Validation of Kidney Donor Profile Index and Estimated Post-Transplant Survival Scores in an Eastern European Transplantation Center—A Seven-Year Retrospective Observational Study
Abstract
:1. Introduction
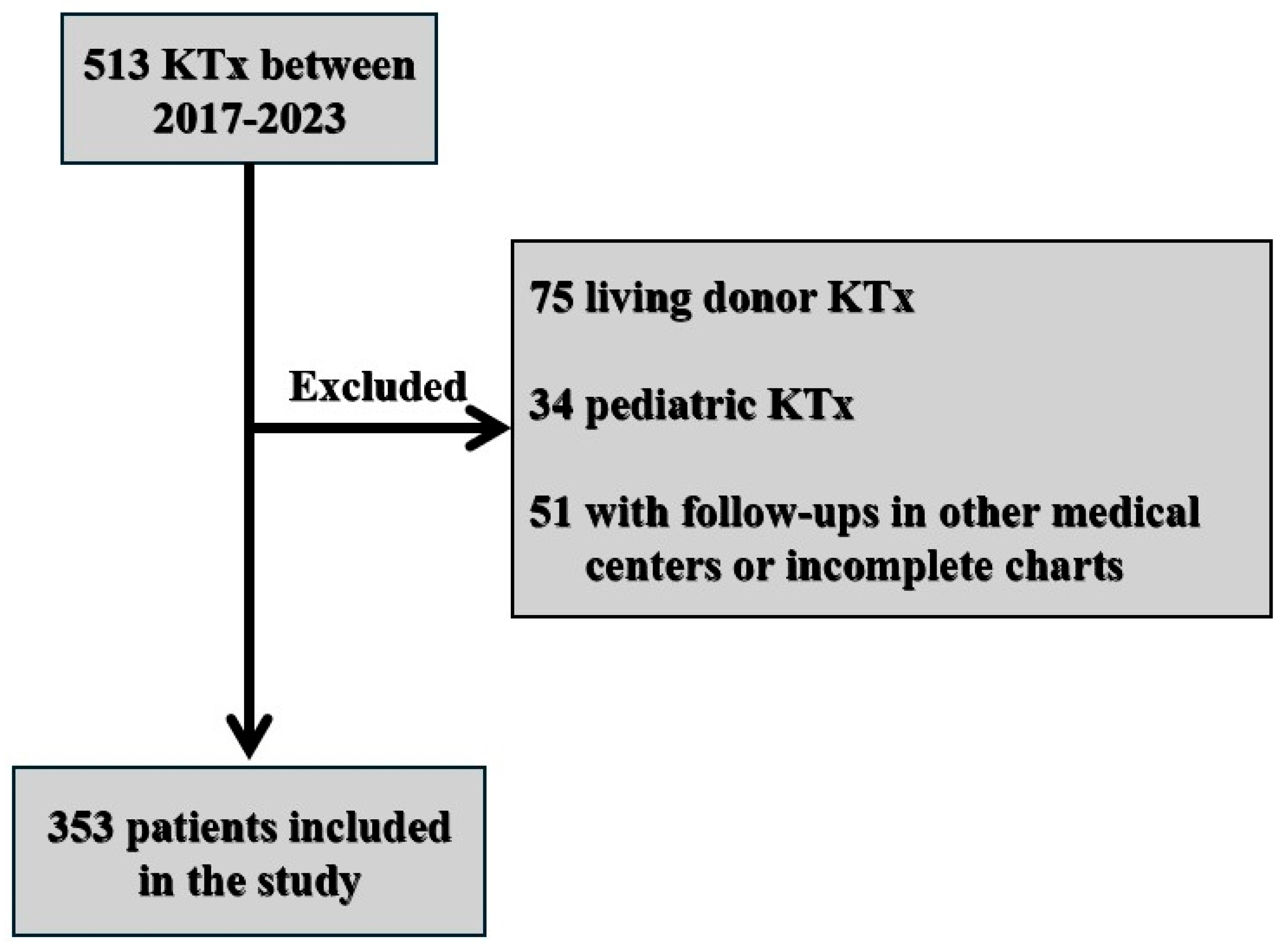
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Coemans, M.; Süsal, C.; Döhler, B.; Anglicheau, D.; Giral, M.; Bestard, O.; Legendre, C.; Emonds, M.-P.; Kuypers, D.; Molenberghs, G.; et al. Analyses of the Short- and Long-Term Graft Survival after Kidney Transplantation in Europe between 1986 and 2015. Kidney Int. 2018, 94, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Summers, D.M.; Johnson, R.J.; Allen, J.; Fuggle, S.V.; Collett, D.; Watson, C.J.; Bradley, J.A. Analysis of Factors That Affect Outcome after Transplantation of Kidneys Donated after Cardiac Death in the UK: A Cohort Study. Lancet 2010, 376, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Stallone, G.; Grandaliano, G. To Discard or Not to Discard: Transplantation and the Art of Scoring. Clin. Kidney J. 2019, 12, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Milford, E.L.; Mackenzie, H.S.; Brenner, B.M. Antigen-Independent Determinants of Cadaveric Kidney Transplant Failure. JAMA 1996, 276, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Melk, A.; Sugianto, R.I.; Zhang, X.; Dahhou, M.; Döhler, B.; Süsal, C.; Sapir-Pichhadze, R.; Wong, G.; Foster, B.J. Influence of Donor Sex and Age on Graft Outcome in Kidney Transplantation. Nephrol. Dial. Transplant. 2024, 39, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Dayoub, J.C.; Cortese, F.; Anžič, A.; Grum, T.; de Magalhães, J.P. The Effects of Donor Age on Organ Transplants: A Review and Implications for Aging Research. Exp. Gerontol. 2018, 110, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Crannell, W.C.; Perkins, J.D.; Leca, N.; Kling, C.E. Deceased Donor Kidneys Are Discarded at Higher Rates When Labeled as High Kidney Donor Profile Index. Am. J. Transplant. 2022, 22, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Aubert, O.; Reese, P.P.; Audry, B.; Bouatou, Y.; Raynaud, M.; Viglietti, D.; Legendre, C.; Glotz, D.; Empana, J.-P.; Jouven, X.; et al. Disparities in Acceptance of Deceased Donor Kidneys Between the United States and France and Estimated Effects of Increased US Acceptance. JAMA Intern. Med. 2019, 179, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Organ Procurement and Transplantation Network. Kidney Donor Profile Index (KDPI) Calculator [Internet]. Available online: https://optn.transplant.hrsa.gov/data/allocation-calculators/kdpi-calculator/ (accessed on 14 March 2024).
