Unusual Presentation of Metastatic Testicular Mixed-Germ Cell Tumor with Intracardiac Extension: A Case Report
Abstract
1. Introduction
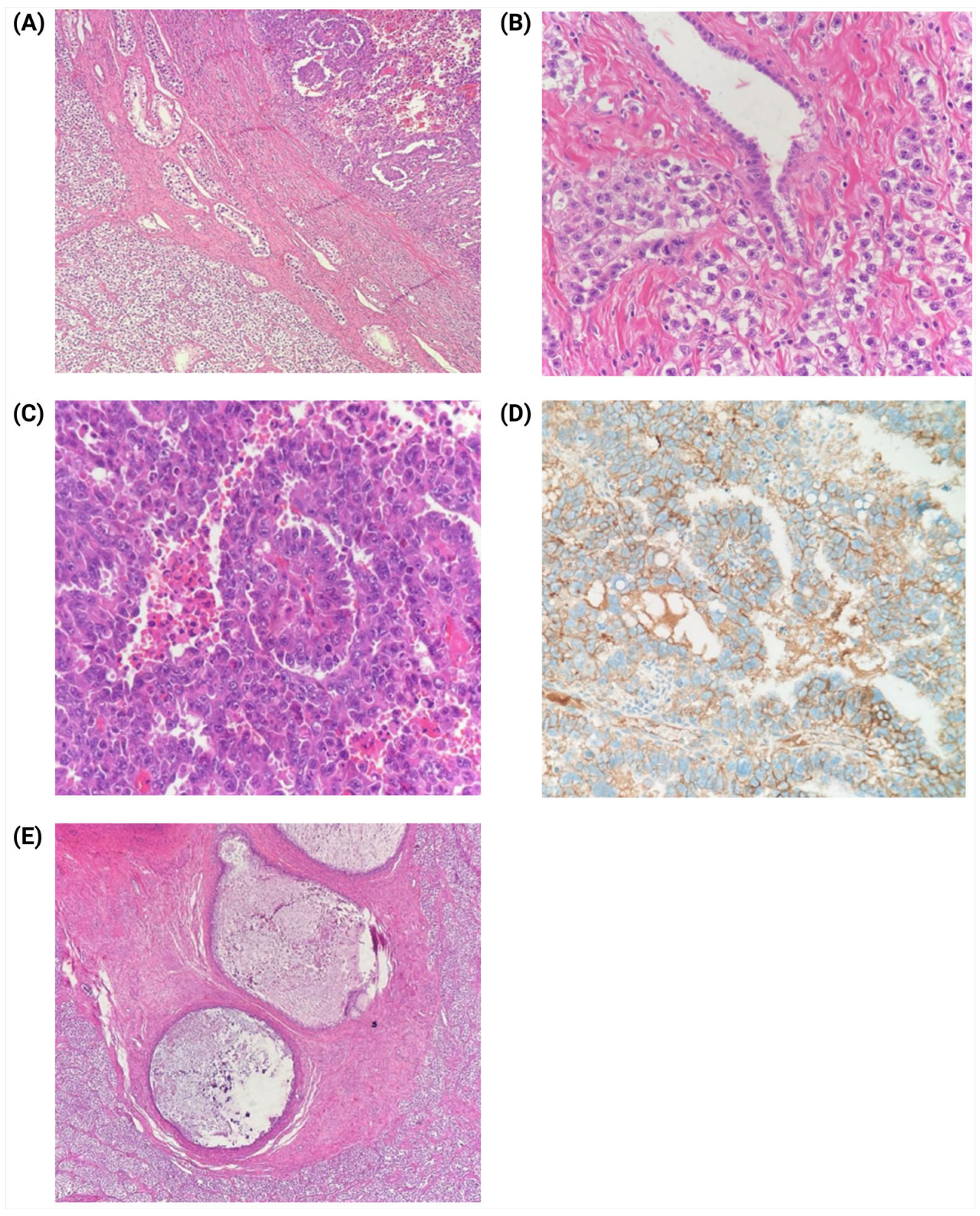
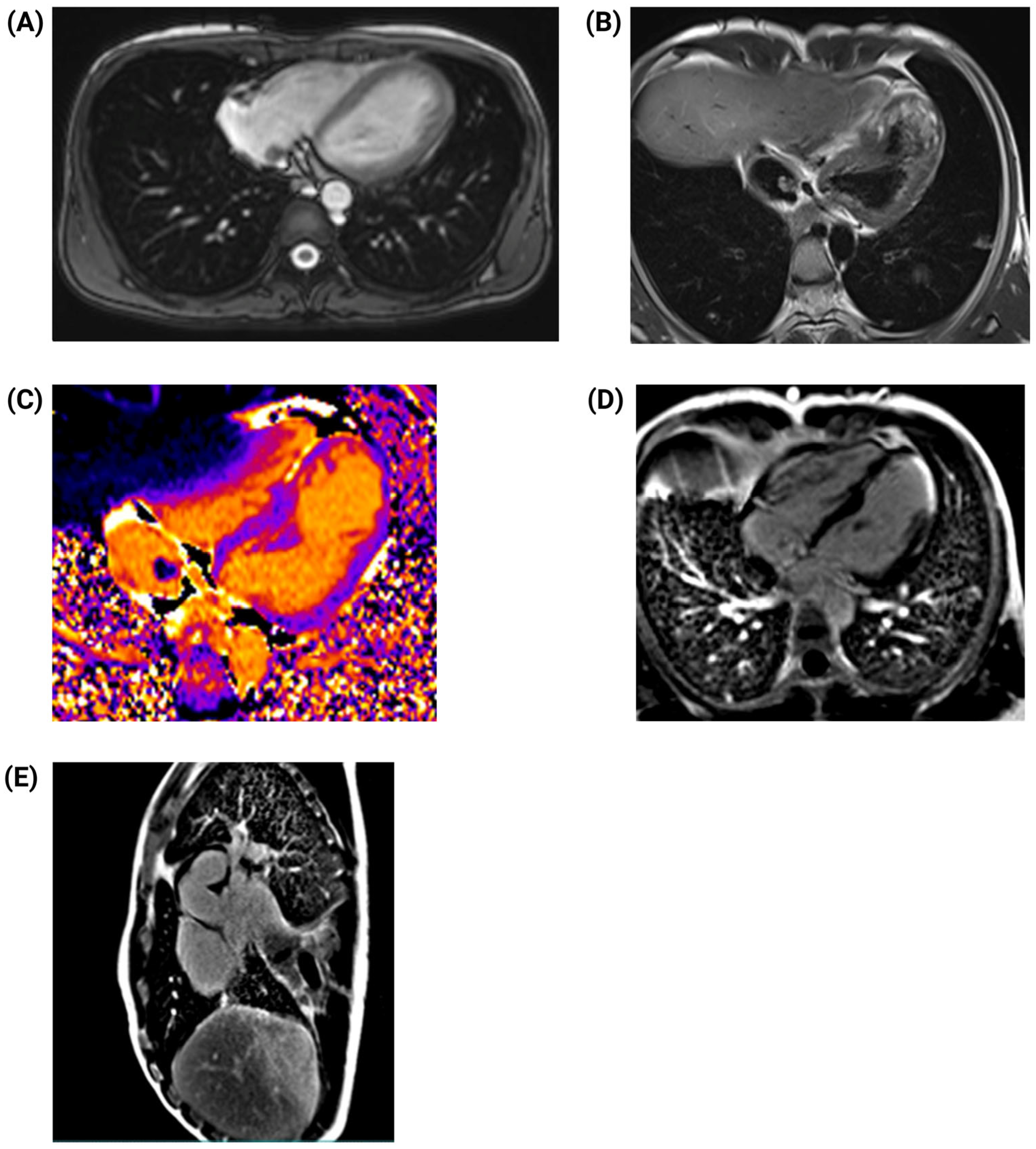
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular Seminoma and Non-Seminoma: ESMO-EURACAN Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Karimi, N.; Wu, X.-N.; Chen, S.-R.; Liu, Y.-X. Testicular Germ Cell Tumor: A Comprehensive Review. Cell. Mol. Life Sci. 2019, 76, 1713–1727. [Google Scholar] [CrossRef]
- Bussani, R.; De-Giorgio, F.; Abbate, A.; Silvestri, F. Cardiac Metastases. J. Clin. Pathol. 2007, 60, 27–34. [Google Scholar] [CrossRef]
- Karigyo, C.J.T.; Pessoa, B.M.S.; Nicacio, S.P.; Terwilliger, E.; Costa, P.; Dos Santos, P.R.; Ernani, V.; Seetharam, M.; Murakami, A.N.; Batalini, F. Cardiac Tumors: Review. Braz. J. Cardiovasc. Surg. 2024, 39, e20230405. [Google Scholar] [CrossRef]
- Casella, M.; Carbucicchio, C.; Dello Russo, A.; Tundo, F.; Bartoletti, S.; Monti, L.; Marana, I.; Giraldi, F.; Tondo, C. Radiofrequency Catheter Ablation of Life-Threatening Ventricular Arrhythmias Caused by Left Ventricular Metastatic Infiltration. Circ. Arrhythm. Electrophysiol. 2011, 4, e7–e10. [Google Scholar] [CrossRef]
- Huseyin, S.; Yüksel, V.; Okyay, A.; Hacıbekiroğlu, İ.; Tastekin, E.; Yilmaztepe, M.; Taylan, G.; Canbaz, S.; Çiçin, İ. A Rare Cause in Etiology of Left Atrial Mass: Metastatic Testicular Germ Cell Tumor. Pol. J. Cardio-Thorac. Surg. 2016, 1, 45–48. [Google Scholar] [CrossRef]
- Bredael, J.J.; Vugrin, D.; Whitmore, W.F. Autopsy Findings in 154 Patients with Germ Cell Tumors of the Testis. Cancer 1982, 50, 548–551. [Google Scholar] [CrossRef]
- Linneweber, J.; Magheli, A.; Lembcke, A.; Christ, T.; Konertz, W. Intracavitary Tumor Spread of a Metastatic Testicular Seminoma in the Left Atrium and Ventricle. J. Thorac. Cardiovasc. Surg. 2011, 141, 583–584. [Google Scholar] [CrossRef]
