Renal Denervation After USA FDA Approval: An Update from an Interventional Cardiologist’s Perspective
Abstract
:1. The Burden of Hypertension and Inadequate Control
2. What Is Renal Denervation?
3. Latest Evidence from Clinical Trials and Registries
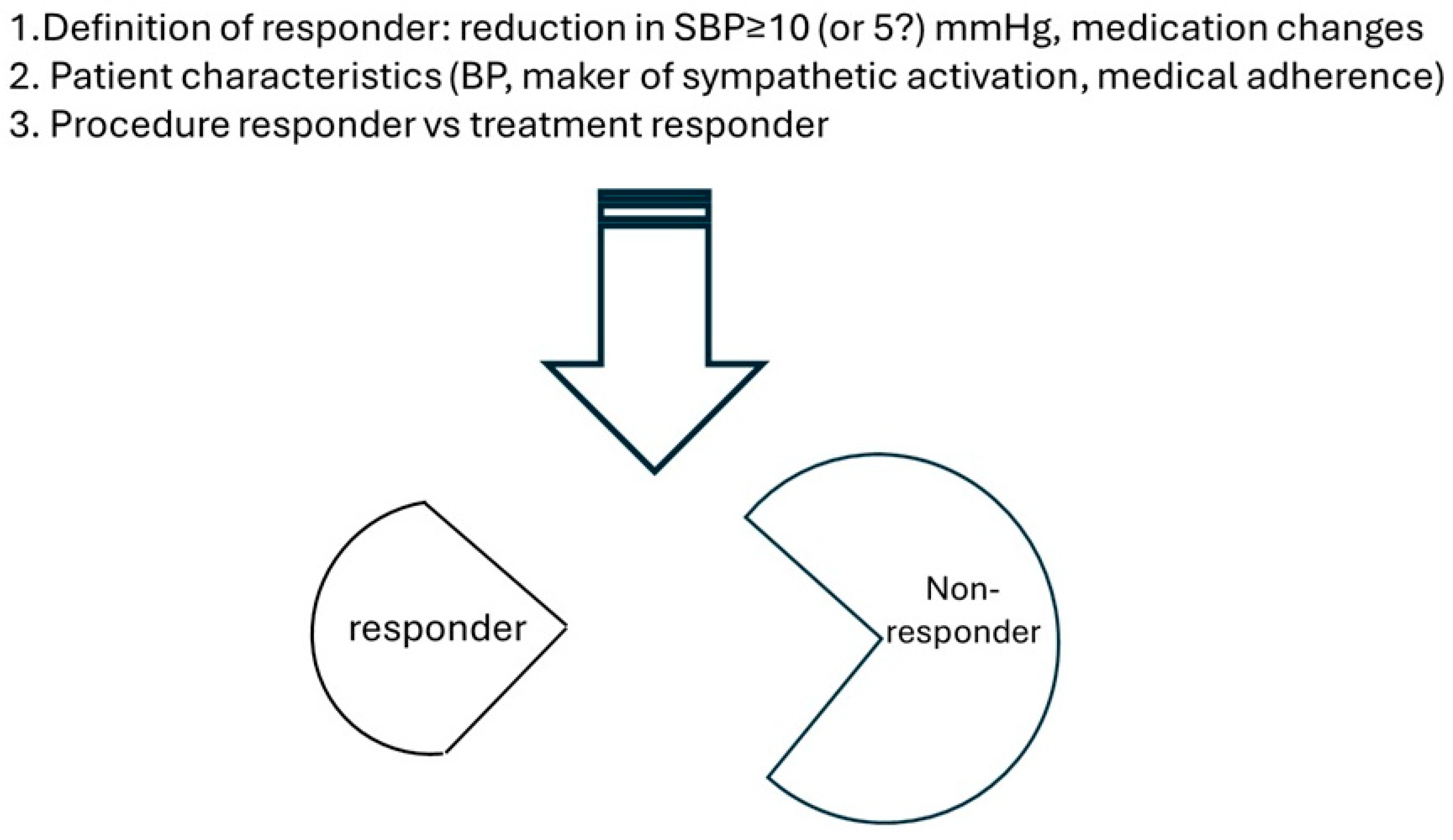
4. Current Challenges in Clinical Practice of Renal Denervation
- Preprocedural Imaging: Noninvasive imaging should be performed to rule out structural causes of secondary hypertension and to assess renal artery anatomy, including the presence of accessory arteries that may impact procedural efficacy.
