Exploring the Potential of Digital Twins in Cancer Treatment: A Narrative Review of Reviews
Abstract
1. Introduction
1.1. Digital Twins: Introductory Overview from Industry to Precision Oncology—Revolutionizing Healthcare Through Virtual Modeling
1.2. Purpose
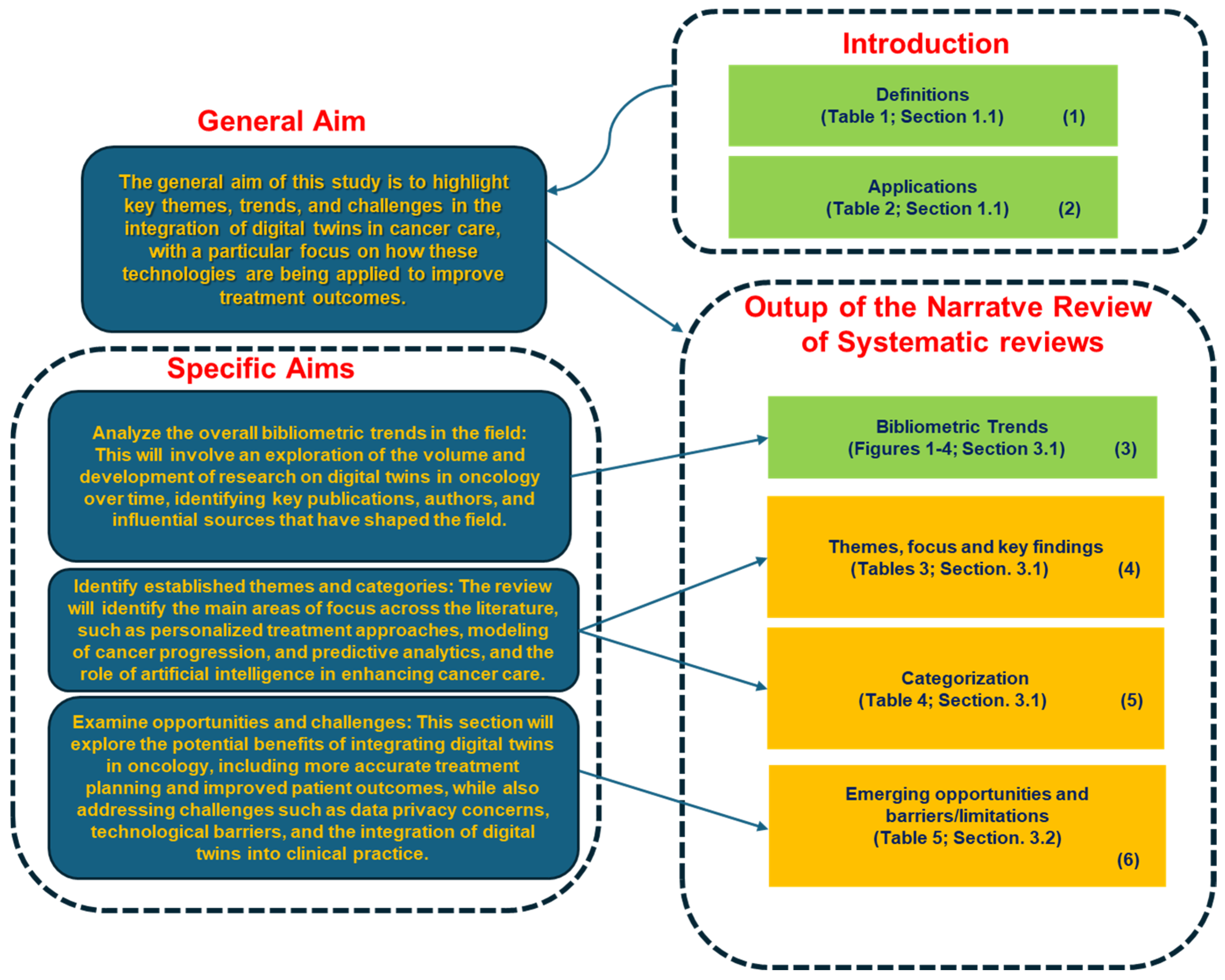
- Analyze the overall bibliometric trends in the field: This will involve an exploration of the volume and development of research on digital twins in oncology over time, identifying key publications, authors, and influential sources that have shaped the field.
- Identify established themes and categories: The review will identify the main areas of focus across the literature, such as personalized treatment approaches, modeling of cancer progression, predictive analytics, and the role of artificial intelligence in enhancing cancer care.
- Examine opportunities and challenges: This section will explore the potential benefits of integrating digital twins in oncology, including more accurate treatment planning and improved patient outcomes, while also addressing challenges such as data privacy concerns, technological barriers, and the integration of digital twins into clinical practice.
2. Methods
| Algorithm 1: Selection Process for Narrative Review on Digital Twins in Oncology |
|
3. Results
3.1. Research Trends and Thematic Insights on Digital Twins in Oncology
3.1.1. Trends
| (digital twins[Title/Abstract]) AND ((oncology[Title/Abstract]) OR (cancer[Title/Abstract]) OR (tumor[Title/Abstract])) (digital twins[Title/Abstract]) |
3.1.2. Emerging Themes and Categorization
Emerging Themes
- Reference: This column reports the references.
- Brief Description: This section provides a concise summary of the study’s key focus or contribution. It outlines the primary research questions or objectives of the study and the general approach used to explore the integration of digital twins in oncology.
- Focus on Oncology: Here, the table specifies how each study is particularly relevant to oncology. It highlights the specific oncological areas or challenges addressed in the study, such as cancer treatment personalization, tumor progression prediction, or optimizing radiotherapy. This column helps to contextualize the research within the specific needs of oncology.
- Innovative Approach: This final column summarizes the novel contributions or methodologies introduced in each study. It emphasizes how digital twin technology or related innovations, such as AI and machine learning, are being utilized to enhance clinical decision-making, personalize treatment plans, or improve cancer care outcomes.
Categorization
- Category:This column organizes the studies into broader research areas, offering a high-level view of the different aspects of digital twin applications in oncology. Categories such as “Foundations and Frameworks,” “Innovative Applications,” “Challenges in Development,” and others help to contextualize the research, allowing readers to easily understand the focus of each study.
- 2.
- Key Topics:The Key Topics column provides a detailed summary of the core subjects covered. It highlights the main concepts, methodologies, and areas of interest discussed in the research, such as computational frameworks, agent-based modeling, and real-time data assimilation. This section enables readers to quickly grasp the specific contributions of each study to the field of oncology.
- 3.
- Key References:The Key References column lists the key studies and sources that underpin the research within each category.
3.2. Opportunities and Challenges
- Study:This column reports the references.
- 2.
- Opportunities:The Opportunities column outlines the potential benefits of applying digital twin technology in oncology. It emphasizes how DTs can enhance clinical decision-making by providing personalized treatment options, predicting cancer treatment responses, and optimizing therapeutic strategies. This section highlights the innovations that digital twin technology brings to oncology, showcasing its value in improving patient care and treatment outcomes.
- 3.
- Barriers:In contrast, the Barriers column identifies the challenges associated with the integration of DTs into oncology. These challenges include issues such as the variability of clinical data, the complexity of real-world clinical environments, and difficulties in validating DT models across diverse patient populations. It also addresses the technological and operational hurdles that need to be overcome to ensure the effective use of digital twin technology in cancer treatment.
