Abstract
Background/Objectives: Oral anticoagulation therapy (OAC) is crucial for reducing the risk of ischemic complications in patients with atrial fibrillation (AF). However, OAC also increases the risk of major bleeding events. The optimal management of OAC in patients with AF undergoing transaortic valve implantation (TAVI) is unclear. This study aimed to compare the efficacy and safety of OAC interruption vs. continuation in patients with AF scheduled for TAVI. Methods: PubMed, EMBASE, and Cochrane were searched to include all pertinent randomized and observational studies. The primary endpoint was the occurrence of net adverse clinical events (NACE), a composite of all-cause death, major vascular complications, and major bleeding at 30-day follow-up. Secondary endpoints included all-cause death, cardiovascular death, major vascular complications, major bleeding, any bleeding, stroke, non-fatal myocardial infarction, and the need for red-packed blood transfusion. Results: A total of three studies and 2773 patients were included in the analysis (1314 were allocated to continuation of OAC therapy and 1459 to interruption of OAC therapy during TAVI). The two study groups experienced a similar rate of NACE (OR = 0.89 [95% CI 0.61 to 1.31], I2 = 77%, p = 0.56) compared to the OAC-interruption group. No significant differences were observed in the rate of all-cause death (p = 0.21), cardiovascular death (p = 0.35), major vascular complications (p = 0.84), major bleeding events (p = 0.47), total bleeding events (p = 0.62), or non-fatal MI (p = 0.55). Interestingly, the OAC-continuation group experienced a lower occurrence of stroke (OR = 0.62 [95% CI 0.39 to 0.97], I2 = 0%, p = 0.04) and the need for red packed blood cells (OR = 0.66 [95% CI 0.50 to 0.86], I2 = 20%, p < 0.01) compared to the OAC-interruption group. Conclusions: In patients with AF undergoing TAVI, there was no significant difference between interruption and continuation of OAC in terms of NACE, composite of all-cause death, major vascular complications, or major bleeding at 30-day follow-up. Of interest, the OAC-continuation group patients experienced lower rates of stroke and the need for blood transfusion.
1. Introduction
Transcatheter aortic valve implantation (TAVI) is an established therapy for patients with symptomatic severe aortic stenosis [1]. It constitutes the standard of care for older patients (≥75 years) and those who are at high risk or unsuitable for surgery.
Approximately one-third of patients scheduled for TAVI are treated with oral anticoagulation (OAC) therapy due to concomitant conditions, especially atrial fibrillation (AF) [2]. OAC therapy is crucial for reducing the risk of ischemic complications in this population; however, it also increases the risk of major bleeding events [3]. Furthermore, the TAVI procedure itself is associated with a significant risk of ischemic complications, mainly due to debris embolization [4,5], as well as major bleeding events, both at the access site and non-access locations [6,7].
With the growing number of TAVI procedures in OAC patients, determining the optimal management of OAC therapy during the periprocedural phase has become a key concern [8], requiring a delicate balance between ischemic and hemorrhagic risks. However, current guidelines remain elusive [2,9], and clinical evidence reports conflicting findings [10,11,12].
This systematic review and meta-analysis aimed to synthesize the current evidence on the continuation versus interruption of OACs during the periprocedural phase in patients who are being treated with OAC undergoing TAVI.
2. Materials and Methods
2.1. Protocol and Registration
This comprehensive review was conducted following the Cochrane Handbook for Systematic Reviews of Interventions and was reported according to the Preferred Reporting Items for Systematic Reviews Incorporating Network Meta-analyses (PRISMA-NMA) guidelines. The meta-analysis protocol was registered on PROSPERO (ID: CRD42024618742).
2.2. Eligibility Criteria
Inclusion in this meta-analysis was restricted to studies that met all the following eligibility criteria: (1) randomized trials or observational studies including patients scheduled for TAVI; (2) studies involving patients prescribed OACs (both direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs)) for concomitant comorbidities; (3) studies comparing interruption to continuation of OACs during the periprocedural phase of TAVI; (4) studies reporting clinical outcomes during the periprocedural phase and at 30-day follow-up.
