Single Injection of Highly Concentrated Hyaluronic Acid Provides Improvement of Knee Joint Arthrokinematic Motion and Clinical Outcomes in Patients with Osteoarthritis—Non-Randomized Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Product Description
2.3. Trial Design and Clinical Assessment
2.4. Statistical Analysis
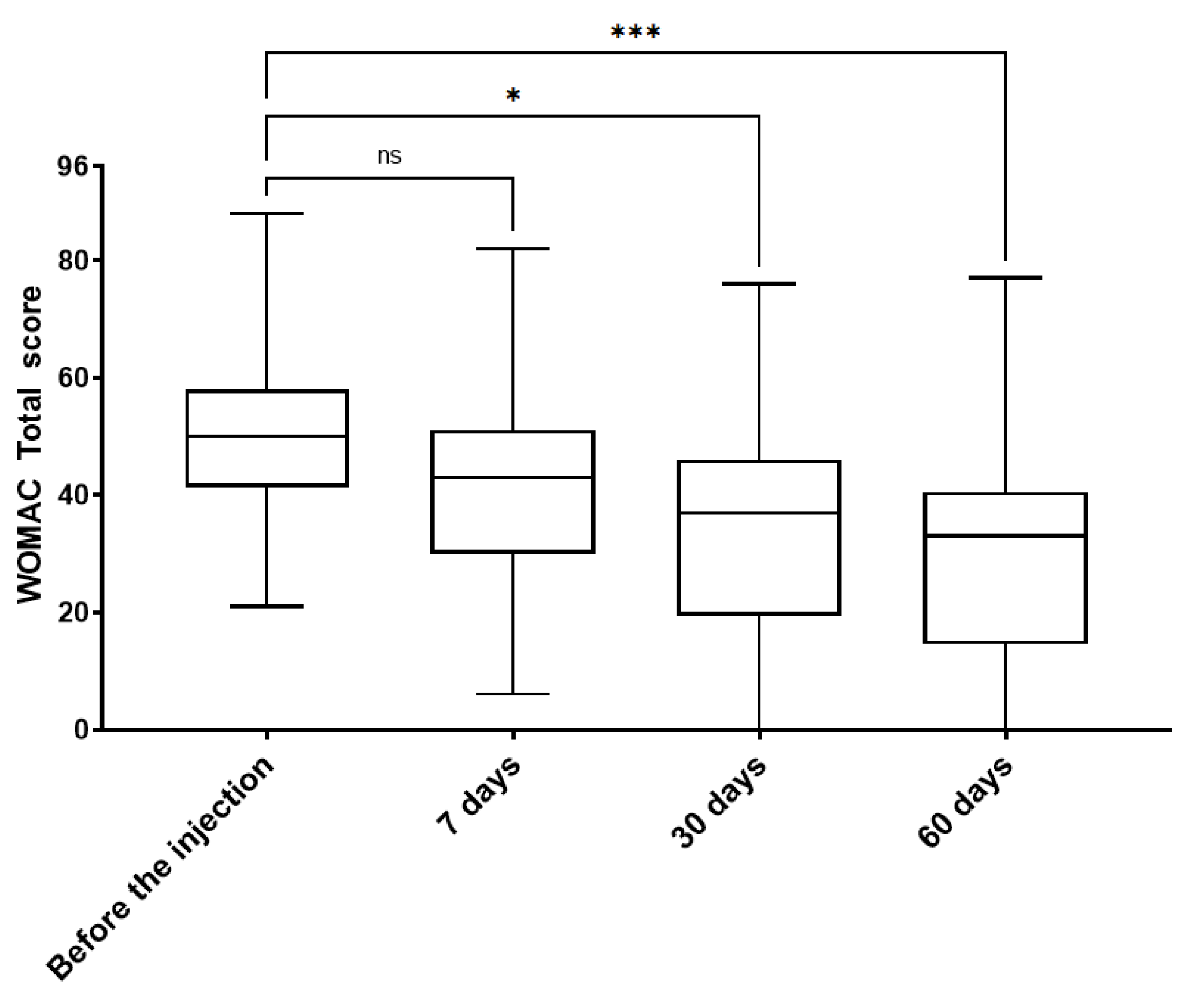
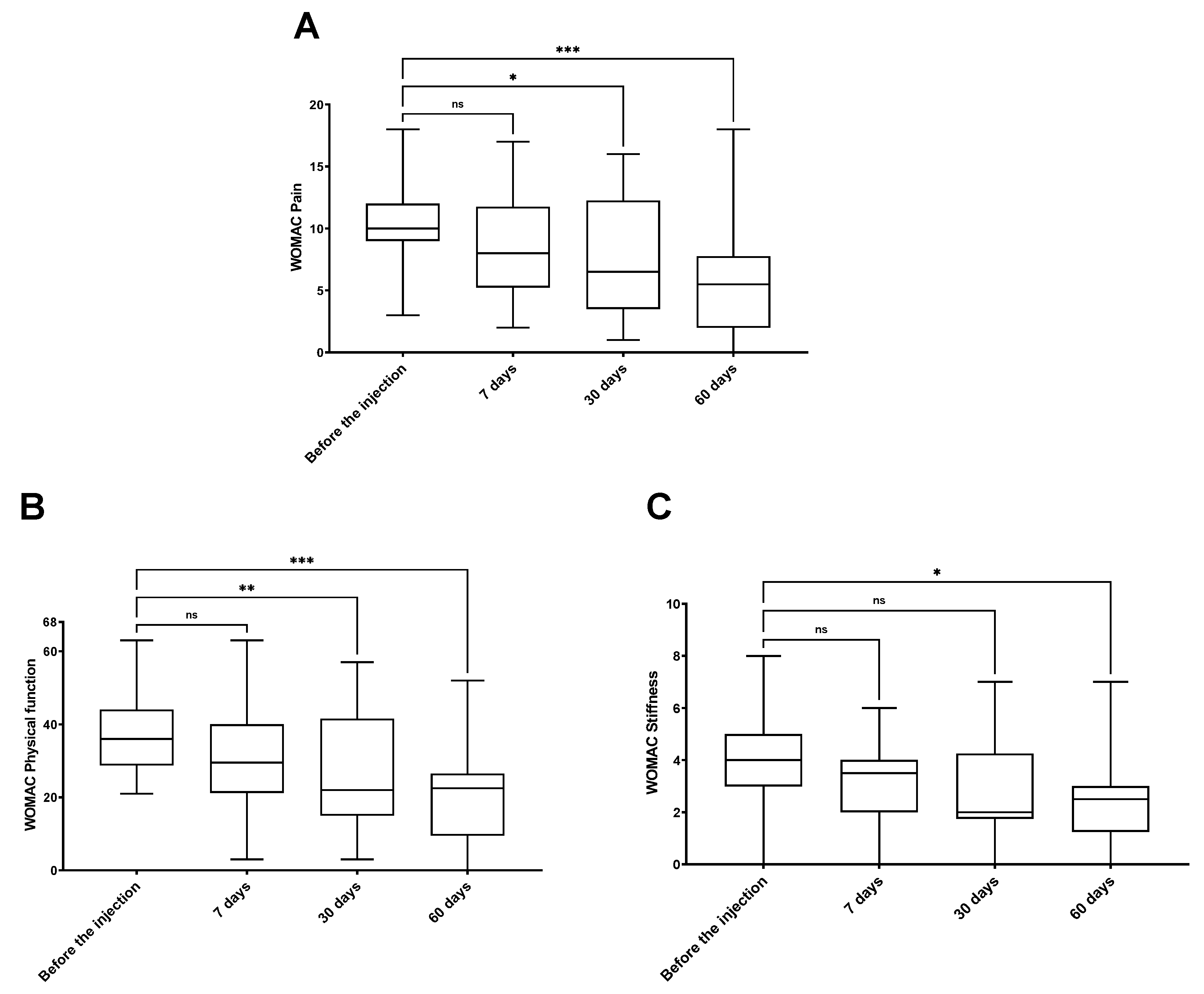
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Nguyen, U.-S.D.T.; Zhang, Y.; Zhu, Y.; Niu, J.; Zhang, B.; Felson, D.T. Increasing Prevalence of Knee Pain and Symptomatic Knee Osteoarthritis: Survey and Cohort Data. Ann. Intern. Med. 2011, 155, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, A.; Gerhardsson De Verdier, M.; Engstrom, G.; Nilsson, P.M.; Mellstrom, C.; Lohmander, L.S.; Englund, M. Prevalence of Knee Pain and Knee OA in Southern Sweden and the Proportion That Seeks Medical Care. Rheumatology 2015, 54, 827–835. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Petersson, I.F.; Björk, J.; Dahlberg, L.E.; Englund, M. The Consultation Prevalence of Osteoarthritis 2030 May Increase by 50%: Prognosis for Sweden. Osteoarthr. Cartil. 2013, 21, S160–S161. [Google Scholar] [CrossRef]
- Bird, H.A. Controversies in the Treatment of Osteoarthritis. Clin. Rheumatol. 2003, 22, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Schurz, J.; Ribitsch, V. Biorheology of Synovial Fluid1. BIR 1987, 24, 385–399. [Google Scholar] [CrossRef]
- Sabaratnam, S.; Arunan, V.; Coleman, P.J.; Mason, R.M.; Levick, J.R. Size Selectivity of Hyaluronan Molecular Sieving by Extracellular Matrix in Rabbit Synovial Joints. J. Physiol. 2005, 567, 569–581. [Google Scholar] [CrossRef]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A Systems Biology Approach to Synovial Joint Lubrication in Health, Injury, and Disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef]
- Ghosh, P.; Guidolin, D. Potential Mechanism of Action of Intra-Articular Hyaluronan Therapy in Osteoarthritis: Are the Effects Molecular Weight Dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef]
- Waddell, D.D.; Kolomytkin, O.V.; Dunn, S.; Marino, A.A. Hyaluronan Suppresses IL-1β-Induced Metalloproteinase Activity from Synovial Tissue. Clin. Orthop. Relat. Res. 2007, 465, 241–248. [Google Scholar] [CrossRef]