4. Discussion
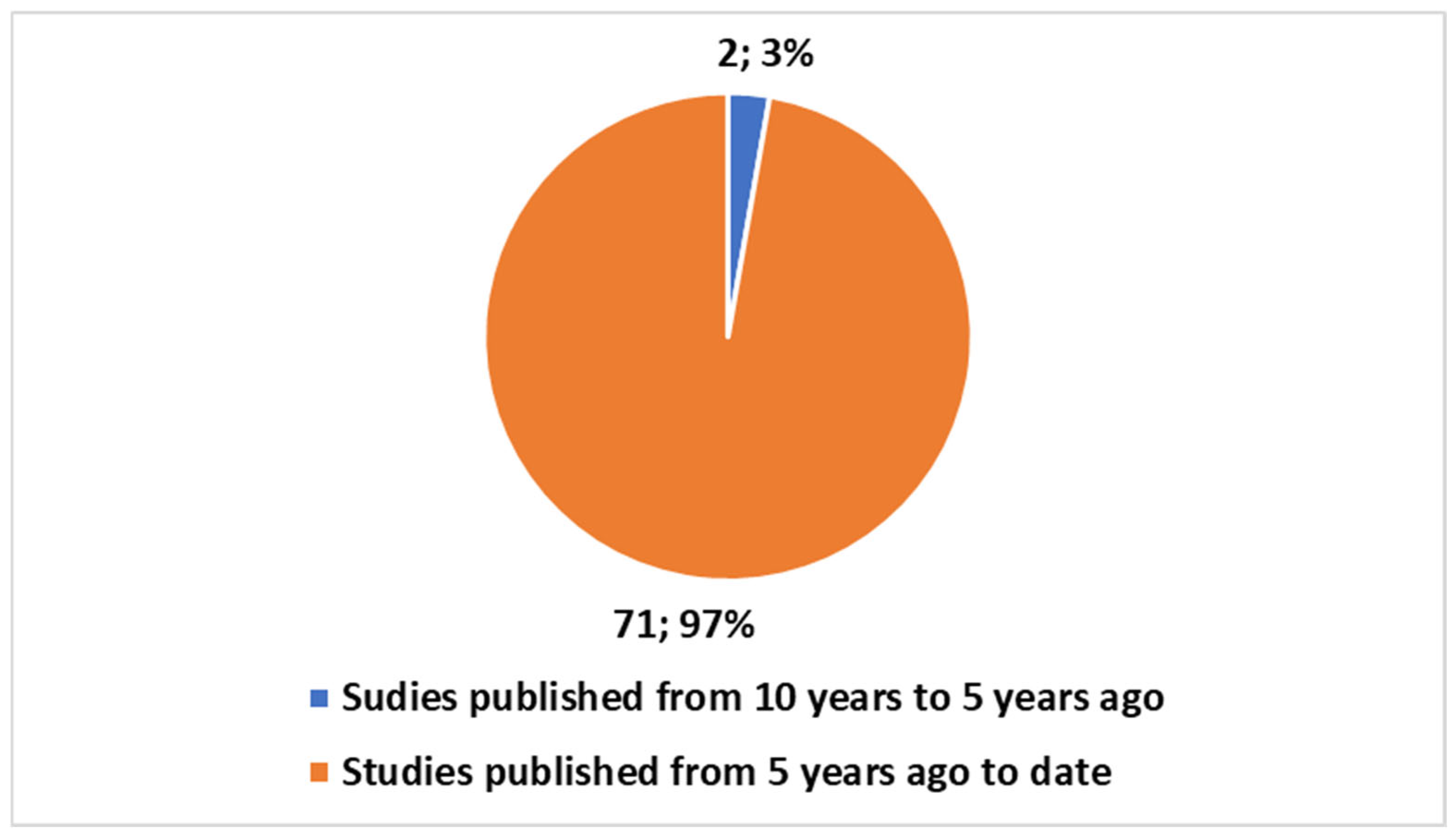
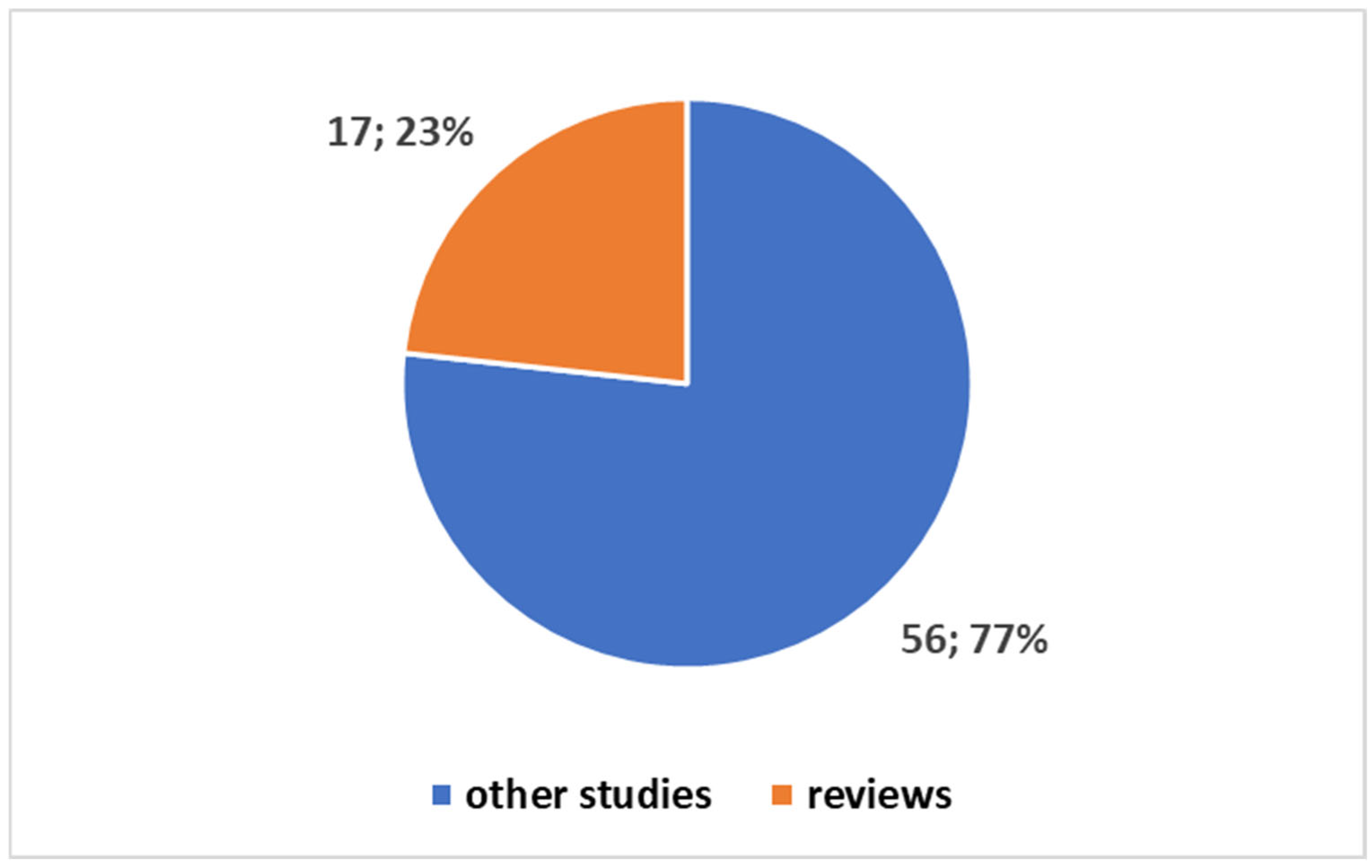
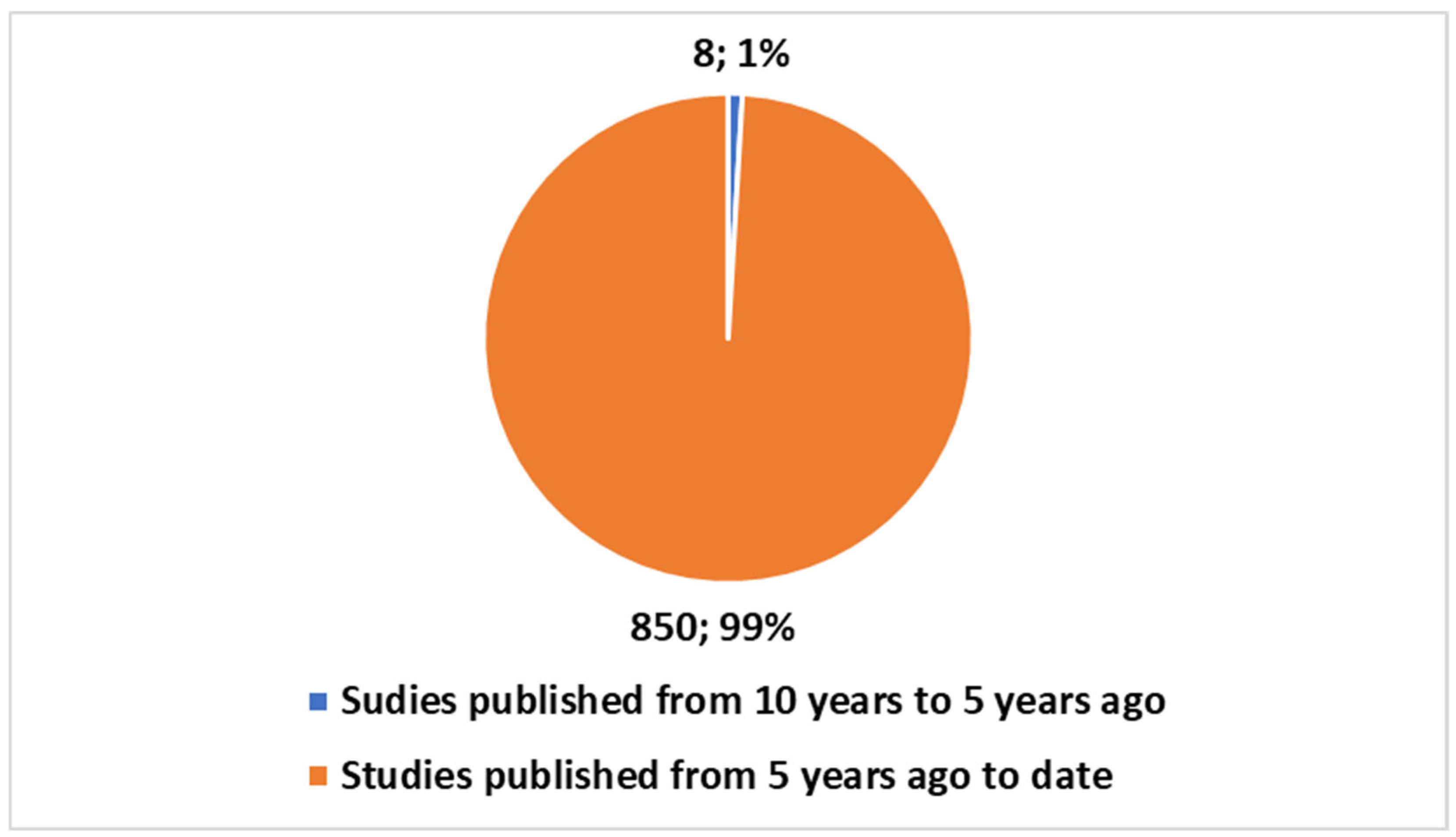
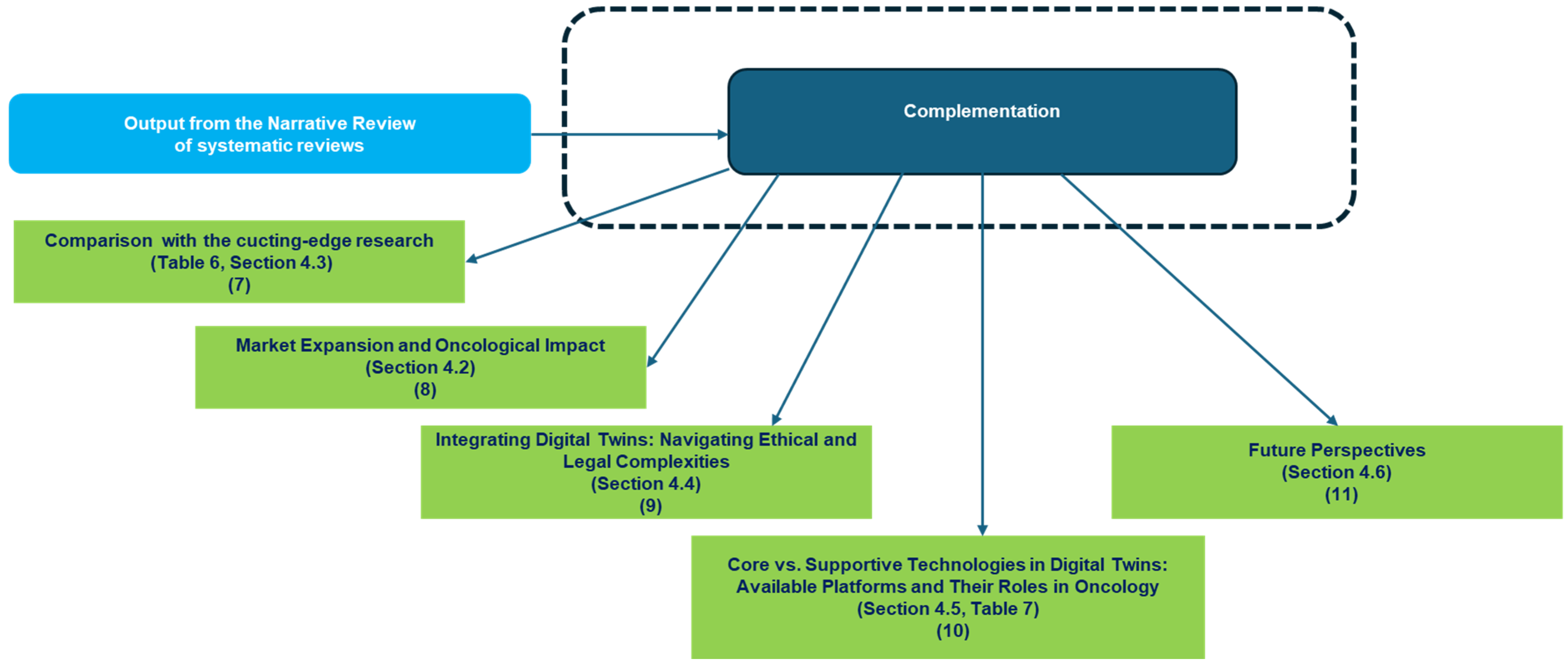
- Block 3: This block represents the bibliometric trends reported in Figure 1, Figure 2, Figure 3 and Figure 4 (Section 3.1). These trends were analyzed to provide an overview of the scientific production in the field.
- Block 4: This block corresponds to the identification of thematic areas, as presented in Table 3 (Section 3.1).
- Block 5: Building upon the thematic categorization, this block recalls the categorization as reported in Table 4 (Section 3.1), enabling a deeper understanding of the different ways DTs are applied in oncology.
- Block 6: This block synthesizes the opportunities and challenges identified in the reviewed studies, as reported in Table 5 (Section 3.2). These findings highlight both the potential benefits of SARSs’ applications in the health domain—and the barriers/limitations
- Block 7 reports a comparison with the cutting-edge research as discussed in Table 6 (Section 4.3).
- Blocks 8 and 9 map the emerging market growth discussed in Section 4.2 and the ethical and legal deepening discussed in Section 4.4
- Block 10 faces the devices and platforms detailing and discussing the Core vs. Supportive Technologies in Table 7 (Section 4.5).
- Block 11 finally identifies the emerging future perspectives (Section 4.6).
4.1. Highlights, Detected Gaps, and Added Values
- 1.
- Opportunities and Barriers
- 2.
- Technical and Clinical Challenges
- 3.
- Interdisciplinary Approaches and AI Integration
- 4.
- Toward a Complementary Perspective: Future Directions, Ethics, and Market Readiness
4.2. The Evolution of Digital Twins: Market Expansion and Oncological Impact