2.3. Search Strategy and Data Extraction
The initial search included the Cochrane Library, EMBASE and PubMed from inception to November 2024 using the keywords (“Transcatheter Aortic Valve Implantation” OR “TAVI” OR “TAVR”) AND (“Continued Anticoagulation” OR “Anticoagulant Continuation”) AND (“Interrupted Anticoagulation” OR “Anticoagulant Interruption”) AND (“Thromboembolism” OR “Thromboembolic Events” OR “Bleeding Complications” OR “Procedure Success”) AND (“Vitamin K Antagonist” OR “VKA” OR “Warfarin” OR “Direct Oral Anticoagulants” OR “DOACs” OR “Factor Xa Inhibitors” OR “Dabigatran” OR “Rivaroxaban” OR “Apixaban” OR “Edoxaban”). The retrieval strategy was applied with customization of the search strings to accommodate the recommendations of each database. No document type or other relevant restrictions were used in the retrieval process, and unpublished articles were excluded. In addition, presentations from major cardiovascular meetings and references of the included studies were also screened. No search limitations by publication date, publication status, or language were applied. All the search results were imported into the Rayyan.ai software for the management of the review process. The screening process was conducted by two independent reviewers (JK and MF) who assessed titles and abstracts for relevance, followed by a full-text review to determine eligibility. A double-blind approach was used to minimize potential selection bias. Reviewer discrepancies were resolved through discussion or consultation with a third reviewer (BC). Only studies that included: (1) Adults ≥18 years; (2) Undergoing TAVI; (3) Indication of anticoagulation for long-term treatment of AF, patients with a mechanical valve or patients with DVT/PE or other rare causes; (4) studies with 2 arms showing a comparison of peri-procedural continuation vs. interruption of OACs; (5) Continuation defined as uninterrupted or minimally interrupted without bridging; (6) Reported outcomes: bleeding, stroke, mortality, valve thrombosis, or vascular complications; (7) RCTs or observational studies; (8) English language; (9) Full text available and available results. Furthermore, studies meeting any of the following exclusion criteria were excluded: (1) No direct comparison of continuation vs. interruption; (2) Non-TAVI procedures (e.g., SAVR); (3) Outcomes reported post-TAVI (beyond the 30 days limit period); (4) Case reports, reviews, editorials, or abstracts; (5) Animal or in vitro studies; (6) Bridging strategies only without continuation/interruption comparison; (7) Duplicate or overlapping data.
2.4. Quality Assessment
We evaluated the risk of bias in randomized and observational studies using version 2 of the Cochrane Risk of Bias assessment tool [13]. Disagreements were resolved through a consensus after discussing the reasons for the discrepancy.
2.5. Statistical Analysis
A quantitative analysis, consisting of three meta-analyses, was conducted using the software “R” (RStudio ver. 2022.07.2) and the package “meta”. Odds ratios (ORs) and confidence intervals (CIs) were employed as a measure of effect size. The threshold for statistical significance of the overall effect size was set at p < 0.05. A random-effects model was employed, and the DerSimonian and Laird method was applied to estimate the between-study variance (τ2). I2 values of 25%, 50%, and 75% were considered indicative of small, moderate, and high levels of heterogeneity, respectively [14].
2.6. Study Endpoints
The primary endpoint was the occurrence of net adverse clinical event (NACE), a composite of all-cause death, major vascular complications, and major bleeding at 30-day follow-up.
Secondary endpoints included all-cause death, cardiovascular death, major vascular complications, major bleeding, any bleeding, stroke, non-fatal myocardial infarction (MI), and the need for red packed blood cells at 30-day follow-up. A qualitative synthesis of the results is provided in Table 1.

Table 1.
Characteristics of included studies.
3. Results
3.1. Qualitative Analysis
A total of nine full-text articles were assessed for eligibility. Of these, six studies were excluded for the following reasons: two were protocols with no results, two did not include an indication for OACs, and two did not report relevant outcomes of interest.
Ultimately, three studies met the inclusion criteria and were included in the final analysis. The flowchart of the systematic search is illustrated in Figure 1. The most relevant clinical and procedural findings from the included articles are summarized in Table 1.