- Kela, K.; Shah, P.; Buradkar, A.; Avula, S.; Pratap Singh, V.; Buch, T. An Unusual Cause of Echodensity in Heart in Patient with Testicular Cancer. Chest 2021, 160, A227. [Google Scholar] [CrossRef]
- Lucà, F.; Parrini, I.; Canale, M.L.; Rao, C.M.; Nucara, M.; Pelaggi, G.; Murrone, A.; Oliva, S.; Bisceglia, I.; Sergi, A.; et al. Cardiac Metastasis: Epidemiology, Pathophysiology, and Clinical Management. Life 2025, 15, 291. [Google Scholar] [CrossRef]
- Isaji, T.; Iwami, K.; Ato, F.; Watanabe, T.; Takahashi, E.; Miyachi, S. Mixed Germ Cell Tumor with a Yolk Sac Tumor Component in the Medulla Oblongata of a 50-Year-Old Patient: A Case Report and Literature Review. Intern. Med. 2023, 62, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.-F.; Li, J.; Tang, B.; Zhu, Y.-Q. Testicular Mixed Germ Cell Tumor: A Case Report. World J. Clin. Cases 2023, 11, 6902–6907. [Google Scholar] [CrossRef]
- Alvarado-Cabrero, I.; Hernández-Toriz, N.; Paner, G.P. Clinicopathologic Analysis of Choriocarcinoma as a Pure or Predominant Component of Germ Cell Tumor of the Testis. Am. J. Surg. Pathol. 2014, 38, 111–118. [Google Scholar] [CrossRef]
- Bonatelli, M.; Silva, E.C.A.; Cárcano, F.M.; Zaia, M.G.; Lopes, L.F.; Scapulatempo-Neto, C.; Pinheiro, C. The Warburg Effect Is Associated with Tumor Aggressiveness in Testicular Germ Cell Tumors. Front. Endocrinol. 2019, 10, 417. [Google Scholar] [CrossRef]
- Silva, E.C.A.; Cárcano, F.M.; Bonatelli, M.; Zaia, M.G.; Morais-Santos, F.; Baltazar, F.; Lopes, L.F.; Scapulatempo-Neto, C.; Pinheiro, C. The Clinicopathological Significance of Monocarboxylate Transporters in Testicular Germ Cell Tumors. Oncotarget 2018, 9, 20386–20398. [Google Scholar] [CrossRef]