4.2.1. Market Trends and Economic Forecasts for Digital Twins
4.2.2. Digital Twins in Healthcare
4.2.3. Digital Twins in Oncology
- Treatment Optimization: Predicting responses to chemotherapy, radiotherapy, and immunotherapy, while personalizing doses to reduce side effects.
- Clinical Trial Simulations: Using digital twins to simulate clinical trials, predicting patient responses, and optimizing trial design.
- Therapeutic Decision Support: Providing real-time data on tumor evolution and how tumors might respond to ongoing treatments, offering valuable support to oncologists.
4.3. Comparison and Contribution of Cutting-Edge Research
- Reference:This column provides the citation of the study or review.
- 2.
- Contribution:The Contribution column outlines the primary focus and key findings of each referenced study. It details how each study contributes to advancing digital twin technology’s application in precision oncology. This includes for example the integration of artificial intelligence (AI) and machine learning (ML) with DTs to personalize cancer treatment, improve diagnostic accuracy, and optimize therapeutic decisions. It also covers the operational challenges, such as the complexities in data technology, algorithm development, and integrating digital twin models into clinical workflows.
- 3.
- Additional Insights:The Additional Insights column provides further reflections or novel findings that emerge from the study. It highlights aspects that are not commonly discussed in the literature or that contribute uniquely to the field. For example, in the study referenced, the use of AI/ML for generating synthetic data (digital twins) is mentioned as an innovative approach to expedite clinical trials, which adds an important dimension to the broader conversation of digital twins in oncology.