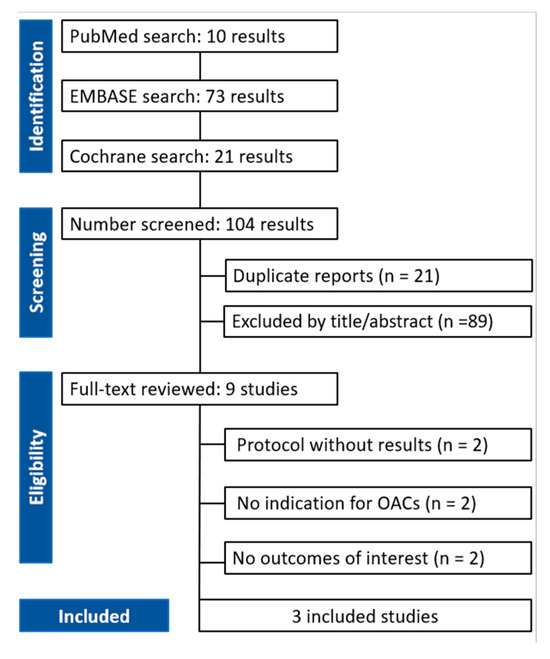
Figure 1.
PRISMA flow diagram of study screening and selection.
A total of three studies met the inclusion criteria and were included in the qualitative and quantitative analysis. Of these, one was a randomized clinical trial, and two were observational studies. The present systematic review included results from 2773 patients who received clinically indicated OAC therapy and underwent successful TAVI: 1314 were allocated to continuation of OAC therapy and 1459 to interruption of OAC therapy during TAVI.
AF was the primary indication for OAC therapy in all the included studies (ranging from 95% to 100%). Among the included studies, only one—the POPular TAVI trial—reported the type of atrial fibrillation at baseline. In this study, paroxysmal atrial fibrillation was present in 192 of 414 patients (46.4%) in the continuation group and 184 of 406 patients (45.3%) in the interruption group. In the continuation group, direct oral anticoagulants (DOACs) were the most commonly used anticoagulants in two studies, while in the study by Brinkert et al. DOACs and VKAs were equally represented.
The prevalence of hypertension and diabetes varied from 77% to 97% and 29% to 48%, respectively, while a history of stroke was observed in 13% to 22% of the included populations.
The results of the quantitative analysis are graphically displayed using Forest plots (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10).
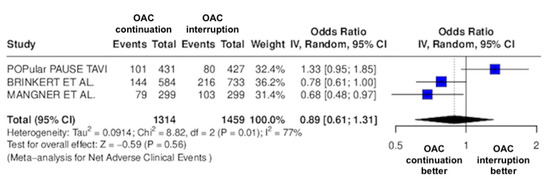
Figure 2.
Odds ratio of NACE between the continuation OAC group and the interruption OAC group [10,11,12].
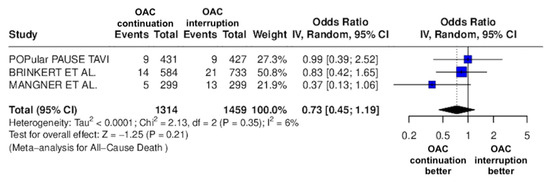
Figure 3.
Odds ratio of all-cause death between the continuation OAC group and the interruption OAC group [10,11,12].
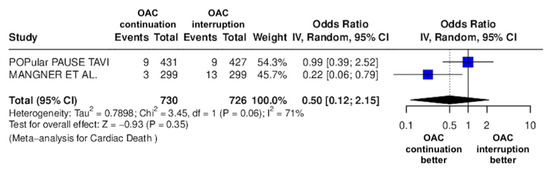
Figure 4.
Odds ratio of cardiac death between the continuation OAC group and the interruption OAC group [11,12].
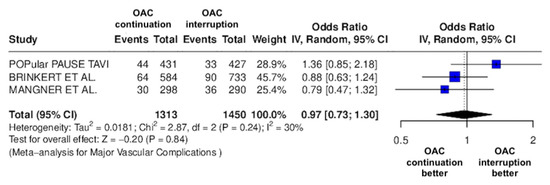
Figure 5.
Odds ratio of major vascular complications between the continuation OAC group and the interruption OAC group [10,11,12].
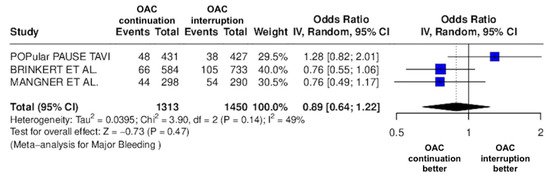
Figure 6.
Odds ratio of major bleeding between the continuation OAC group and the interruption OAC group [10,11,12].
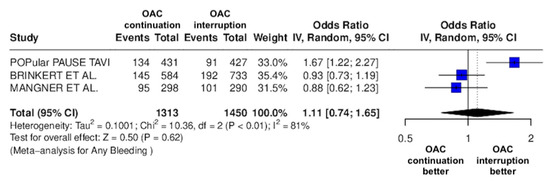
Figure 7.