- Contrast-Induced Nephropathy (CIN) Risk: As RDN relies on contrast-based imaging, proper hydration strategies and judicious contrast use are essential, particularly in patients with preexisting renal impairment.
- Pain Management: Due to the nonselective nature of nerve ablation, adequate analgesia and sedation are necessary to manage procedural pain and improve patient comfort.
- Anticoagulation and Antiplatelet Management: Unfractionated heparin should be administered during the procedure to maintain an activated clotting time (ACT) > 250 s. Additionally, aspirin loading followed by low-dose aspirin for one month post-procedure is recommended to minimize thrombotic risks.
5. Topics of Interest
6. Perspective
- Careful patient selection to identify those who will derive the most benefit;
- A dedicated multidisciplinary approach to ensure comprehensive management;
- Ongoing research to refine procedural techniques and further define the ideal patient profile.
Author Contributions
Funding
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.T.; Jaeger, B.C.; Foti, K.; Ghazi, L.; Wozniak, G.; Muntner, P. Trends in Blood Pressure Control among US Adults With Hypertension, 2013–2014 to 2021–2023. Am. J. Hypertens. 2025, 38, 120–128. [Google Scholar] [CrossRef]
- Esler, M.D.; Osborn, J.W.; Schlaich, M.P. Sympathetic Pathophysiology in Hypertension Origins: The Path to Renal Denervation. Hypertension 2024, 81, 1194–1205. [Google Scholar] [CrossRef]
- Zintel, H.A.; Mackie, J.A.; Sellers, A.M.; Jeffers, W.A.; Hafkenschiel, J.H.; Lindauer, M.A. Results of thoracolumbar sympathectomy for essential hypertension; three-to-seven-year follow-up of one hundred patients. AMA Arch. Surg. 1955, 71, 215–222. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Persu, A.; Fadl Elmula, F.E.M.; Jin, Y.; Os, I.; Kjeldsen, S.E.; Staessen, J.A. Renal Denervation After Symplicity HTN-3—Back to Basics. Review of the Evidence. Eur. Cardiol. 2014, 9, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Haberman, D.; Chitturi, K.R.; Lupu, L.; Wermers, J.P.; Waksman, R. Overview of the 2023 FDA Circulatory System Devices Advisory Panel meeting on the Recor Paradise Ultrasound-Based Renal Denervation System. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2024, 104, 34–43. [Google Scholar] [CrossRef]
- Chitturi, K.R.; Haberman, D.; Wermers, J.P.; Waksman, R. Overview of the 2023 FDA Circulatory System Devices Advisory Panel Meeting on the Symplicity Spyral Renal Denervation System. Am. Heart J. 2024, 269, 108–117. [Google Scholar] [CrossRef]
- Kandzari, D.E. Long-term Safety and Efficacy of Radiofrequency Renal Denervation in the Presence of Antihypertensive Drugs: 24-Month Results From the SPYRAL HTN-ON MED Randomized Trial. 2024. Available online: https://www.tctmd.com/slide/long-term-safety-and-efficacy-radiofrequency-renal-denervation-presence-antihypertensive (accessed on 28 October 2024).