| Reference | Contribution | Additional Insights |
|---|---|---|
| [45] Integration of AI/ML and Digital Twins in Precision Oncology | This study explores the convergence of AI/ML technologies with digital twins (DTs) to enhance precision oncology. It emphasizes the integration of multi-dimensional, multi-omic, spatial pathology, and radiomic data to enable personalized treatment strategies, improving diagnostic accuracy and therapeutic decisions. The study also highlights operational challenges like data technology, algorithm development, and data sharing in clinical workflows. | The application of AI/ML in generating synthetic data (digital twins) and its role in expediting clinical trials is a novel aspect not fully covered in previous studies. |
| [46] Theranostic Digital Twins for Precision Radiopharmaceutical Therapy | This research discusses the use of theranostic digital twins (TDTs) for personalizing radiopharmaceutical therapy (RPT). It integrates clinical, biomarker, image-based, and dosimetric data to optimize treatment, moving away from the one-size-fits-all approach. The study also addresses the social, ethical, and regulatory challenges in TDT implementation, such as data sharing, consent, and reimbursement. | Focuses specifically on theranostic applications and real-time treatment monitoring, providing additional insights into how DTs can personalize radiotherapy—an area not previously explored in-depth. |
| [47] Computational Oncology for In Silico Clinical Trials | This study explores the use of quantitative system pharmacology (QSP) models, virtual patients, and digital twins in predicting tumor responses to treatments, particularly for immuno-oncology therapies. The approach aims to reduce clinical trial time and costs. | The application of digital twins in simulating virtual patients for clinical trials, especially in immuno-oncology, offers a novel perspective not addressed in earlier studies. |
| [48] Cancer Patient Digital Twins for Immune Response | This work introduces cancer patient digital twins (CPDTs) for analyzing immune responses to metastasis. It utilizes multiscale mathematical models to simulate immune surveillance in cancer progression, highlighting how digital twins can dissect cancer complexity at the individual level. | The study provides new insights into immune responses and metastasis, incorporating computational models to analyze immune system interactions with cancer—extending digital twins’ application beyond tumor dynamics. |
| [49] Prostate Cancer Digital Twin Framework | This study presents a framework for creating personalized digital twins in prostate cancer (PCa). It integrates clinical MRI data and simulates tumor growth using methods like the Finite Element Method (FEM), along with patient-specific biomarkers like PSA levels. The study also employs a multi-objective optimization process for model adjustments. | This case study offers a concrete example of digital twin creation for prostate cancer, focusing on MRI data integration and computational simulations to predict tumor growth and PSA dynamics, providing valuable insights for DT development in specific cancer types. |
| [50] Generalizing Digital Twins and Complexity Data Science | This study broadens the concept of digital twins, positioning them as part of a larger field that connects complexity science with data science. The paper introduces the term “complexity data science” and explores the historical, theoretical, and future implications of this approach. | The integration of complexity science with digital twins opens new interdisciplinary pathways, suggesting that digital twins can be a cross-domain tool for scientific exploration beyond traditional applications. |
| [51] Digital Twins in Neuroscience: Modeling Brain Functions and Pathology | The study applies digital twin technology to model brain tumors, offering insights into how brain structure and function can be impacted by tumors. This method is directly translatable to oncology, particularly in understanding tumor progression, treatment responses, and the effects of therapies on brain function, which is essential for personalized care in brain tumor patients. | The interdisciplinary approach combines neuroscience and digital twin technology, offering valuable insights into tumor dynamics and treatment planning in oncology. These insights could be extended beyond brain tumors to other areas of cancer care, supporting a more personalized, data-driven approach to treatment in oncology. |
| [52] Optimizing Fentanyl Dosing with Physics-Based Digital Twins | Focuses on using physics-based digital twins (PBDTs) to personalize fentanyl dosing for cancer patients, incorporating clinical and physiological parameters. The study outlines a clinical protocol for validating PBDT-guided dosing and emphasizes therapeutic drug monitoring. | This approach applies digital twins in pain management, particularly in oncology, offering a personalized and clinically relevant strategy to optimize opioid dosing and minimize risks for advanced cancer patients. |
| [53] Promoting FAIR Data Sharing in Biomedical Research | Presents a digital twin-based system to facilitate FAIR sharing of sensitive biomedical data. The system allows non-IT-affine users to generate synthetic data and enrich existing datasets, promoting open science and reproducibility in research. | By making data sharing easier for non-technical users and fostering collaboration, this work advances the integration of digital twins into data management systems, significantly enhancing the accessibility and utility of biomedical data. |
| [54] Predicting Treatment Outcomes with Mathematical Models and Digital Twins | Investigates mathematical models and digital twins to predict treatment outcomes for lung cancer patients undergoing immune checkpoint inhibitor therapy. The study integrates a delayed response model to predict disease progression with high accuracy. | This research shows how digital twins, in combination with mathematical models, can provide clinicians with actionable insights, improving treatment decision-making in oncology and helping predict patient outcomes with precision. |
| [55] Machine Learning and Radiomics in Non-Small Cell Lung Cancer | Explores the value of healthy organ data alongside tumor tissue for survival prediction in non-small cell lung cancer using PET/CT images and radiomics. | PET and CT Organomics significantly enhance survival prediction models. This approach is key for developing digital twins in oncology, improving patient outcomes. |
| [56] Digital Twins for OvCa Patients and Caregivers | Creates digital twins of OvCa patients and caregivers, using data from forums, interviews, expert input, and clinical notes to represent diverse cancer trajectories and needs. | The digital twins enable AI model training in scenarios with limited user data, offering a promising tool for personalized healthcare and decision support systems in cancer care. |
| [57] Cell State Transition Assessment for Breast Cancer | Applies the cSTAR approach to analyze phosphoproteomic data from breast cancer cell lines, identifying key signaling nodes and causal connections within core control networks. | cSTAR models offer a mechanistic understanding of cancer subtypes and suggest strategies for normalizing signaling networks, potentially offering therapeutic avenues for breast cancer. |
| [58] Physics-Based Digital Twin for Transdermal Fentanyl Delivery | Develops a physics-based digital twin to optimize fentanyl delivery through transdermal patches, considering factors like skin characteristics, temperature, and activity levels. | Temperature regulation and personalized patch placement improve fentanyl delivery consistency, reducing variability in pain management outcomes, offering a tool for clinical decision-making. |
| [59] Simplifying Patient-Specific CFD Simulations for Hepatocellular Carcinoma Treatment | Evaluates simplification strategies for CFD simulations in hepatocellular carcinoma treatment, reducing computational time while maintaining accuracy. | Grid coarsening effectively reduces computational time by 45%, maintaining accuracy, and improving the efficiency of patient-specific simulations for personalized cancer treatment. |
| [60] Applications of Digital Twins in Laboratory Medicine | Explores digital twins in laboratory medicine, focusing on personalized treatment plans, biological variation data, and AI-driven lab test interpretation. | Digital twins combined with AI and synthetic data can revolutionize laboratory medicine, improving personalized diagnostics and treatment planning, and enhancing healthcare precision. |
4.4. Integrating Digital Twins: Navigating Ethical and Legal Complexities
4.5. Core vs. Supportive Technologies in Digital Twins: Available Platforms and Their Roles in Oncology
| Reference | Device/Platform Name | Function in Oncology Digital Twin | Category |
|---|---|---|---|
| [65] | ModCell™ | Mechanistic modeling system for predicting tumor response to specific therapies, integrating omic data and in silico simulations. | Core |
| [66] | Cytocast™ Digital Twin | Platform that creates a digital twin of the patient using multi-omic and clinical data to simulate drug efficacy and side effects. | Core |
| [67] | Quibim QP-Insights | Cloud platform for the management and quantitative analysis of multi-omic data, supporting precision medicine and oncology research. | Support |
| [68] | CERTAINTY Virtual Twin | European project developing a virtual twin for supporting personalized cancer treatment, focusing on advanced therapies like CAR-T cells. | Support |
| [69] | Q Bio Gemini | Platform for creating a digital twin of the entire human body, integrating genetic, biochemical, imaging data, and more for health monitoring. | Support |
| [70] | IBM Watson for Oncology | AI platform for analyzing oncology data, providing therapeutic recommendations based on scientific evidence. | Support |
- Reference: This column cites the source of the device or platform, providing a reference for further exploration.
- Device/Platform Name: The specific name of the technology, system, or platform that is being utilized for oncology digital twin development.
- Function in Oncology Digital Twin: A brief description of how each device or platform contributes to the use of digital twins in oncology, focusing on its core functionalities and how it enhances personalized cancer treatment, tumor modeling, or therapy simulation.
- Category: This column categorizes the device or platform, for instance, whether it is considered a core or auxiliary component (supportive technology) in oncology digital twin systems.
- ModCell™: This platform stands as an example of how mechanistic modeling can predict tumor responses to therapies. By integrating omic data and performing in silico simulations, ModCell™ enables the construction of a highly personalized digital twin, essential for exploring how a particular tumor might behave under different treatments. This predictive capability can significantly improve decision-making processes in oncology, making it a core tool in the development of precision therapies. Though ModCell™ is often applied in oncology, it can be adapted to other clinical areas that require predictive modeling of disease mechanisms.
- Cytocast™ Digital Twin: Similarly to ModCell™, Cytocast™ leverages multi-omic and clinical data to create a digital twin of the patient. Its strength lies in simulating not just the tumor’s behavior but also the potential side effects of treatments. By providing a more holistic view of treatment outcomes, Cytocast™ helps doctors tailor therapy choices, balancing efficacy and minimizing risks for patients. While Cytocast™ is especially valuable in oncology, its modeling capabilities are applicable in other therapeutic areas where understanding complex treatment responses is critical.
- Quibim QP-Insights: While not a digital twin in itself, Quibim QP-Insights provides valuable data processing capabilities, helping oncology teams manage and analyze multi-omic data. By extracting relevant insights, it enhances the foundation on which a digital twin can be built, improving the precision and predictive power of models. This tool is particularly useful in oncology, where large datasets need to be analyzed to support personalized treatment plans.
- CERTAINTY Virtual Twin: Although this platform is part of an ongoing European research project, it is an example of how virtual twin technology is being applied to personalized cancer treatment. It focuses on advanced therapies, like CAR-T cell treatments, providing simulations that inform both clinical trials and patient-specific treatment planning. Although it is still in the development phase, its potential to support digital twins in oncology is immense. CERTAINTY is not yet a fully realized product but shows significant promise in advancing virtual twin applications for oncology and beyond.
- Q Bio Gemini: Though Q Bio Gemini is primarily focused on whole-body health monitoring, its ability to create a comprehensive digital twin of a patient that integrates genetic, biochemical, and imaging data makes it a valuable resource in the oncology space. While it is not exclusively cancer-focused, the data it provides can be crucial in understanding how cancer develops and progresses, supporting digital twin models for personalized care. Q Bio Gemini’s wide-ranging health data make it applicable to a variety of clinical fields, not just oncology.
- IBM Watson for Oncology: Powered by AI, IBM Watson for Oncology analyzes vast amounts of oncology data to provide actionable insights and therapeutic recommendations. While not a traditional digital twin platform, its AI-driven support aids clinicians in making informed decisions, enhancing the digital twin models by feeding them with evidence-based, up-to-date treatment strategies. Watson’s ability to process and interpret large datasets is a key supportive feature for personalized cancer treatment.