Odds ratio of total bleeding events between the continuation OAC group and the interruption OAC group [10,11,12].
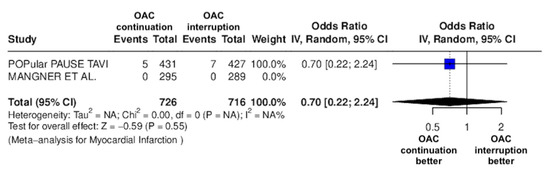
Figure 8.
Odds ratio of non-fatal MI between the continuation OAC group and the interruption OAC group [11,12].
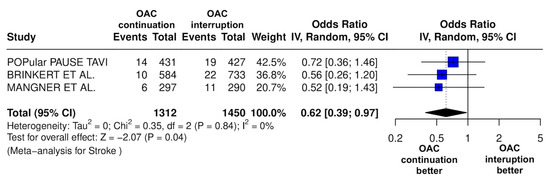
Figure 9.
Odds ratio of stroke between the continuation OAC group and the interruption OAC group [10,11,12].
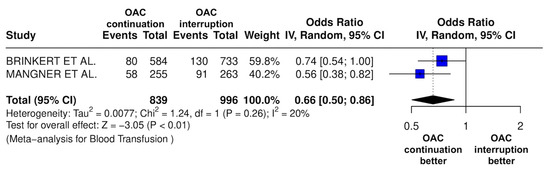
Figure 10.
Odds ratio of the need for red packed blood cells between the continuation OAC group and the interruption OAC group [10,12].
3.2. Risk of Bias Assessment
The major findings of the risk-of-bias assessment are reported in Supplementary Table S1. One open-label study was considered at low risk of bias, and two had some concerns.
3.3. Quantitative Analysis
Nine meta-analyses were performed to compare the safety and efficacy of continuation versus interruption of OAC therapy during TAVI. Three studies reported data on NACE, all-cause death, major vascular complications, major bleeding, any bleeding, and stroke, while two investigated the occurrence of cardiovascular death, non-fatal MI, and the need for red packed blood cells.
The continuation OAC group experienced a similar rate of NACE (OR = 0.89 [95% CI 0.61 to 1.31], I2 = 77%, p = 0.56, Figure 2) as the interruption OAC group. There were no significant differences between the continuation OAC group and the interruption OAC group for the occurrence of all-cause death (OR = 0.73 [95% CI 0.45 to 1.19], I2 = 6%, p = 0.21, Figure 3), cardiovascular death (OR = 0.50 [95% CI 0.12 to 2.15], I2 = 71%, p = 0.35, Figure 4), major vascular complications (OR = 0.97 [95% CI 0.73 to 1.30], I2 = 30%, p = 0.84, Figure 5), major bleeding events (OR = 0.89 [95% CI 0.64 to 1.22], I2 = 49%, p = 0.47, Figure 6), any bleeding events (OR = 1.11 [95% CI 0.74 to 1.65], I2 = 81%, p = 0.62, Figure 7), or non-fatal MI (OR = 0.70 [95% CI 0.22 to 2.24], p = 0.55, Figure 8). Interestingly, the continuation OAC group experienced a lower occurrence of stroke (OR = 0.62 [95% CI 0.39 to 0.97], I2 = 0%, p = 0.04, Figure 9) and the need for red packed blood cells (OR = 0.66 [95% CI 0.50 to 0.86], I2 = 20%, p < 0.01, Figure 10) compared to the interruption OAC group (Table 2).

Table 2.
Summary of the Baseline Characteristics.
4. Discussion
To our knowledge, this is the first systematic review and meta-analysis comparing the efficacy and safety of continuation versus interruption of OACs during the periprocedural phase of TAVI in patients with a concomitant clinical indication for OAC.
The main findings from the pooled analyses were as follows: (1) there was no significant difference between continuation and interruption of OACs in terms of NACE, all cause-death, cardiovascular death, major vascular complications, major bleeding events, any bleeding events, or non-fatal MI; and (2) the continuation OAC group showed a lower occurrence of stroke and a lower need of red packed blood cells compared to the continuation group.
Despite advancements in technical skills and the minimization of delivery system caliber, the risk of adverse events during the periprocedural phase of TAVI remains significant. Patients are often frail, particularly those receiving OACs, with a 30-day adverse event rate of 15.6%, as reported in the study by Van Ginkel et al. [11].