- Kandzari, D.E.; Townsend, R.R.; Kario, K.; Mahfoud, F.; Weber, M.A.; Schmieder, R.E.; Pocock, S.; Tsioufis, K.; Konstantinidis, D.; Choi, J.; et al. Safety and Efficacy of Renal Denervation in Patients Taking Antihypertensive Medications. J. Am. Coll. Cardiol. 2023, 82, 1809–1823. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Böhm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet Lond. Engl. 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Mahfoud, F.; Kandzari, D.E.; Kario, K.; Townsend, R.R.; Weber, M.A.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; Dimitriadis, K.; Choi, J.W.; et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): A randomised, sham-controlled trial. Lancet Lond. Engl. 2022, 399, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.J.; Kirtane, A.J.; Azizi, M.; Mahfoud, F.; Basile, J.; Daemen, J.; Saxena, M.; Thackeray, L.; McGuire, M.; Claude, L.; et al. 36-month durability of ultrasound renal denervation for hypertension resistant to combination therapy in RADIANCE-HTN TRIO. Hypertens. Res. 2024, 47, 3467–3472. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Weber, M.A.; Pathak, A.; Zidar, J.P.; Saxena, M.; David, S.W.; Schmieder, R.E.; Janas, A.J.; Langer, C.; Persu, A.; et al. Effect of Alcohol-Mediated Renal Denervation on Blood Pressure in the Presence of Antihypertensive Medications: Primary Results From the TARGET BP I Randomized Clinical Trial. Circulation 2024, 149, 1875–1884. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Ren, C.; Ma, G.; Bu, P.; Fu, G.; Chen, H.; Han, Z.; Li, Y.; Li, J.; et al. The NetrodTM six-electrode radiofrequency renal denervation system for uncontrolled hypertension: A sham-controlled trial. Eur. Heart J. 2024, 45, 4761–4764. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Mahfoud, F.; Li, W.; Dong, H.; Yu, J.; Yu, S.; Chen, X.; Wang, P.; Li, Z.; Lauder, L.; et al. Efficacy and Safety of Catheter-Based Radiofrequency Renal Denervation in Chinese Patients with Uncontrolled Hypertension: The Randomized, Sham-Controlled, Multi-Center Iberis-HTN Trial. Circulation 2024, 150, 1588–1598. [Google Scholar] [CrossRef]
- Zidar, J.P. 3-Month Primary Efficacy Data from the REDUCED-1 Pilot Study. 2024. Available online: https://interventionalnews.com/tivus-renal-denervation-system-completes-enrolment/ (accessed on 6 February 2024).
- Mahfoud, F.; Azizi, M.; Daemen, J.; Sharp, A.S.P.; Patak, A.; Iglesias, J.F.; Kirtane, A.; Fisher, N.D.L.; Scicli, A.; Lobo, M.D. Real-world experience with ultrasound renal denervation utilizing home blood pressure monitoring: The Global Paradise System registry study design. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2024, 113, 1375–1383. [Google Scholar] [CrossRef]
- Mahfoud, F.; Mancia, G.; Schmieder, R.E.; Ruilope, L.; Narkiewicz, K.; Schlaich, M.; Williams, B.; Ribichini, F.; Weil, J.; Almerri, K.; et al. Outcomes Following Radiofrequency Renal Denervation According to Antihypertensive Medications: Subgroup Analysis of the Global SYMPLICITY Registry DEFINE. Hypertension 2023, 80, 1759–1770. [Google Scholar] [CrossRef]
- Barbato, E.; Azizi, M.; Schmieder, R.E.; Lauder, L.; Böhm, M.; Brouwers, S.; Bruno, R.M.; Dudek, D.; Kahan, T.; Kandzari, D.E.; et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2023, 44, 1313–1330. [Google Scholar] [CrossRef]
- Boffa, R.J.; Constanti, M.; Floyd, C.N.; Wierzbicki, A.S.; Guideline Committee. Hypertension in adults: Summary of updated NICE guidance. BMJ 2019, 367, l5310. [Google Scholar] [CrossRef]