4.6. Future Directions
4.7. Limitations
- Expand Data Sources: Integrate conference proceedings, preprints, and gray literature to capture emerging trends and novel applications.
- Enhance Cross-Cultural Insights: Conduct studies across different linguistic and cultural contexts to better understand region-specific adaptations and challenges.
- Integrate Primary Research: Complement reviews with qualitative and mixed-methods studies to capture real-world implementation challenges and patient experiences.
- Develop Standardized Evaluation Frameworks: Establish universal benchmarks for assessing digital twin effectiveness, usability, and ethical compliance.
- Explore Long-Term Social and Psychological Effects: Investigate the impact of digital twins on healthcare providers, patient trust, and the broader ethical landscape.
- Promote Interdisciplinary Collaboration: Foster collaboration among oncologists, engineers, ethicists, and policymakers to ensure responsible and impactful digital twin development.
5. Conclusions
Funding
Conflicts of Interest
References
- Available online: https://www.gartner.com/en/information-technology/glossary/digital-twin (accessed on 22 March 2025).
- Available online: https://www.digitaltwinconsortium.org/initiatives/the-definition-of-a-digital-twin/ (accessed on 22 March 2025).
- Available online: https://ntrs.nasa.gov/api/citations/20230008294/downloads/%20Digital%20Twin%20Consortium%202023%20Meeting%20vickers%20revd%20final.pdf (accessed on 22 March 2025).
- Available online: https://www.iso.org/standard/75066.html (accessed on 22 March 2025).
- Available online: https://www.weforum.org/stories/2022/05/digital-twin-technology-virtual-model-tech-for-good (accessed on 22 March 2025).
- Shen, M.D.; Chen, S.B.; Ding, X.D. The effectiveness of digital twins in promoting precision health across the entire population: A systematic review. NPJ Digit. Med. 2024, 7, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital twins for health: A scoping review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuriakose, S.M.; Joseph, J.; Rajimol, A.; Kollinal, R. The Rise of Digital Twins in Healthcare: A Mapping of the Research Landscape. Cureus 2024, 16, e65358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drummond, D.; Coulet, A. Technical, Ethical, Legal, and Societal Challenges with Digital Twin Systems for the Management of Chronic Diseases in Children and Young People. J. Med. Internet Res. 2022, 24, e39698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappon, G.; Facchinetti, A. Digital Twins in Type 1 Diabetes: A Systematic Review. J. Diabetes Sci. Technol. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sager, S. Digital twins in oncology. J. Cancer Res. Clin. Oncol. 2023, 149, 5475–5477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernandez-Boussard, T.; Macklin, P.; Greenspan, E.J.; Gryshuk, A.L.; Stahlberg, E.; Syeda-Mahmood, T.; Shmulevich, I. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat. Med. 2021, 27, 2065–2066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://datascience.cancer.gov/news-events/blog/digital-twins-cancer-not-if-when-how-and-why (accessed on 22 March 2025).
- Shen, S.; Qi, W.; Liu, X.; Zeng, J.; Li, S.; Zhu, X.; Dong, C.; Wang, B.; Shi, Y.; Yao, J.; et al. From virtual to reality: Innovative practices of digital twins in tumor therapy. J. Transl. Med. 2025, 23, 348. [Google Scholar] [CrossRef]
- Available online: https://dl.acm.org/doi/fullHtml/10.1145/3543873.3587688 (accessed on 22 March 2025).
- Chaudhuri, A.; Pash, G.; Hormuth, D.A., II; Lorenzo, G.; Kapteyn, M.; Wu, C.; Lima, E.A.B.F.; Yankeelov, T.E.; Willcox, K. Predictive digital twin for optimizing patient-specific radiotherapy regimens under uncertainty in high-grade gliomas. Front. Artif Intell. 2023, 6, 1222612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumini, M.; Teodori, F.; Isolan, L. Digital twins in dosimetry and radiotherapy, a survey and some applications. Radiat. Phys. Chem. 2024, 218, 111649. [Google Scholar] [CrossRef]
- Available online: https://health.ec.europa.eu/document/download/6e4f5ecb-ea9c-445a-a661-7147809aa255_en?filename=policy_20241126_js02_en.pdf&prefLang (accessed on 22 March 2025).
- Available online: https://www.appliedclinicaltrialsonline.com/view/new-regulatory-road-clinical-trials-digital-twins (accessed on 22 March 2025).
- Available online: https://legacyfileshare.elsevier.com/promis_misc/ANDJ%20Narrative%20Review%20Checklist.pdf (accessed on 22 March 2025).
- Santos, C.S.; Amorim-Lopes, M. Externally validated and clinically useful machine learning algorithms to support patient-related decision-making in oncology: A scoping review. BMC Med. Res. Methodol. 2025, 25, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Loscalzo, J.; Mahmud, A.K.M.F.; Aly, D.M.; Rzhetsky, A.; Zitnik, M.; Benson, M. Digital twins as global learning health and disease models for preventive and personalized medicine. Genome Med. 2025, 17, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, J.; Lafata, K.; Kim, E.; Yao, C.; Lin, F.; Rattay, T.; Nori, H.; Katsoulakis, E.; Lee, C.I. Artificial intelligence across oncology specialties: Current applications and emerging tools. BMJ Oncol. 2024, 3, e000134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tortora, M.; Pacchiano, F.; Ferraciolli, S.F.; Criscuolo, S.; Gagliardo, C.; Jaber, K.; Angelicchio, M.; Briganti, F.; Caranci, F.; Tortora, F.; et al. Medical Digital Twin: A Review on Technical Principles and Clinical Applications. J. Clin. Med. 2025, 14, 324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sel, K.; Hawkins-Daarud, A.; Chaudhuri, A.; Osman, D.; Bahai, A.; Paydarfar, D.; Willcox, K.; Chung, C.; Jafari, R. Survey and perspective on verification, validation, and uncertainty quantification of digital twins for precision medicine. NPJ Digit. Med. 2025, 8, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ștefănigă, S.A.; Cordoș, A.A.; Ivascu, T.; Feier, C.V.I.; Muntean, C.; Stupinean, C.V.; Călinici, T.; Aluaș, M.; Bolboacă, S.D. Advancing Precision Oncology with Digital and Virtual Twins: A Scoping Review. Cancers 2024, 16, 3817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kemkar, S.; Tao, M.; Ghosh, A.; Stamatakos, G.; Graf, N.; Poorey, K.; Balakrishnan, U.; Trask, N.; Radhakrishnan, R. Towards verifiable cancer digital twins: Tissue level modeling protocol for precision medicine. Front. Physiol. 2024, 15, 1473125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Koelzer, V.H. Towards generative digital twins in biomedical research. Comput. Struct. Biotechnol. J. 2024, 23, 3481–3488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deasy, J.O. Data Science Opportunities to Improve Radiotherapy Planning and Clinical Decision Making. Semin. Radiat. Oncol. 2024, 34, 379–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Arulraj, T.; Ippolito, A.; Popel, A.S. From virtual patients to digital twins in immuno-oncology: Lessons learned from mechanistic quantitative systems pharmacology modeling. NPJ Digit. Med. 2024, 7, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdollahi, H.; Yousefirizi, F.; Shiri, I.; Brosch-Lenz, J.; Mollaheydar, E.; Fele- Paranj, A.; Shi, K.; Zaidi, H.; Alberts, I.; Soltani, M.; et al. Theranostic digital twins: Concept, framework and roadmap towards personalized radiopharmaceutical therapies. Theranostics 2024, 14, 3404–3422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metzcar, J.; Jutzeler, C.R.; Macklin, P.; Köhn-Luque, A.; Brüningk, S.C. A review of mechanistic learning in mathematical oncology. Front. Immunol. 2024, 15, 1363144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laubenbacher, R.; Mehrad, B.; Shmulevich, I.; Trayanova, N. Digital twins in medicine. Nat. Comput. Sci. 2024, 4, 184–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yankeelov, T.E.; Hormuth, D.A., II; Lima, E.A.B.F.; Lorenzo, G.; Wu, C.; Okereke, L.C.; Rauch, G.M.; Venkatesan, A.M.; Chung, C. Designing clinical trials for patients who are not average. iScience 2023, 27, 108589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terranova, N.; Venkatakrishnan, K. Machine Learning in Modeling Disease Trajectory and Treatment Outcomes: An Emerging Enabler for Model-Informed Precision Medicine. Clin. Pharmacol. Ther. 2024, 115, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lorenzo, G.; Hormuth, D.A., II; Lima, E.A.B.F.; Slavkova, K.P.; DiCarlo, J.C.; Virostko, J.; Phillips, C.M.; Patt, D.; Chung, C.; et al. Integrating mechanism-based modeling with biomedical imaging to build practical digital twins for clinical oncology. Biophys. Rev. 2022, 3, 021304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinton, P. Computational models in inflammatory bowel disease. Clin. Transl. Sci. 2022, 15, 824–830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.marketsandmarkets.com/Market-Reports/digital-twin-market-225269522.html (accessed on 22 March 2025).
- Available online: https://www.grandviewresearch.com/industry-analysis/digital-twin-market (accessed on 22 March 2025).