- Perri, A.; Lofaro, D.; Bossio, S.; Maltese, L.; Casaburi, I.; Tucci, L.; La Vignera, S.; Aversa, A.; Aquila, S.; Rago, V. Different Expression Patterns of Metabolic Reprogramming Proteins in Testicular Germ Cell Cancer. Endocrines 2022, 3, 578–589. [Google Scholar] [CrossRef]
- Belge, G.; Hennig, F.; Dumlupinar, C.; Grobelny, F.; Junker, K.; Radtke, A.; Dieckmann, K.-P. Graded Expression of microRNA-371a-3p in Tumor Tissues, Contralateral Testes, and in Serum of Patients with Testicular Germ Cell Tumor. Oncotarget 2020, 11, 1462–1473. [Google Scholar] [CrossRef]
- Gilligan, T.; Lin, D.W.; Adra, N.; Bagrodia, A.; Feldman, D.R.; Yamoah, K.; Aggarwal, R.; Chandrasekar, T.; Costa, D.; Drakaki, A.; et al. NCCN Guidelines® Insights: Testicular Cancer, Version 2.2025: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2025, 23, e250018. [Google Scholar] [CrossRef]
- Butany, J.; Leong, S.W.; Carmichael, K.; Komeda, M. A 30-Year Analysis of Cardiac Neoplasms at Autopsy. Can. J. Cardiol. 2005, 21, 675–680. [Google Scholar]
- Buckley, O.; Madan, R.; Kwong, R.; Rybicki, F.J.; Hunsaker, A. Cardiac Masses, Part 1: Imaging Strategies and Technical Considerations. Am. J. Roentgenol. 2011, 197, W837–W841. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Blankstein, R.; Padera, R.F. Tumors Metastatic to the Heart. Circulation 2013, 128, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus Statement on Endomyocardial Biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Hendrickson, A.W.; Moynihan, T.J. Oncologic Emergencies: Pathophysiology, Presentation, Diagnosis, and Treatment. CA Cancer J. Clin. 2011, 61, 287–314. [Google Scholar] [CrossRef]




| Test | Results | Reference Range |
|---|---|---|
| WBC | 8.89 × 103/μL | 3.4–9.7 × 103/μL |
| Neutrophil | 6.53 × 103/μL | 2.2–4.8 × 103/μL |
| Lymphocyte | 1.75 × 103/μL | 1.1–3.2 × 103/μL |
| Hemoglobin | 14.9 g/dL | 14.0–18.0 g/dL |
| Platelet | 361,000/μL | 130,000–400,000/μL |
| Lactate dehydrogenase (LDH) | 1683 U/L | 135–225 U/L |