Until a few years ago, interruption of OAC therapy was considered the best option to minimize the risk of major periprocedural bleeding due to the use of large devices for vascular access, especially in frail patients. However, emerging evidence has challenged this approach, and current guidelines remain unclear regarding the optimal management of OAC therapy during the periprocedural phase of TAVI. Therefore, real-world clinical practice involves a variety of approaches, with different OAC types (DOACs vs. VKAs) being used, and the decision to continue or interrupt therapy largely remains at the discretion of the operator.
The results of our meta-analysis suggest that continuing OAC therapy is as safe and effective as interrupting it. The rate of NACE at 30 days was comparable between the two groups (24.7% vs. 27.3% in the OAC continuation group vs. 27.3%, 399/1459 in the OAC interruption group). However, high heterogeneity across studies may influence the reliability of these results.
It is worth noting that several factors related to patient characteristics and procedural features contribute to the occurrence of adverse events [5,6]. A meticulous TAVI planning strategy aimed at minimizing vascular access and device sizes may have prognostic relevance [15].
A major finding of our meta-analysis is that continuing OAC therapy during the periprocedural phase of TAVI was associated with a lower incidence of early stroke events. This is not surprising, as atrial fibrillation is an independent predictor of stroke after TAVI [16], and aggressive OAC therapy may have prognostic relevance in this population.
Of note, our results highlighted that the OAC continuation group required fewer red blood cell transfusions compared to the OAC interruption group. Although mechanistic explanations cannot be definitively drawn from our results, the lack of bridging therapy with heparin may help explain these findings. Previous studies have shown that bridging therapy with heparin is associated with higher rates of pocket hematomas in patients undergoing pacemaker or defibrillator surgery (Essebag et al., 2019) [16] as well as increased minor bleeding in patients undergoing catheter ablation of AF [17] compared to the OAC continuation strategy.
One possible contributor to the high heterogeneity observed in bleeding outcomes (I2 = 81%) is the variability in the type of oral anticoagulation used across studies. For instance, Mangner et al. provided detailed breakdowns of both VKAs and various DOACs (rivaroxaban, apixaban, edoxaban, dabigatran), whereas the POPular TAVI trial did not specify the anticoagulant type used. Brinkert et al. included patients on both VKAs and DOACs but did not distinguish outcomes based on anticoagulant class. Given the known differences in bleeding risk profiles between VKAs and DOACs—and even among individual DOACs—this variability likely influenced the pooled bleeding estimates and contributed to the observed heterogeneity. Another factor likely contributing to the high heterogeneity in bleeding outcomes (I2 = 81%) is the use of different bleeding classification systems across studies. While Brinkert et al. and Magnéner et al. utilized the VARC criteria, the POPular TAVI trial used the BARC definition. These bleeding scales differ in thresholds and categorization of events, which may have led to inconsistencies in event reporting and outcome interpretation across the studies.
The observed heterogeneity in NACE outcomes (I2 = 77%) may also stem from differences in how this composite endpoint was defined across studies. In Brinkert et al., NACE included vascular complications, stroke, and mortality. The POPular TAVI trial used a broader composite that included cardiovascular death, stroke, myocardial infarction, major vascular complications, and major bleeding. Meanwhile, Magnéner et al. defined NACE as a combination of life-threatening or major bleeding, stroke, and all-cause mortality. These inconsistencies in endpoint definitions can significantly influence event rates and compromise direct comparability across studies.
Limitations
This study has several limitations. First, the analysis included only three randomized and observational studies with a relatively low number of patients. Second, OAC therapy included both DOACs and VKAs, which differ significantly in their pharmacokinetic properties, potentially introducing bias. Third, several factors contributing to adverse outcomes, such as the use of embolic protection devices, the type of vascular closure devices, and the percentage of patients receiving bridging therapy with heparin, were not accounted for in the analysis. Finally, the lack of patient-level data precluded the identification of individuals who might derive greater benefit from continuing OAC therapy during the periprocedural phase of TAVI.
One important limitation is that the type of atrial fibrillation—whether paroxysmal, persistent, or permanent—was not specified in the included studies, with the exception of the POPular TAVI trial. This limits our ability to evaluate the potential impact of AF subtype on clinical outcomes. Given that different AF types are associated with varying risks of thromboembolism and bleeding, the distribution of AF subtypes may influence both procedural and long-term outcomes, as well as the risk–benefit balance of continuing versus interrupting anticoagulation.