- Available online: https://www.marketsandmarkets.com/Market-Reports/digital-twins-in-healthcare-market-74014375.html (accessed on 22 March 2025).
- Available online: https://www.grandviewresearch.com/industry-analysis/healthcare-digital-twins-market-report (accessed on 22 March 2025).
- Available online: https://www.linkedin.com/pulse/digital-twins-healthcare-market-worth-211-billion-2028-snehal-gupta-olxzf (accessed on 22 March 2025).
- Available online: https://event.eortc.org/ena2024/2024/10/25/digital-twin-prediction/ (accessed on 22 March 2025).
- Available online: https://ascopost.com/news/october-2024/scientists-develop-a-digital-twin-model-to-predict-cancer-treatment-responses/ (accessed on 22 March 2025).
- Fountzilas, E.; Pearce, T.; Baysal, M.A.; Chakraborty, A.; Tsimberidou, A.M. Convergence of evolving artificial intelligence and machine learning techniques in precision oncology. NPJ Digit. Med. 2025, 8, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strigari, L.; Schwarz, J.; Bradshaw, T.; Brosch-Lenz, J.; Currie, G.; El-Fakhri, G.; Jha, A.K.; Mežinska, S.; Pandit-Taskar, N.; Roncali, E.; et al. Computational Nuclear Oncology Toward Precision Radiopharmaceutical Therapies: Ethical, Regulatory, and Socioeconomic Dimensions of Theranostic Digital Twins. J. Nucl. Med. 2025, 66, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Arulraj, T.; Ippolito, A.; Popel, A.S. Quantitative Systems Pharmacology Modeling in Immuno-Oncology: Hypothesis Testing, Dose Optimization, and Efficacy Prediction. Handb. Exp. Pharmacol 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.L.; Aguilar, B.; Getz, M.; Shmulevich, I.; Macklin, P. A multiscale model of immune surveillance in micrometastases gives insights on cancer patient digital twins. NPJ Syst. Biol. Appl. 2024, 10, 144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Benito, Á.; García-Aznar, J.M.; Gómez-Benito, M.J.; Pérez, M.Á. Patient-specific prostate tumour growth simulation: A first step towards the digital twin. Front. Physiol. 2024, 15, 1421591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emmert-Streib, F.; Cherifi, H.; Kaski, K.; Kauffman, S.; Yli-Harja, O. Complexity data science: A spin-off from digital twins. PNAS Nexus 2024, 3, pgae456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fekonja, L.S.; Schenk, R.; Schröder, E.; Tomasello, R.; Tomšič, S.; Picht, T. The digital twin in neuroscience: From theory to tailored therapy. Front. Neurosci. 2024, 18, 1454856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cukic, M.; Annaheim, S.; Bahrami, F.; Defraeye, T.; De Nys, K.; Jörger, M. Is personal physiology-based rapid prediction digital twin for minimal effective fentanyl dose better than standard practice: A pilot study protocol. BMJ Open 2024, 14, e085296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jean-Quartier, C.; Stryeck, S.; Thien, A.; Vrella, B.; Kleinschuster, J.; Spreitzer, E.; Wali, M.; Mueller, H.; Holzinger, A.; Jeanquartier, F. Unlocking biomedical data sharing: A structured approach with digital twins and artificial intelligence (AI) for open health sciences. Digit. Health 2024, 10, 20552076241271769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scibilia, K.R.; Schlicke, P.; Schneller, F.; Kuttler, C. Predicting resistance and pseudoprogression: Are minimalistic immunoediting mathematical models capable of forecasting checkpoint inhibitor treatment outcomes in lung cancer? Math. Biosci. 2024, 376, 109287. [Google Scholar] [CrossRef] [PubMed]
- Salimi, Y.; Hajianfar, G.; Mansouri, Z.; Sanaat, A.; Amini, M.; Shiri, I.; Zaidi, H. Organomics: A Concept Reflecting the Importance of PET/CT Healthy Organ Radiomics in Non-Small Cell Lung Cancer Prognosis Prediction Using Machine Learning. Clin. Nucl. Med. 2024, 49, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thaker, K.; Hui, V.; Brusilovsky, P.; He, D.; Donovan, H.; Lee, Y.J. Utilizing Digital Twin to Create Personas Representing Ovarian Cancer Patients and Their Families. Stud. Health Technol. Inform. 2024, 315, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Rukhlenko, O.S.; Imoto, H.; Tambde, A.; McGillycuddy, A.; Junk, P.; Tuliakova, A.; Kolch, W.; Kholodenko, B.N. Cell State Transition Models Stratify Breast Cancer Cell Phenotypes and Reveal New Therapeutic Targets. Cancers 2024, 16, 2354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahrami, F.; Psikuta, A.; Rossi, R.M.; Dommann, A.; Defraeye, T. Exploring the thermally-controlled fentanyl transdermal therapy to provide constant drug delivery by physics-based digital twins. Eur. J. Pharm. Sci. 2024, 200, 106848. [Google Scholar] [CrossRef] [PubMed]
- Bomberna, T.; Maleux, G.; Debbaut, C. Simplification strategies for a patient-specific CFD model of particle transport during liver radioembolization. Comput. Biol. Med. 2024, 178, 108732. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M. Dynamic mirroring: Unveiling the role of digital twins, artificial intelligence and synthetic data for personalized medicine in laboratory medicine. Clin. Chem. Lab. Med. 2024, 62, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Garske, B.; Holz, W.; Ekardt, F. Digital twins in sustainable transition: Exploring the role of EU data governance. Front. Res. Metr. Anal. 2024, 9, 1303024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuersten, A. Health Digital Twins, Legal Liability, and Medical Practice. Am. J. Bioeth. 2023, 23, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.D.; Krauthammer, M.; Biller-Andorno, N. The Use and Ethics of Digital Twins in Medicine. J. Law Med. Ethics. 2022, 50, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D. Making Compassionate Use More Useful: Using real-world data, real-world evidence and digital twins to supplement or supplant randomized controlled trials. Pac. Symp. Biocomput. 2021, 26, 38–49. [Google Scholar] [PubMed]
- Available online: https://www.alacris.de/digital-twin/ (accessed on 22 March 2025).
- Available online: https://cytocast.com/cytocast-digital-twin-platform/ (accessed on 22 March 2025).
- Available online: https://quibim.com/qp-insights/ (accessed on 22 March 2025).
- Available online: https://www.certainty-virtualtwin.eu/ (accessed on 22 March 2025).
- Available online: https://www.q.bio/ (accessed on 22 March 2025).
- Available online: https://www.ibm.com/mysupport/s/topic/0TO500000002PWlGAM/watson-for-oncology?language=it (accessed on 22 March 2025).