- Altman, R.D.; Dasa, V.; Takeuchi, J. Review of the Mechanism of Action for Supartz FX in Knee Osteoarthritis. CARTILAGE 2018, 9, 11–20. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Pałka, J.A.; Jarząbek, K.; Wołczyński, S. Hyaluronic Acid Counteracts Interleukin-1-Induced Inhibition of Collagen Biosynthesis in Cultured Human Chondrocytes. Pharmacol. Res. 2006, 54, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Forsey, R.; Fisher, J.; Thompson, J.; Stone, M.; Bell, C.; Ingham, E. The Effect of Hyaluronic Acid and Phospholipid Based Lubricants on Friction within a Human Cartilage Damage Model. Biomaterials 2006, 27, 4581–4590. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-Articular Hyaluronan (Hyaluronic Acid) and Hylans for the Treatment of Osteoarthritis: Mechanisms of Action. Arthritis Res. Ther. 2003, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- McConnell, S.; Kolopack, P.; Davis, A.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A Review of Its Utility and Measurement Properties. Arthritis Rheum. 2001, 45, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Orfanos, G.; Gibson, W.; Banks, T.; Mcconaghie, G.; Banerjee, R. Viscosupplementation with High Molecular Weight Hyaluronic Acid for Hip Osteoarthritis: A Systematic Review and Meta-Analysis of Randomised Control Trials of the Efficacy on Pain, Functional Disability, and the Occurrence of Adverse Events. Acta Chir. Orthop. Traumatol. Cech. 2024, 91, 109–119. [Google Scholar] [CrossRef]
- Łysiak, A.; Froń, A.; Bączkowicz, D.; Szmajda, M. Vibroarthrographic Signal Spectral Features in 5-Class Knee Joint Classification. Sensors 2020, 20, 5015. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Majorczyk, E.; Kręcisz, K. Age-Related Impairment of Quality of Joint Motion in Vibroarthrographic Signal Analysis. BioMed Res. Int. 2015, 2015, 591707. [Google Scholar] [CrossRef]
- Kręcisz, K.; Bączkowicz, D.; Kawala-Sterniuk, A. Using Nonlinear Vibroartrographic Parameters for Age-Related Changes Assessment in Knee Arthrokinematics. Sensors 2022, 22, 5549. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Estimation of Differences in Selected Indices of Vibroacoustic Signals between Healthy and Osteoarthritic Patellofemoral Joints as a Potential Non-Invasive Diagnostic Tool. J. Phys.: Conf. Ser. 2021, 2130, 012009. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Kręcisz, K.; Borysiuk, Z. Analysis of Patellofemoral Arthrokinematic Motion Quality in Open and Closed Kinetic Chains Using Vibroarthrography. BMC Musculoskelet. Disord. 2019, 20, 48. [Google Scholar] [CrossRef]