- Chia, Y.C.; Wan Ahmad, W.A.; Fong, A.Y.Y.; Rosman, A.; Abdul Rahman, A.R.; Choo, G.H.; Lim, S.K.; Abu Bakar, M.Z.; Ong, T.K. 2022 Malaysian Working Group Consensus Statement on Renal Denervation for management of arterial hypertension. Hypertens. Res. 2022, 45, 1111–1122. [Google Scholar] [CrossRef]
- Cluett, J.L.; Blazek, O.; Brown, A.L.; East, C.; Ferdinand, K.C.; Fisher, N.D.L.; Ford, C.D.; Griffin, K.A.; Mena-Hurtado, C.I.; Sarathy, H.; et al. Renal Denervation for the Treatment of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2024, 81, e135–e148. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Kim, B.-K.; Aoki, J.; Wong, A.Y.-T.; Lee, Y.-H.; Wongpraparut, N.; Nguyen, Q.N.; Ahmad, W.A.W.; Lim, S.T.; Ong, T.K.; et al. Renal Denervation in Asia: Consensus Statement of the Asia Renal Denervation Consortium. Hypertension 2020, 75, 590–602. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Swaminathan, R.V.; East, C.A.; Feldman, D.N.; Fisher, N.D.; Garasic, J.M.; Giri, J.S.; Kandzari, D.E.; Kirtane, A.J.; Klein, A.; Kobayashi, T.; et al. SCAI Position Statement on Renal Denervation for Hypertension: Patient Selection, Operator Competence, Training and Techniques, and Organizational Recommendations. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101121. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-D.; Chiang, C.-E.; Chao, T.-H.; Cheng, H.-M.; Wu, Y.-W.; Wu, Y.-J.; Lin, Y.-H.; Chen, M.Y.-C.; Ueng, K.-C.; Chang, W.-T.; et al. 2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension. Acta Cardiol. Sin. 2022, 38, 225–325. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N. Engl. J. Med. 2009, 361, 878–887. [Google Scholar] [CrossRef]
- Ukena, C.; Cremers, B.; Ewen, S.; Böhm, M.; Mahfoud, F. Response and non-response to renal denervation: Who is the ideal candidate? EuroIntervention 2013, 9 (Suppl. R), R54–R57. [Google Scholar] [CrossRef]
- Schmieder, R.; Burnier, M.; East, C.; Tsioufis, K.; Delaney, S. Renal Denervation: A Practical Guide for Health Professionals Managing Hypertension. Interv. Cardiol. Lond. Engl. 2023, 18, e06. [Google Scholar] [CrossRef]
- Ammar, S.; Ladich, E.; Steigerwald, K.; Deisenhofer, I.; Joner, M. Pathophysiology of renal denervation procedures: From renal nerve anatomy to procedural parameters. EuroIntervention 2013, 9 (Suppl. R), R89–R95. [Google Scholar] [CrossRef]
- CPT® Editorial Summary of Panel Actions September 2024. 2024. Available online: https://www.ama-assn.org/system/files/sept-2024-summary-of-panel-actions.pdf (accessed on 14 May 2025).
- CMS. FY 2025 IPPS Final Rule Home Page. 2024. Available online: https://www.govinfo.gov/content/pkg/FR-2024-08-28/pdf/2024-17021.pdf (accessed on 14 May 2025).
- Schmieder, R.E. Renal denervation in patients with chronic kidney disease: Current evidence and future perspectives. Nephrol. Dial. Transplant. 2023, 38, 1089–1096. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, F.; Ye, R.; Zhang, X.; Peng, Y.; Chen, X.; Liu, K. Medication Changes After Renal Denervation: Current Evidence and Patient Perspectives. J. Am. Heart Assoc. 2024, 13, e037187. [Google Scholar] [CrossRef]