| Human chorionic gonadotropin subunit b HCG-b | 11.4 IU/L | <5 IU/L |
| a-fetoprotein (AFP) | 3490 UI/mL | 0–10 UI/mL |
| Post-surgery and first chemotherapy profile | ||
| Lactate dehydrogenase (LDH) | 261.00 | 135–225 U/L |
| a-fetoprotein (AFP) | 429.00 | 0–10 UI/mL |
| Human chorionic gonadotropin subunit b HCG-b | 0.2 IU/L | <5 IU/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Cadena, M.; Rodríguez-Arcentales, F.; Lata, W.; Sacoto, K.M.; Guerrero, L.; Narváez Inca, K.; Arias-Intriago, M.; Ortiz-Prado, E.; Izquierdo-Condoy, J.S. Unusual Presentation of Metastatic Testicular Mixed-Germ Cell Tumor with Intracardiac Extension: A Case Report. J. Clin. Med. 2025, 14, 3564. https://doi.org/10.3390/jcm14103564
Rojas-Cadena M, Rodríguez-Arcentales F, Lata W, Sacoto KM, Guerrero L, Narváez Inca K, Arias-Intriago M, Ortiz-Prado E, Izquierdo-Condoy JS. Unusual Presentation of Metastatic Testicular Mixed-Germ Cell Tumor with Intracardiac Extension: A Case Report. Journal of Clinical Medicine. 2025; 14(10):3564. https://doi.org/10.3390/jcm14103564
Chicago/Turabian StyleRojas-Cadena, Marlon, Felipe Rodríguez-Arcentales, Williams Lata, Karla Mera Sacoto, Luis Guerrero, Katherin Narváez Inca, Marlon Arias-Intriago, Esteban Ortiz-Prado, and Juan S. Izquierdo-Condoy. 2025. "Unusual Presentation of Metastatic Testicular Mixed-Germ Cell Tumor with Intracardiac Extension: A Case Report" Journal of Clinical Medicine 14, no. 10: 3564. https://doi.org/10.3390/jcm14103564
APA StyleRojas-Cadena, M., Rodríguez-Arcentales, F., Lata, W., Sacoto, K. M., Guerrero, L., Narváez Inca, K., Arias-Intriago, M., Ortiz-Prado, E., & Izquierdo-Condoy, J. S. (2025). Unusual Presentation of Metastatic Testicular Mixed-Germ Cell Tumor with Intracardiac Extension: A Case Report. Journal of Clinical Medicine, 14(10), 3564. https://doi.org/10.3390/jcm14103564