- Kręcisz, K.; Bączkowicz, D. Analysis and Multiclass Classification of Pathological Knee Joints Using Vibroarthrographic Signals. Comput. Methods Programs Biomed. 2018, 154, 37–44. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Skiba, G.; Szmajda, M.; Vařeka, I.; Falkowski, K.; Laudner, K. Effects of Viscosupplementation on Quality of Knee Joint Arthrokinematic Motion Analyzed by Vibroarthrography. Cartilage 2021, 12, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Bączkowicz, D.; Kręcisz, K. Vibroarthrography in the Evaluation of Musculoskeletal System—A Pilot Study. Ortop. Traumatol. Rehabil. 2013, 15, 407–416. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Majorczyk, E. Joint Motion Quality in Vibroacoustic Signal Analysis for Patients with Patellofemoral Joint Disorders. BMC Musculoskelet. Disord. 2014, 15, 426. [Google Scholar] [CrossRef]
- Tanaka, N.; Hoshiyama, M. Vibroarthrography in Patients with Knee Arthropathy. J. Back. Musculoskelet. Rehabil. 2012, 25, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bączkowicz, D.; Majorczyk, E. Joint Motion Quality in Chondromalacia Progression Assessed by Vibroacoustic Signal Analysis. PMR 2016, 8, 1065–1071. [Google Scholar] [CrossRef]
- ISO Standard No. 14155:2020; Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice. 2018. Available online: https://www.iso.org/standard/71690.html (accessed on 1 May 2025).
- Bannuru, R.R.; Natov, N.S.; Dasi, U.R.; Schmid, C.H.; McAlindon, T.E. Therapeutic Trajectory Following Intra-Articular Hyaluronic Acid Injection in Knee Osteoarthritis—Meta-Analysis. Osteoarthr. Cartil. 2011, 19, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Mensitieri, M.; Ambrosio, L.; Iannace, S.; Nicolais, L.; Perbellini, A. Viscoelastic Evaluation of Different Knee Osteoarthritis Therapies. J. Mater. Sci: Mater. Med. 1995, 6, 130–137. [Google Scholar] [CrossRef]
- Arrich, J. Intra-Articular Hyaluronic Acid for the Treatment of Osteoarthritis of the Knee: Systematic Review and Meta-Analysis. Can. Med. Assoc. J. 2005, 172, 1039–1043. [Google Scholar] [CrossRef]