- DiBona, G.F. Renal innervation and denervation: Lessons from renal transplantation reconsidered. Artif. Organs 1987, 11, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Grisk, O.; Frey, B.A.; Uber, A.; Rettig, R. Sympathetic activity in early renal posttransplantation hypertension in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1737–R1744. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.; de Nijs, R.; Kjær Olsen, L.; Kamper, A.-L.; Evi Bang, L.; Frimodt-Møller, M.; Kelbæk, H.; Schwartz Sørensen, S.; Kjær, A.; Feldt-Rasmussen, B.; et al. Renal 123I-MIBG Uptake before and after Live-Donor Kidney Transplantation. Diagnostics 2020, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- Fengler, K.; Reimann, P.; Rommel, K.-P.; Kresoja, K.-P.; Blazek, S.; Unterhuber, M.; Besler, C.; von Roeder, M.; Böhm, M.; Desch, S.; et al. Comparison of Long-Term Outcomes for Responders Versus Non-Responders Following Renal Denervation in Resistant Hypertension. J. Am. Heart Assoc. 2021, 10, e022429. [Google Scholar] [CrossRef]
- Böhm, M.; Tsioufis, K.; Kandzari, D.E.; Kario, K.; Weber, M.A.; Schmieder, R.E.; Townsend, R.R.; Kulenthiran, S.; Ukena, C.; Pocock, S.; et al. Effect of Heart Rate on the Outcome of Renal Denervation in Patients With Uncontrolled Hypertension. J. Am. Coll. Cardiol. 2021, 78, 1028–1038. [Google Scholar] [CrossRef]
- Mahfoud, F.; Townsend, R.R.; Kandzari, D.E.; Kario, K.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; David, S.; Patel, K.; Rao, A.; et al. Changes in Plasma Renin Activity After Renal Artery Sympathetic Denervation. J. Am. Coll. Cardiol. 2021, 77, 2909–2919. [Google Scholar] [CrossRef]
- Fengler, K.; Rommel, K.-P.; Kriese, W.; Kresoja, K.-P.; Blazek, S.; Obradovic, D.; Feistritzer, H.-J.; Lücke, C.; Gutberlet, M.; Desch, S.; et al. Assessment of arterial stiffness to predict blood pressure response to renal sympathetic denervation. EuroIntervention 2022, 18, e686–e694. [Google Scholar] [CrossRef]
- Delles, C.; Schmieder, R.E.; Daly, R.; Kannenkeril, D.; Bosch, A.; Lauder, L.; Kunz, M.; Böhm, M.; Hamilton, G.; Schmieder, R.S.; et al. Response of Blood Pressure to Renal Denervation Is Not Associated With Genetic Variants. Hypertension 2025, 82, 118–125. [Google Scholar] [CrossRef]
- Persu, A.; Gordin, D.; Jacobs, L.; Thijs, L.; Bots, M.L.; Spiering, W.; Miroslawska, A.; Spaak, J.; Rosa, J.; de Jong, M.R.; et al. Blood pressure response to renal denervation is correlated with baseline blood pressure variability: A patient-level meta-analysis. J. Hypertens. 2018, 36, 221–229. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Schmieder, R.E. Wilder’s principle: Pre-treatment value determines post-treatment response. Eur. Heart J. 2015, 36, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Ladich, E.; Edelman, E.R.; Markham, P.; Stanley, J.R.L.; Keating, J.; Kolodgie, F.D.; Virmani, R.; Joner, M. Methodological standardization for the pre-clinical evaluation of renal sympathetic denervation. JACC Cardiovasc. Interv. 2014, 7, 1184–1193. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y.; Lu, C.; Lu, Z.; Hu, J.; Wang Yue Ge, J.; Jiang, H.; Yao, C.; Yan, X.; Ma, W.; et al. Efficacy and safety of sympathetic mapping and ablation of renal nerves for the treatment of hypertension (SMART): 6-month follow-up of a randomised, controlled trial. EClinicalMedicine 2024, 72, 102626. [Google Scholar] [CrossRef] [PubMed]
- Balaji, P.; Liu, X.; Tran, V.T.; Barry, M.A.; Vien, A.; Yang, E.; Nguyen, D.M.; Patel, U.; Lu, J.; Alvarez, S.; et al. Abolition of Aorticorenal Ganglia Pacing Responses Improves Denervation Efficacy. Hypertension 2025, 82, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Kip, K.E.; Hollabaugh, K.; Marroquin, O.C.; Williams, D.O. The problem with composite end points in cardiovascular studies: The story of major adverse cardiac events and percutaneous coronary intervention. J. Am. Coll. Cardiol. 2008, 51, 701–707. [Google Scholar] [CrossRef]
