. Med. 2025, 14, 3582. https://doi.org/10.3390/jcm14103582
Camard M, Moises A, Bourdic K, Venditti L, Denier C, Henry J, Sterpu R, David P, De Menthon M, Lambotte O, et al. Cognitive Impairment in ANCA-Associated Vasculitis: A Cross-Sectional Pilot Study. Journal of Clinical Medicine. 2025; 14(10):3582. https://doi.org/10.3390/jcm14103582
Chicago/Turabian StyleCamard, Marion, Ana Moises, Katia Bourdic, Laura Venditti, Christian Denier, Julien Henry, Raluca Sterpu, Perla David, Mathilde De Menthon, Olivier Lambotte, and et al. 2025. "Cognitive Impairment in ANCA-Associated Vasculitis: A Cross-Sectional Pilot Study" Journal of Clinical Medicine 14, no. 10: 3582. https://doi.org/10.3390/jcm14103582
APA StyleCamard, M., Moises, A., Bourdic, K., Venditti, L., Denier, C., Henry, J., Sterpu, R., David, P., De Menthon, M., Lambotte, O., Petit, A.-C., Babin, M., Noel, N., & Urbain, F. (2025). Cognitive Impairment in ANCA-Associated Vasculitis: A Cross-Sectional Pilot Study. Journal of Clinical Medicine, 14(10), 3582. https://doi.org/10.3390/jcm14103582