- Petrella, R.J.; DiSilvestro, M.D.; Hildebrand, C. Effects of Hyaluronate Sodium on Pain and Physical Functioning in Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arch. Intern. Med. 2002, 162, 292–298. [Google Scholar] [CrossRef]
- Miltner, O.; Schneider, U.; Siebert, C.H.; Niedhart, C.; Niethard, F.U. Efficacy of Intraarticular Hyaluronic Acid in Patients with Osteoarthritis—a Prospective Clinical Trial. Osteoarthr. Cartil. 2002, 10, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Govoni, E.; Bacchini, P. Intra-Articular Hyaluronic Acid in the Treatment of Osteoarthritis of the Knee: Clinical and Morphological Study. Clin. Exp. Rheumatol. 1998, 16, 441–449. [Google Scholar] [PubMed]
- Altman, R.D.; Moskowitz, R. Intra-Articular Sodium Hyaluronate Reduces Pain and Improves Function in Osteoarthritis of Knee. J. Rheumatol. 2000, 25, 2203–2212. [Google Scholar]
- Huang, T.-L.; Chang, C.-C.; Lee, C.-H.; Chen, S.-C.; Lai, C.-H.; Tsai, C.-L. Intra-Articular Injections of Sodium Hyaluronate (Hyalgan®) in Osteoarthritis of the Knee. a Randomized, Controlled, Double-Blind, Multicenter Trial in the Asian Population. BMC Musculoskelet. Disord. 2011, 12, 221. [Google Scholar] [CrossRef]
- Huskisson, E. Hyaluronic Acid in the Treatment of Osteoarthritis of the Knee. Rheumatology 1999, 38, 602–607. [Google Scholar] [CrossRef]
- Gimeno Del Sol, M.; Trueba Davalillo, C.Á.; Trueba Vasavilbaso, C.; Navarrete Álvarez, J.M.; Coronel Granado, M.P.; García Jiménez, O.A.; Gil Orbezo, F. Clinical Efficacy of Intra-Articular Injections in Knee Osteoarthritis: A Prospective Randomized Study Comparing Hyaluronic Acid and Betamethasone. Open Access Rheumatol. Res. Rev. 2015, 7, 9–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neustadt, D.H. Long-Term Efficacy and Safety of Intra-Articular Sodium Hyaluronate (Hyalgan) in Patients with Osteoarthritis of the Knee. Clin. Exp. Rheumatol. 2003, 21, 307–311. [Google Scholar]
- Kolarz, G.; Kotz, R.; Hochmayer, I. Long-Term Benefits and Repeated Treatment Cycles of Intra-Articular Sodium Hyaluronate (Hyalgan) in Patients with Osteoarthritis of the Knee. Semin. Arthritis Rheum. 2003, 32, 310–319. [Google Scholar] [CrossRef]