- Haider, S.A.; Wagener, M.; Iqbal, T.; Shahzad, S.; Del Sole, P.A.; Leahy, N.; Murphy, D.; Sharif, R.; Ullah, I.; Sharif, F. Does renal denervation require cardiovascular outcome-driven data? Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2024, 47, 2633–2643. [Google Scholar] [CrossRef]
- Kario, K.; Pickering, T.G.; Umeda, Y.; Hoshide, S.; Hoshide, Y.; Morinari, M.; Murata, M.; Kuroda, T.; Schwartz, J.E.; Shimada, K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: A prospective study. Circulation 2003, 107, 1401–1406. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Hoshide, S.; Kario, K. Difference between morning and evening home blood pressure and cardiovascular events: The J-HOP Study (Japan Morning Surge-Home Blood Pressure). Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2021, 44, 1597–1605. [Google Scholar] [CrossRef]
- Kario, K.; Mahfoud, F.; Kandzari, D.E.; Townsend, R.R.; Weber, M.A.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; Brar, S.; Hettrick, D.A.; et al. Long-term reduction in morning and nighttime blood pressure after renal denervation: 36-month results from SPYRAL HTN-ON MED trial. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2023, 46, 280–288. [Google Scholar] [CrossRef]
- Mahfoud, F.; Townsend, R.R.; Kandzari, D.E.; Mancia, G.; Whitbourn, R.; Lauder, L.; Bhatt, D.L.; Kario, K.; Schmieder, R.E.; Schlaich, M.; et al. Long-Term, Patient-Level Analysis of Radiofrequency Renal Denervation in the SYMPLICITY Clinical Trial Program. JACC Adv. 2025, 4, 101606. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Harada, K.; Hishiki, Y.; Kario, K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J. Clin. Hypertens. 2019, 21, 853–858. [Google Scholar] [CrossRef]
- Goyal, A.; Jain, H.; Verma, A.; Jain, J.; Shamim, U.; Kanagala, S.G.; Motwani, J.; Dey, R.C.; Chunawala, Z.; Sohail, A.H.; et al. The role of renal denervation in cardiology and beyond: An updated comprehensive review and future directives. Curr. Probl. Cardiol. 2024, 49, 102196. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Hettrick, D.A.; Böhm, M.; Kandzari, D.E.; Kario, K.; Mahfoud, F.; Tsioufis, K.; Weber, M.A.; Esler, M.D.; Townsend, R.R. Novel approaches to define responders to interventional treatment in hypertension: Insights from the SPYRAL HTN-OFF and HTN-ON MED trials. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2025, 48, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Shimada, H.; Imazato, K.; Sako, H.; Udo, A.; Taniguchi, K.; Morisaki, S.; Imamura, I.; Urata, H.; Arima, H.; et al. Impact of renal denervation on quality of life (How does renal denervation contribute to improving hypertension treatment affected by poor medication adherence?). Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2024, 47, 2652–2658. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Cao, K.N.; Ryschon, A.M.; Sharp, A.S.P.; Pietzsch, J.B. Catheter-Based Radiofrequency Renal Denervation in the United States: A Cost-Effectiveness Analysis Based on Contemporary Evidence. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102234. [Google Scholar] [CrossRef]
- Watson, N.; Earle, W.; Kandzari, D.E.; Kirtane, A.; Mahfound, F.; Cluett, J.; Krawisz, A.; Juraschek, S.; Secemsky, E. National Estimates of Patient Eligibility for Renal Denervation Therapy Post-FDA Approval. Circulation 2024, 150, 4125667. [Google Scholar] [CrossRef]
| Technology and Catheter | Design | Access Site and Size | Ablation Sites | Status |
|---|---|---|---|---|
| Radiofrequency | ||||