Another limitation is the lack of data regarding the presence of bicuspid aortic valves across the included studies. None of the trials reported whether patients had bicuspid morphology at baseline, which is known to be associated with altered hemodynamics and a potentially higher risk of valve thrombosis. This limits our ability to assess whether bicuspid anatomy may have influenced outcomes related to anticoagulation continuation or interruption.
Information regarding the specific type of transcatheter heart valve (THV) implanted was not consistently reported across the included studies. While Brinkert et al. and Mangner et al. stated that CE-marked valves were used, they did not specify whether balloon-expandable or self-expanding valves were employed. The POPular TAVI trial did not report valve type at all. Given that valve type can influence procedural complexity, bleeding risk, and post-procedural outcomes, this lack of detail may have contributed to residual confounding and limited our ability to explore valve-related outcome differences. Furthermore, there are no data about the dimensions of the valves implanted in the patients.
5. Conclusions
No statistical significance between the continuation and interruption of OACs in terms of NACE was observed in the present meta-analysis. These results are adequate for any adult age group, gender, or indication for OACs. However, given that only three studies were included, the statistical power and generalizability of the findings are limited. Therefore, the results cannot be generalized across all age groups, genders, or indications for OAC use. Future randomized studies focusing on DOACs with different dosages are required to identify the optimal periprocedural management of OAC therapy in patients with AF scheduled for TAVI.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14103563/s1, Table S1: Risk of bias summary for randomized studies (RoB 2).
Author Contributions
Conceptualization, J.K.; methodology, J.K. and M.F.; validation, B.C. and G.A.; formal analysis, F.L.G. and D.D.; investigation, J.H.; resources, F.L.G. and D.D.; writing—original draft preparation, J.K., M.F. and J.H.; writing—review and editing, B.C. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | Atrial Fibrillation |
| OAC | Oral Anticoagulation |
| TAVI | Transcatheter Aortic Valve Implantation |
| NACE | Net Adverse Clinical Events |
| AKI | Acute Kidney Injury |
| ESRD | End-Stage Renal Disease |
| PCI | Percutaneous Coronary Intervention |
| VKA | Vitamin K Antagonist |
| DOAC | Direct Oral Anticoagulant |
| MI | Myocardial Infarction |
| MACE | Major Adverse Cardiovascular Events |
| ICH | Intracranial Hemorrhage |
| RCT | Randomized Controlled Trial |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| INR | International Normalized Ratio |
| TIA | Transient Ischemic Attack |
| PVL | Paravalvular Leak |
| RoB | Risk of bias |
References
- Rahhab, Z.; El Faquir, N.; Tchetche, D.; Delgado, V.; Kodali, S.; Mara Vollema, E.; Bax, J.; Leon, M.B.; Van Mieghem, N.M. Expanding the indications for transcatheter aortic valve implantation. Nat. Rev. Cardiol. 2020, 17, 75–84. Available online: https://pubmed.ncbi.nlm.nih.gov/31527743/ (accessed on 15 March 2025). [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. Available online: https://pubmed.ncbi.nlm.nih.gov/39210723/ (accessed on 18 March 2025). [CrossRef] [PubMed]
- Rubboli, A.; Becattini, C.; Verheugt, F.W. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J. Cardiol. 2011, 3, 351. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3224868/ (accessed on 18 March 2025). [CrossRef] [PubMed]
- Arboix, A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J. Clin. Cases WJCC 2015, 3, 418. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4419105/ (accessed on 18 March 2025). [CrossRef] [PubMed]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchétché, D.; Chandrasekhar, J.; De Brito, F.S.; Barbanti, M.; Kornowski, R.; Latib, A.; D’onofrio, A.; Ribichini, F.; et al. Predictors, Incidence, and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation Complicated by Stroke. Circ. Cardiovasc. Interv. 2019, 12, e007546. Available online: https://pubmed.ncbi.nlm.nih.gov/30871358/ (accessed on 26 January 2025). [CrossRef] [PubMed]