- Farì, G.; Mancini, R.; Dell’Anna, L.; Ricci, V.; Della Tommasa, S.; Bianchi, F.P.; Ladisa, I.; De Serio, C.; Fiore, S.; Donati, D.; et al. Medial or Lateral, That Is the Question: A Retrospective Study to Compare Two Injection Techniques in the Treatment of Knee Osteoarthritis Pain with Hyaluronic Acid. J. Clin. Med. 2024, 13, 1141. [Google Scholar] [CrossRef]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. [Google Scholar] [CrossRef]
- Neogi, T.; Colloca, L. Placebo Effects in Osteoarthritis: Implications for Treatment and Drug Development. Nat. Rev. Rheumatol. 2023, 19, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, K.; Skiba, G.; Czerner, M.; Szmajda, M.; Bączkowicz, D. Effects of Viscosupplementation on Knee Joint Arthrokinematics—Pilot Study. Ortop. Traumatol. Rehabil. 2018, 20, 409–419. [Google Scholar] [CrossRef]
- Altman, R.D.; Bedi, A.; Karlsson, J.; Sancheti, P.; Schemitsch, E. Product Differences in Intra-Articular Hyaluronic Acids for Osteoarthritis of the Knee. Am. J. Sports Med. 2016, 44, 2158–2165. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Nijboer, C.H.; Pappalardo, G.; Pasurka, M.; Betsch, M.; Kubach, J. Comparison of Different Molecular Weights of Intra-Articular Hyaluronic Acid Injections for Knee Osteoarthritis: A Level I Bayesian Network Meta-Analysis. Biomedicines 2025, 13, 175. [Google Scholar] [CrossRef]
- Wang, C.-T.; Lin, Y.-T.; Chiang, B.-L.; Lin, Y.-H.; Hou, S.-M. High Molecular Weight Hyaluronic Acid Down-Regulates the Gene Expression of Osteoarthritis-Associated Cytokines and Enzymes in Fibroblast-like Synoviocytes from Patients with Early Osteoarthritis. Osteoarthr. Cartil. 2006, 14, 1237–1247. [Google Scholar] [CrossRef]
- Smith, M.M.; Ghosh, P. The Synthesis of Hyaluronic Acid by Human Synovial Fibroblasts Is Influenced by the Nature of the Hyaluronate in the Extracellular Environment. Rheumatol. Int. 1987, 7, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Hjelm, R.; Lärkner, H.; Nord, L.I.; Karlsson, A.; Wiebensjö, Å.; Höglund, A.U.; Kenne, A.H.; Näsström, J. Intra-Articular Duration of DurolaneTM after Single Injection into the Rabbit Knee. Cartilage 2011, 2, 384–388. [Google Scholar] [CrossRef] [PubMed]
- McGrath, A.F.; McGrath, A.M.; Jessop, Z.M.; Gandham, S.; Datta, G.; Dawson-Bowling, S.; Cannon, S.R. A Comparison of Intra-Articular Hyaluronic Acid Competitors in the Treatment of Mild to Moderate Knee Osteoarthritis. J. Arthritis 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Bellamy, N.; Campbell, J.; Welch, V.; Gee, T.L.; Bourne, R.; Wells, G.A. Viscosupplementation for the Treatment of Osteoarthritis of the Knee. Cochrane Database Syst. Rev. 2006, 2014, CD005321. [Google Scholar] [CrossRef]