| Source | Definition | Emphasis |
|---|---|---|
| Gartner (2020) [1] | “A virtual representation of a real-world entity or system that uses real-time data to simulate behaviors and enhance decision-making.” | Dynamic real-time data integration, behavior modeling, and decision support optimization. This definition emphasizes the importance of integrating real-time data from the physical world into the digital model, enabling the simulation of behaviors that can enhance the decision-making process in dynamic environments. It highlights the application of digital twin (DT) technology as a decision support tool. |
| Digital Twin Consortium [2] | “An accurate virtual representation of an object, system, or process that continuously updates with real data to support monitoring, analysis, and optimization.” | Real-time continuous data synchronization, high model accuracy, and data-driven decision-making for process optimization. This source highlights continuous real-time updates to ensure the digital twin remains highly accurate over time. It underscores the role of the digital twin in supporting data analytics and optimization to improve processes and systems within industries. |
| NASA (2012) [3] | “A digital model of a physical system that integrates data, simulations, and analytics to understand, predict, and optimize its operation.” | Comprehensive integration of analytics, predictive modeling, and operational optimization through simulations. NASA’s definition focuses on the ability of digital twins to integrate a variety of data types (including simulations and analytics) to predict behaviors and optimize operations of physical systems. It emphasizes the utility of digital twins for long-term operational efficiency and predictive maintenance. |
| ISO 23247-1:2021 [4] | “A set of interconnected digital models that replicate the characteristics of a physical entity, enabling bidirectional interactions between the real and virtual worlds.” | Interconnected, multi-dimensional digital models with bidirectional interaction for seamless integration between virtual and physical environments. ISO’s definition emphasizes the interconnectedness of digital models that replicate real-world entities. The concept of bidirectional interaction stands out, ensuring that changes in the physical world are reflected in the digital twin and vice versa, which is essential for continuous monitoring and optimization. The alignment with Industry 4.0 highlights the advancement towards smart, automated industrial processes. |
| Domain | DT Applications | Technologies/Data Integrated | Purpose |
|---|---|---|---|
| Imaging (MRI, PAI) | Real-time tumor modeling; monitoring of tumor microenvironment changes | MRI, photoacoustic imaging, ultrasound, CT scans | Enhanced tumor visualization; dynamic tracking of tumor evolution |
| Diagnosis | Predictive modeling of cancer onset and progression; risk stratification | Genomics, liquid biopsies, histopathology images | Early detection; accurate staging and prognosis |
| Chemotherapy | Simulation of pharmacokinetics and pharmacodynamics; optimization of drug regimens | Pharmacogenomics, treatment response data | Predict therapeutic efficacy; reduce toxicity |
| Radiotherapy | Personalized radiation dose planning; adaptation during treatment | CT, MRI, radiomics data | Maximize tumor control; minimize damage to healthy tissue |
| Photodynamic Therapy (PDT) | Optimization of light dosimetry and photosensitizer administration | Optical imaging; tissue oxygenation measurements | Improve therapeutic outcomes; reduce side effects |
| Photothermal Therapy (PTT) | Simulation of heat distribution; optimization of energy delivery | Thermal imaging; nanoparticle distribution models | Precision ablation of tumors; preservation of healthy tissue |
| Reference | Brief Description | Focus on Oncology | Innovative Approach |
|---|---|---|---|
| [21] | Scoping review mapping externally validated machine learning (ML)-based models in cancer care, assessing their performance, clinical utility, and model relationships. | Although the focus is on machine learning, it contributes to the integration of ML with digital twin concepts in oncology by improving clinical decision-making and cancer patient care. | Integration of ML with digital twin concepts for oncology decision support. |
| [22] | Discusses digital twins (DTs) for personalized treatments in oncology, addressing challenges like data integration and real-time simulation for patient-specific treatments. | Direct focus on oncology with applications in cancer treatment, disease prevention, and personalized therapies. | Digital twins (DTs) used to simulate disease and treatment pathways, enabling more personalized cancer care. |
| [23] | Review on the integration of AI and DTs in oncology, focusing on the usage of imaging, pathology, radiotherapy, and genomics. | Oncological applications including imaging and genomics-driven therapies powered by DTs. | AI and DTs work together to enable precision medicine, improving diagnostic accuracy and treatment effectiveness in oncology. |
| [24] | Exploration of digital twins (DTs) for improving healthcare, particularly in oncology and neurology, including challenges in data integration. | Oncology-specific applications, such as diagnostic simulations and predictive modeling for cancer progression thanks to the advancing medical knowledge in neuroscience. | Demonstrates the potential of digital twin technology to model patient-specific oncology scenarios, allowing for tailored treatment plans, while simultaneously highlighting the importance of neuroscience in understanding complex tumor behaviors and brain–cancer interactions. |
| [25] | Focus on the role of verification, validation, and uncertainty quantification (VVUQ) for digital twins, with applications in oncology. | Direct focus on the use of DTs in oncology, particularly in ensuring the safety and efficacy of patient simulations. | VVUQ framework helps ensure the reliability and clinical utility of DTs in oncology. |
| [26] | Scoping review of digital twins (DTHs) and virtual twins (VTHs) in oncology, examining technical solutions, challenges, and credibility. | Broad coverage of oncology types including breast, lung, and colorectal cancers, focusing on diagnosis, therapy, and monitoring. | Digital twins in oncology for personalized diagnosis and treatment simulations, helping in therapy optimization. |
| [27] | Discusses advancements in computational methodologies for precision oncology, with an emphasis on cancer digital twins (DTs) for patient-specific decision-making. | Focuses on cancer digital twins for precision oncology, integrating patient-specific data and mathematical models for improved clinical decisions. | Development of cancer digital twins using agent-based modeling and integration of tumor microenvironment simulations. |
| [28] | Outlines the potential of generative digital twins (GDTs) in biomedical research, focusing on the creation of high-fidelity virtual replicas for oncology applications. | Applies generative digital twins in oncology for personalized diagnostics and treatments. | Use of generative AI to create spatially resolved digital representations of tumors and biological entities. |
| [29] | Reviews the use of digital twins in radiotherapy, focusing on personalized cancer treatments guided by mechanistic patient-specific simulations. | Oncology focus on personalized radiotherapy and the use of digital twins to guide treatment adaptations based on patient-specific data. | Personalized digital twins in radiotherapy to improve tumor control while minimizing damage to healthy tissues. |
| [30] | Discusses the challenges of generating virtual patient populations and digital twins for immuno-oncology, with a focus on personalized models for specific clinical settings. | Immuno-oncology applications, focusing on creating digital twins for cancer treatment personalization. | Development of digital twins for immuno-oncology research, with study-specific models tailored to clinical needs. |
| [31] | Proposes theranostic digital twins (TDTs) to personalize radiopharmaceutical therapy (RPT) based on patient-specific data to optimize treatment and minimize toxicity. | Focus on radiopharmaceutical therapy in oncology, using TDTs to optimize radiation dose and minimize side effects. | Theranostic digital twins (TDTs) that personalize cancer treatment based on real patient data, improving precision and reducing risks. |
| [32] | Explores the potential of mechanistic learning (ML) combining mathematical modeling and machine learning in oncology, with applications in tumor prediction and response modeling. | Focus on oncology research, utilizing mechanistic learning for tumor response prediction and time-to-event modeling. | Synergistic use of mechanistic mathematical modeling and machine learning, including the integration of digital twins in oncology. |
| [33] | Provides an overview of medical digital twins, highlighting their application in oncology and cardiology, and discusses major challenges such as data integration and privacy. | Focuses on digital twins in oncology for personalized treatments and diagnostics. | Emphasizes the use of digital twins to personalize medicine and improve patient outcomes, while addressing challenges in data integration. |
| [34] | Describes a computational framework for personalized clinical trials using digital twins, mathematical modeling, and data assimilation. | Applies digital twins in oncology to simulate patient-specific interventions and optimize treatment outcomes. | Use of digital twins in personalized clinical trials, applying optimal control theory and data assimilation to refine predictions and interventions. |
| [35] | Discusses model-informed precision medicine using AI and ML, focusing on the integration of digital twins in drug development, particularly in oncology. | Focuses on oncology and immuno-oncology, using digital twins to personalize treatments based on multi-dimensional biomarker data. | Use of AI/ML to integrate real-world and -omics data, enabling model-informed precision medicine with digital twins for better treatment outcomes. |
| [36] | Reviews the potential of digital twins in oncology, specifically through image-guided, mechanism-based, tissue-scale mathematical modeling. | Focuses on oncology, particularly using digital twins for tumor dynamics and patient-specific care. | Development of image-guided, mechanism-based digital twins to model tumor behavior and personalize cancer treatment. |
| [37] | Discusses the application of AI and digital twins in precision medicine for inflammatory bowel disease (IBD) and their potential use in oncology. | While focused on IBD, the review highlights the potential of digital twins in oncology, particularly in predictive modeling and precision dosing. | Combines mechanistic modeling and AI to create digital twins for personalized treatments in IBD and oncology. |
| Category | Key Topics | Key References |
|---|---|---|
| 1. Foundations and Frameworks for Digital Twins in Oncology | Computational frameworks for precision oncology using digital twins (DTs), agent-based modeling for simulating cancer dynamics, patient-specific data for personalized treatments, data assimilation to continuously update models | [27,33,34] |
| 2. Innovative Applications of Digital Twins in Oncology | Application of DTs in radiotherapy for personalized treatment planning, tumor control, minimizing normal tissue damage using patient-specific DT models, development of theranostic digital twins (TDTs) to optimize radiopharmaceutical therapies and minimize toxicity | [29,31,36] |
| 3. Technological Integration: Data, Machine Learning, and Modeling | Integration of AI and ML techniques with DTs to enhance precision medicine in oncology, enabling the use of real-world data, and improving drug development and treatment strategies, hybrid models combining mechanistic and AI-driven approaches for creating DTs, model-informed precision medicine using DTs for optimized treatment planning | [21,35,37] |
| 4. Challenges in Digital Twin Development | Key challenges in creating spatially resolved generative DTs for oncology, such as integrating multi-modal data (e.g., imaging, genomics) and biological complexity, generating immuno-oncology-specific DTs tailored to clinical settings, the difficulty of overcoming cancer heterogeneity using DTs | [26,28,30] |