- Piccolo, R.; Pilgrim, T.; Franzone, A.; Valgimigli, M.; Haynes, A.; Asami, M.; Lanz, J.; Räber, L.; Praz, F.; Langhammer, B.; et al. Frequency, Timing, and Impact of Access-Site and Non-Access-Site Bleeding on Mortality Among Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1436–1446. Available online: https://pubmed.ncbi.nlm.nih.gov/28728657/ (accessed on 18 March 2025). [CrossRef] [PubMed]
- Avvedimento, M.; Real, C.; Nuche, J.; Farjat-Pasos, J.; Galhardo, A.; Trinh, K.H.; Robichaud, M.; Delarochellière, R.; Paradis, J.-M.; Poulin, A.; et al. Incidence, Predictors, and Prognostic Impact of Bleeding Events After TAVR According to VARC-3 Criteria. JACC Cardiovasc. Interv. 2023, 16, 2262–2274. Available online: https://pubmed.ncbi.nlm.nih.gov/37676226/ (accessed on 26 January 2025). [CrossRef] [PubMed]
- Ya’Qoub, L.; Arnautovic, J.; Sharkawi, M.; AlAasnag, M.; Jneid, H.; Elgendy, I.Y. Antithrombotic Management for Transcatheter Aortic Valve Implantation. J. Clin. Med. 2023, 12, 7632. Available online: https://pubmed.ncbi.nlm.nih.gov/38137701/ (accessed on 26 January 2025). [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Brinkert, M.; Keller, L.S.; Moriyama, N.; Cuculi, F.; Bossard, M.; Lehnick, D.; Kobza, R.; Laine, M.; Nietlispach, F.; Toggweiler, S. Safety and Efficacy of Transcatheter Aortic Valve Replacement with Continuation of Oral Anticoagulation. J. Am. Coll. Cardiol. 2019, 73, 2004–2005. Available online: https://pubmed.ncbi.nlm.nih.gov/31000005/ (accessed on 26 January 2025). [CrossRef] [PubMed]
- van Ginkel, D.J.; Bor, W.L.; Aarts, H.M.; Dubois, C.; De Backer, O.; Rooijakkers, M.J.; Rosseel, L.; Veenstra, L.; van der Kley, F.; van Bergeijk, K.H.; et al. Continuation versus Interruption of Oral Anticoagulation during TAVI. N. Engl. J. Med. 2024, 392, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Crusius, L.; Haussig, S.; Woitek, F.J.; Kiefer, P.; Stachel, G.; Leontyev, S.; Schlotter, F.; Spindler, A.; Höllriegel, R.; et al. Continued Versus Interrupted Oral Anticoagulation During Transfemoral Transcatheter Aortic Valve Implantation and Impact of Postoperative Anticoagulant Management on Outcome in Patients with Atrial Fibrillation. Am. J. Cardiol. 2019, 123, 1134–1141. Available online: https://pubmed.ncbi.nlm.nih.gov/30658919/ (accessed on 26 January 2025). [CrossRef] [PubMed]
- Risk of Bias 2 (RoB 2) Tool|Cochrane Methods [Internet]. Available online: https://methods.cochrane.org/risk-bias-2 (accessed on 15 March 2025).
- Higgins, J.P.T.; López-López, J.A.; Becker, B.J.; Davies, S.R.; Dawson, S.; Grimshaw, J.M.; McGuinness, L.A.; Moore, T.H.M.; Rehfuess, E.A.; Thomas, J.; et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob. Health 2019, 4 (Suppl. S1), e000858. Available online: https://pubmed.ncbi.nlm.nih.gov/30775014/ (accessed on 18 March 2025).
- Barbanti, M.; Gulino, S.; Costa, G.; Tamburino, C. Optimization and simplification of transcatheter aortic valve implantation therapy. Expert Rev. Cardiovasc. Ther. 2018, 16, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Essebag, V.; Healey, J.S.; Joza, J.; Nery, P.B.; Kalfon, E.; Leiria, T.L.L.; Verma, A.; Ayala-Paredes, F.; Coutu, B.; Sumner, G.L.; et al. Effect of direct oral anticoagulants, warfarin, and antiplatelet agents on risk of device pocket hematoma: Combined analysis of BRUISE CONTROL 1 and 2. Circ. Arrhythmia Electrophysiol. 2019, 12, e007545. [Google Scholar] [CrossRef] [PubMed]
- Biase, L.; Di Burkhardt, J.D.; Santangeli, P.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Themistoclakis, S.; Rossillo, A.; Lakkireddy, D.; et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation nagement results from the role of coumadin in preventing thromboembolism in atrial fibrillation (AF) patients undergoing catheter ablation (COMPARE) randomized trial. Circulation 2014, 129, 2638–2644. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).