- Bowden, D.J.; Byrne, C.A.; Alkhayat, A.; Eustace, S.J.; Kavanagh, E.C. Injectable Viscoelastic Supplements: A Review for Radiologists. Am. J. Roentgenol. 2017, 209, 883–888. [Google Scholar] [CrossRef]
- Falkowski, K.; Madej, W.; Hubeny, J. Single Intra-Articular Injection of High-Molecular and Highly Concentrated Hyaluronic Acid Improves Arthrokinematics in the Knee Joint, Resulting in a Significant Clinical Outcome. Osteoarthr. Cartil. 2024, 32, S590–S591. [Google Scholar] [CrossRef]



| Inclusion Criteria | Exclusion Criteria |
| Intra-articular injections of steroids or other injectables up to one year before the study; History of knee joint trauma during last 2 years or any trauma with articular cartilage-related damage; Occurrence of knee joint dysfunctions other than osteoarthritis, including symptomatic arthritis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falkowski, K.; Bączkowicz, D. Single Injection of Highly Concentrated Hyaluronic Acid Provides Improvement of Knee Joint Arthrokinematic Motion and Clinical Outcomes in Patients with Osteoarthritis—Non-Randomized Clinical Study. J. Clin. Med. 2025, 14, 3557. https://doi.org/10.3390/jcm14103557
Falkowski K, Bączkowicz D. Single Injection of Highly Concentrated Hyaluronic Acid Provides Improvement of Knee Joint Arthrokinematic Motion and Clinical Outcomes in Patients with Osteoarthritis—Non-Randomized Clinical Study. Journal of Clinical Medicine. 2025; 14(10):3557. https://doi.org/10.3390/jcm14103557
Chicago/Turabian StyleFalkowski, Krzysztof, and Dawid Bączkowicz. 2025. "Single Injection of Highly Concentrated Hyaluronic Acid Provides Improvement of Knee Joint Arthrokinematic Motion and Clinical Outcomes in Patients with Osteoarthritis—Non-Randomized Clinical Study" Journal of Clinical Medicine 14, no. 10: 3557. https://doi.org/10.3390/jcm14103557
APA StyleFalkowski, K., & Bączkowicz, D. (2025). Single Injection of Highly Concentrated Hyaluronic Acid Provides Improvement of Knee Joint Arthrokinematic Motion and Clinical Outcomes in Patients with Osteoarthritis—Non-Randomized Clinical Study. Journal of Clinical Medicine, 14(10), 3557. https://doi.org/10.3390/jcm14103557