| 5. Clinical Utility and Impact of Digital Twins in Oncology | The potential of DTs to improve clinical decision-making, assist in patient-specific treatment planning, and enhance treatment outcomes by simulating real-time patient responses, mechanistic learning models used with DTs for optimizing treatment protocols in oncology | [24,32] |
| 6. Future Directions and Long-term Goals for Digital Twins in Oncology | Long-term potential of DTs in oncology, focusing on their ability to improve personalized treatments, address challenges like data integration, privacy concerns, and enhance the understanding of tumor dynamics for more effective treatment planning | [27,33] |
| 7. Cross-Disciplinary Approaches | Cross-disciplinary applications of DTs in oncology and other fields (e.g., IBD), the integration of AI-driven hybrid models for creating and utilizing DTs to enhance therapeutic responses, collaborative approaches to data analysis for DTs across multiple domains | [35,37] |
| 8. Personalized Trials and Patient-Centered Models | Development of computational frameworks for personalized clinical trials using DTs, real-time data assimilation to continuously refine patient models, optimizing trial designs by integrating patient-specific data with DTs to simulate treatment outcomes | [34] |
| Study | Opportunities | Barriers |
|---|---|---|
| [21] | Enhancement of clinical decision-making: DTs can integrate machine learning models into oncology practice to provide personalized treatment decisions based on patient data. Improved treatment strategies: DTs help optimize therapy selection by predicting the response to different cancer treatments. | Data variability: Challenges in dealing with heterogeneous clinical data, which can affect the reliability of the DTs. Clinical validation: Difficulty in validating DT models in diverse, real-world clinical settings due to the complexity of the cancer patient population. |
| [22] | Personalized treatment plans: DTs can predict and simulate patient-specific responses, allowing oncologists to fine-tune therapies for each patient. Early diagnosis and intervention: The use of predictive models to detect cancer and identify the most effective treatment modalities early on. | Complexity of modeling: Difficulty in developing accurate, patient-specific DTs due to the complexity and variability of biological data. Scalability: Issues with applying predictive models across a large and diverse patient base in real-world clinical settings. |
| [23] | Optimization of therapy regimens: DTs can simulate and predict optimal chemotherapy or radiotherapy protocols tailored to individual patients, increasing treatment efficacy. Enhancement of treatment outcomes: Personalized models can improve patient survival rates by tailoring therapies to specific tumor dynamics. | Computational demand: Real-time simulations for patient-specific treatment planning can be computationally expensive and resource-intensive. Data limitations: Insufficient or low-quality patient data limits the ability of DTs to accurately simulate patient-specific responses. |
| [24] | Revolutionizing oncology care: Digital health twins (DHTs) can integrate large-scale data, including genetic and environmental factors, to offer truly personalized care. AI integration: Using AI to enhance the predictive accuracy of DTs, improving treatment and monitoring in oncology. | Infrastructure challenges: Existing healthcare systems may lack the resources or infrastructure to adopt and implement DT technologies. Regulatory hurdles: Challenges in regulatory approval and validation of digital health twins for clinical use. |
| [25] | Precision dosing: DTs can be used to personalize chemotherapy and radiotherapy dosing for cancer patients, reducing toxicity and improving effectiveness. Integration of multi-omics data: DTs can integrate genomic, transcriptomic, and proteomic data for better precision in treatment planning. | Data sparsity: Lack of comprehensive and high-resolution patient data limits the ability of DTs to accurately model cancer progression and therapy responses. High computational resources: Generating and updating DTs requires significant computational power, which may not be feasible in all clinical settings. |
| [26] | Multi-modal data integration: The ability of DTs to combine imaging, genomics, and clinical data can provide a more holistic view of cancer progression, enhancing personalized treatment strategies. Improved accuracy: Mathematical modeling can improve the precision of DTs in predicting tumor behavior and therapeutic responses. | Data integration issues: Integrating multi-modal data (e.g., imaging, genomics) is complex and may result in inaccurate or incomplete models. Biological complexity: Capturing the heterogeneity of cancer through DTs is difficult, especially when accounting for tumor mutations and microenvironment variations. |
| [27] | Agent-based modeling: The use of computational frameworks based on DTs can simulate patient-specific treatment plans using agent-based models, which predict tumor growth and response to therapies. Precision in treatment strategies: DTs improve the ability to personalize treatment plans and optimize therapy efficacy for individual patients. | Computational resource requirements: DTs demand high computational power for simulations, especially for large-scale clinical use. Data dependency: The accuracy of DTs heavily depends on high-quality patient-specific data, which may not always be available. |
| [28] | Spatially resolved DTs: The development of generative DTs that can simulate tumor behavior in a 3D spatial context offers more accurate predictions of tumor growth and treatment responses. Data-driven insights: DTs can provide deeper insights into tumor biology, helping oncologists tailor treatment strategies effectively. | Challenges in data integration: Combining clinical, imaging, and genomic data to create spatially accurate DTs is a complex task. Tumor heterogeneity: Cancer’s inherent biological variability complicates the development of universal DTs for different tumor types. |
| [29] | Radiotherapy personalization: DTs allow for the simulation of radiotherapy treatment plans that are tailored to the individual patient’s tumor and surrounding tissues, minimizing normal tissue damage. Tumor control optimization: DTs can predict the efficacy of different radiotherapy protocols, enhancing treatment outcomes. | Patient response variability: Variations in how different patients respond to radiotherapy can challenge the effectiveness of DT-based simulations. Dependence on advanced imaging: Accurate tumor modeling through DTs requires high-quality imaging techniques, which may not be universally available. |
| [30] | Immunotherapy optimization: DTs can help personalize immuno-oncology treatments by simulating the immune response and optimizing therapeutic protocols based on patient-specific data. Targeted immunotherapy approaches: DTs can simulate tumor–immune interactions, enabling more targeted immunotherapies for specific patient profiles. | Limited immune data: Accurate DT models require detailed immune system data, which is often sparse or difficult to standardize. Difficulty in immune simulation: Simulating immune responses is inherently complex, and current models may not fully capture immune system dynamics. |
| [31] | Theranostic digital twins (TDTs): These DTs can be used to personalize radiopharmaceutical therapies, minimizing toxicity while optimizing treatment efficacy based on patient-specific data. Personalized therapeutic optimization: TDTs can adjust treatment strategies for radiopharmaceutical therapies, enhancing precision and minimizing side effects. | Data quality and quantity: Reliable data on radiopharmaceutical interactions with tumors is needed to optimize the efficacy of TDTs. Clinical implementation challenges: Scaling the use of TDTs in clinical practice requires overcoming significant barriers in regulatory approval and clinical validation. |
| [32] | Mechanistic learning: Integrating mechanistic learning with DTs could improve decision-making processes by providing insights into cancer progression and response to treatment. Improved clinical outcomes: DTs hold the potential to bridge the gap between preclinical research and real-world clinical outcomes, leading to better therapeutic results. | Complex data integration: DTs require the integration of multiple data types (e.g., clinical, genomic, and imaging), which can be challenging and may lead to inconsistencies. Clinical validation difficulty: Ensuring that DT models align with actual patient outcomes in diverse oncology settings is a significant hurdle. |
| [33] | Transformative potential: DTs can revolutionize personalized oncology treatments by integrating patient-specific data with computational models for more accurate therapy predictions. Long-term use in precision oncology: The long-term goal of DTs in oncology is to develop more precise, efficient, and individualized treatment plans for cancer patients. | Privacy concerns: The large-scale use of patient data in DT models raises privacy and ethical issues, particularly with sensitive health information. Data integration hurdles: Integrating diverse datasets (genomic, clinical, imaging) while maintaining data privacy is a key challenge for DTs. |
| [34] | Personalized clinical trials: DTs can help design clinical trials that are more personalized, integrating real-time data assimilation and mathematical models to enhance patient recruitment and therapy customization. Optimizing clinical trial outcomes: By using DTs to simulate patient responses, clinical trial success rates may be improved. | Real-time data integration: Incorporating real-time data into DT models presents significant computational challenges and can affect model accuracy. Resource-intensive simulations: The computational costs of running personalized simulations for clinical trials may limit widespread adoption. |
| [35] | Model-informed precision medicine: Integrating AI/ML with DTs enhances the precision of oncology treatments, leading to better-targeted drug development and more effective patient care. Clinical trial design optimization: AI-powered DTs can help design more efficient and personalized clinical trials. | Data integration complexity: Using diverse, real-world data for AI/ML applications in DTs can complicate model accuracy and reliability. Scalability and infrastructure needs: High computational power is required for deploying AI/ML-driven DTs across clinical systems. |
| [36] | Personalized oncology treatments: Medical imaging and tissue-scale mathematical modeling are used to develop patient-specific DTs, improving tumor treatment personalization. Simulation of tumor dynamics: DTs can simulate tumor growth and response to therapy, enhancing treatment precision. | Advanced imaging requirements: DTs require high-quality imaging data to ensure accurate tumor modeling and predictions. Complexity of modeling tumor dynamics: Capturing the diverse and dynamic nature of tumors in a digital model is a significant challenge. |
| [37] | Hybrid AI/Mechanistic models: Combining AI with mechanistic modeling for DTs can improve therapy precision in oncology and other medical areas, like inflammatory bowel disease (IBD). Improved therapeutic response: Hybrid models can provide more accurate predictions of patient outcomes, leading to better-targeted therapies. | Data integration challenges: Integrating AI and mechanistic models requires diverse datasets and poses significant challenges in terms of consistency and accuracy. Computational burden: Hybrid models require substantial computational resources to simulate and predict patient outcomes accurately. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giansanti, D.; Morelli, S. Exploring the Potential of Digital Twins in Cancer Treatment: A Narrative Review of Reviews. J. Clin. Med. 2025, 14, 3574. https://doi.org/10.3390/jcm14103574
Giansanti D, Morelli S. Exploring the Potential of Digital Twins in Cancer Treatment: A Narrative Review of Reviews. Journal of Clinical Medicine. 2025; 14(10):3574. https://doi.org/10.3390/jcm14103574
Chicago/Turabian StyleGiansanti, Daniele, and Sandra Morelli. 2025. "Exploring the Potential of Digital Twins in Cancer Treatment: A Narrative Review of Reviews" Journal of Clinical Medicine 14, no. 10: 3574. https://doi.org/10.3390/jcm14103574
APA StyleGiansanti, D., & Morelli, S. (2025). Exploring the Potential of Digital Twins in Cancer Treatment: A Narrative Review of Reviews. Journal of Clinical Medicine, 14(10), 3574. https://doi.org/10.3390/jcm14103574