| Symplicity Spyral Catheter (Medtronic, Minneapolis, MN, USA) | Multiple electrode (4 monopolar electrode), helical design, 60 s per ablation cycle | F (6Fr) | Main and accessory arteries, including branches (same catheter diameter 3–8 mm) | FDA approval 2023 |
| Netrod (Shanghai Golden Leaf MedTec Co., Ltd., Shanghai, China) | Multiple electrode (6 monopolar electrode), basket-shaped tip, 120 s per cycle | F (8Fr) | Main and accessible renal arterial vessels (same catheter diameter 3–12 mm) | Sham-controlled trial (EHJ 2024) |
| Iberis (Angiocare and Terumo, Somerset, NJ, USA) | Multielectrode (4 monopolar electrode), helical design, 60 s per ablation cycle | F/R (6Fr) | Main and accessory arteries, including branches (same catheter diameter 3–8 mm) | Iberis-HTN trial, sham controlled, blinded (Circulation 2024) |
| Ultrasound | ||||
| Paradise (RECOR, Palo Alto, CA, USA) | Piezoelectric ceramic transducer within a fluid-cooled, low-pressure balloon, 7 s per emission | F (7Fr) | Main and accessory arteries, including branches (different catheters for diameter 3–8 mm) | FDA approval 2023 |
| TIVUS (SoniVie, Rehovot, Israel) | Nonocclusive, free floating in the lumen, high ablation depth | R (4Fr) | One size fits all catheter | REDUCED-1 Pilot study (TCT 2024) THRIVE trial (ongoing) |
| Neurolysis | ||||
| Peregrine (Ablative Solutions, Wakefield, MA, USA) | Infusion catheter with three extendable ultrathin microneedles into perivascular space of renal arteries (0.6 mL dehydrated alcohol per treatment) | F (7Fr) | Main and accessory arteries (supply >20% renal parenchyma, maximum of one accessory artery treated per side), including branches (diameter 3–7 mm) | Target BP trial (Circulation 2024) |
| Study | Year of Pub | # of Patients | Patient Characteristics | Device | Medications | Primary Endpoint | Additional Data |
|---|---|---|---|---|---|---|---|
| Symplicity HTN-3 | 2014 | RDN = 364 Sham = 171 | RH (5.1 vs. 5.2 meds in RDN and sham) | Symplicity Flex catheter (radiofrequency) | Protocol called for stable background of medications, but 39% of patients changed medications during study. | Mean change in office SBP at 6 months was −14 ± 24 in the RDN group vs. −12 ± 26 in the sham group (p = 0.26) | 3-year follow-up showed a further reduction in office SBP of 26.4 ± 25.9 in RDN vs. 5.7 ± 24.4 in the sham (p ≤ 0.0001). |
| RADIANCE-HTN SOLO | 2018 | RDN = 74 Sham = 72 | Ambulatory BP ≥ 135/85 and <170/105 after a 4-week d/c of up to 2 meds | Paradise (ultrasound) | No background antihypertensive medications | Reduction in daytime ambulatory SBP with RDN (−8.5 ± 9.3), Sham (−2.2 ± 10.0); p = 0.0001 | For 51 patients in the RDN group with 36-month follow-up, office BP decreased by 18/11 ± 15/9 from baseline (p < 0.001 for both). |
| SPYRAL OFF MEDS | 2020 | RDN = 166 Sham = 165 | 150 ≤ Office SBP < 180, and office DBP ≥ 90, and 140 ≤ mean 24 h SBP < 170 | Spyral catheter (radiofrequency) | No background antihypertensive medications | Reduction in 24 h SBP at 3 months was −4.7 [−6.4, −2.9] in RDN and −0.6 [−2.1, 0.9] in sham | Patients meeting ‘escape criteria’ (office SBP ≥ 180) were lower in the RDN (9.6%) than in the sham (17.0%) within 3 months (p = 0.032). |
| RADIANCE-HTN TRIO | 2021 | RDN = 69 Sham = 67 | Office BP ≥ 140/90 while on at least 3 medications of different classes including a diuretic for at least 4 wks | Paradise (ultrasound) | Stable dose of a triple medication pill (amlodipine, valsartan and HCTZ) | Reduction in daytime ambulatory SBP at 2 months in RDN (−8.0 [IQR –16.4 to 0.0]) vs. Sham (–3.0 [–10.3 to 1.8]) adjusted p = 0.022. | Similar 6-month daytime ambulatory BP measurements in both groups (138.3 ± 15.1 with uRDN vs. 139.0 ± 14.3 in the sham group although fewer medications were added in the RDN group (0.7 ± 1.0 vs. 1.1 ± 1.1 medications; p = 0.045) |
| RADIANCE II | 2023 | RDN = 150 Sham = 74 | 140/90 ≤ Office BP ≤180/120 mm Hg while at least 4 wks on 0–2 medications of different classes | Paradise (ultrasound) | No background antihypertensive medications | Mean reduction in daytime ambulatory SBP at 2 months was −7.9 ± 11.6 with RDN vs. −1.8 ± 9.5 (p < 0.001) | Controlled daytime ambulatory BP was achieved in 18.8% of RDN group and 4.8% in the sham procedure group |
| SPYRAL ON MEDS (expansion) | 2023 | RDN = 206 Sham = 131 | Uncontrolled HTN (2.2 vs. 2.3 meds in RDN and Sham) | Spyral catheter (radiofrequency) | Stable dose of 1–3 antihypertensive medications | Reduction in mean 24 h ambulatory SBP at 6 months RDN (−6.5 ± 10.7) vs. sham (−4.5 ± 10.3); p = 0.12. | Office SBP (−9.9 ± 13.9 vs. −5.1 ± 13.2; p = 0.0015) and DBP (−5.2 ± 8.8 vs. −3.3 ± 8.2; p = 0.041) were lower in RDN at 6 months |
| TARGET BP 1 | 2024 | RDN = 148 Sham = 153 | 150/90 ≤ Office BP ≤180/90, and 135 ≤ mean 24 h SBP ≤170 mm Hg despite on 2 to 5 medications | Peregrine alcohol-mediated RDN | Stable dose of antihypertensive medications | 24 h ambulatory SBP at 3 months was −10.0 ± 14.2 in the RDN and −6.8 ± 12.1 in the sham; p = 0.0487. | No significant differences in office SBP or DBP between the RDN and sham control groups. At 30 days, major adverse event rate was 4.7% for the RDN group and none for the sham control group (p = 0.007) |
| Society | Year | Role as Adjunctive Therapy | Indications | Contra- Indications/Uncertainties | |||||
|---|---|---|---|---|---|---|---|---|---|
| Resistant HTN | Uncontrolled HTN Due to Intolerance | Uncontrolled HTN Due to Nonadherence | ABPM Confirmation | R/O 2nd Causes | Favor Patients with High Cardiovascular Risks | ||||
| SCAI | 2021, 2023 | Y | + | + | + | N/A | + | + | NS * |
| AHA | 2024 | Y | + | + | + | N/A | + | N/A | Y ** |
| ESH *** | 2023 | Y | + | + | + | + | + | N/A | NS |
| ESC | 2023 | Y | + | + | + | + | + | + | NS # |
| Netherland ## | 2022 | Y | + | + | + | + | + | N/A | NS |
| Britain /Ireland ### | 2019, 2023 | N | + | N/A | NS | ||||
| Taiwan | 2022 | Y | + | + | + | N/A | + | + @ | NS |
| Japan | 2024 | Y | + | + | + | N/A | + | N/A | Y @@ |
| Malasia @@@ | 2022 | Y | + | + | + | N/A | + | + | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Belford, P.M.; Stouffer, G.A. Renal Denervation After USA FDA Approval: An Update from an Interventional Cardiologist’s Perspective. J. Clin. Med. 2025, 14, 3554. https://doi.org/10.3390/jcm14103554
Zhang J, Belford PM, Stouffer GA. Renal Denervation After USA FDA Approval: An Update from an Interventional Cardiologist’s Perspective. Journal of Clinical Medicine. 2025; 14(10):3554. https://doi.org/10.3390/jcm14103554
Chicago/Turabian StyleZhang, Jiandong, Peter M. Belford, and George A. Stouffer. 2025. "Renal Denervation After USA FDA Approval: An Update from an Interventional Cardiologist’s Perspective" Journal of Clinical Medicine 14, no. 10: 3554. https://doi.org/10.3390/jcm14103554
APA StyleZhang, J., Belford, P. M., & Stouffer, G. A. (2025). Renal Denervation After USA FDA Approval: An Update from an Interventional Cardiologist’s Perspective. Journal of Clinical Medicine, 14(10), 3554. https://doi.org/10.3390/jcm14103554