- Transplant Models. KDPI and EPTS [Internet]. Available online: http://www.transplantmodels.com/kdpi-epts/ (accessed on 14 March 2024).
- Fabbian, F.; De Giorgi, A.; Manfredini, F.; Lamberti, N.; Forcellini, S.; Storari, A.; Todeschini, P.; Gallerani, M.; La Manna, G.; Mikhailidis, D.P.; et al. Impact of Comorbidity on Outcome in Kidney Transplant Recipients: A Retrospective Study in Italy. Intern. Emerg. Med. 2016, 11, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Elec, F.I.; Bolboacă, S.D.; Muntean, A.; Elec, A.D.; Cismaru, C.; Lupşe, M.; Oltean, M. Comparing the First and Second Wave of COVID-19 in Kidney Transplant Recipients: An East-European Perspective. Eur. Surg. Res. 2022, 63, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pestana, J.; Cristelli, M.P.; Foresto, R.D.; Tedesco-Silva, H.; Requião-Moura, L.R. The Higher COVID-19 Fatality Rate Among Kidney Transplant Recipients Calls for Further Action. Transplantation 2022, 106, 908–910. [Google Scholar] [CrossRef]
- Oltean, M.; Søfteland, J.M.; Bagge, J.; Ekelund, J.; Felldin, M.; Schult, A.; Magnusson, J.; Friman, V.; Karason, K. COVID-19 in Kidney Transplant Recipients: A Systematic Review of the Case Series Available Three Months into the Pandemic. Infect. Dis. 2020, 52, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Taber, D.J.; DuBay, D.; McGillicuddy, J.W.; Nadig, S.; Bratton, C.F.; Chavin, K.D.; Baliga, P.K. Impact of the New Kidney Allocation System on Perioperative Outcomes and Costs in Kidney Transplantation. J. Am. Coll. Surg. 2017, 224, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Coca, A.; Arias-Cabrales, C.; Valencia, A.L.; Burballa, C.; Bustamante-Munguira, J.; Redondo-Pachón, D.; Acosta-Ochoa, I.; Crespo, M.; Bustamante, J.; Mendiluce, A.; et al. Validation of a Survival Benefit Estimator Tool in a Cohort of European Kidney Transplant Recipients. Sci. Rep. 2020, 10, 17109. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.A.; McDonald, S.P.; Snyder, J.J.; Salkowski, N.; Chadban, S.J. External Validation of the Estimated Posttransplant Survival Score for Allocation of Deceased Donor Kidneys in the United States. Am. J. Transplant. 2014, 14, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Murray, E.; Thomson, P.C.; Mark, P.B.; Clancy, M.J.; Asher, J. The New UK National Kidney Allocation Scheme With Maximized “R4-D4” Kidney Transplants: Better Patient-to-Graft Longevity Matching May Be at the Cost of More Resources. Exp. Clin. Transplant. 2021, 19, 1133–1141. [Google Scholar] [CrossRef] [PubMed]


| All Patients n = 353 100% | KDPI < 35% n = 80 22.7% | KDPI 35–85% n = 172 48.7% | KDPI > 85% n = 101 28.6% | p | |
|---|---|---|---|---|---|
| Age, years, median, (IQR) | 46.7 (55–40) | 36 (42.8–28.3) | 48 (53–42) | 57 (61–52) | p < 0.001 *** |
| Sex | |||||
| Females, N (%) | 125 (35.4) | 31 (38.8) | 48 (27.9) | 46 (45.5) | |
| Males, N (%) | 228 (64.6) | 49 (61.2) | 124 (72.1) | 55 (54.5) | |
| BMI | 26.4 ± 4.4 | 24.4 ± 4.5 | 26.5 ± 4.1 | 27.6 ± 4.3 | p < 0.001 ** |
| Donor terminal creatinine mg/dL, median (IQR) | 1.03 (1.6–0.7) | 0.8 (1.25–0.64) | 1.04 (1.47–0.78) | 1.29 (1.91–0.83) | p < 0.001 *** |
| HLA mismatches, median (IQR) | 4 (4–3) | 4 (4.25–3) | 4 (4–3) | 4 (5–3) | p = 0.28 * |
| History of arterial hypertension, n (%) | 267 (75.6) | 48 (60) | 133 (77.3) | 86 (85.1) | p = 0.001 * |
| History of type 2 diabetes, n (%) | 27 (7.6) | 1 (1.25) | 11 (6.39) | 15 (17.44) | p = 0.001 * |
| Mean dialysis time before KTx, years, median (IQR) | 2 (4–0.9) | 2 (4.5–0.75) | 2 (4–0.7) | 2 (4–1) | p = 0.427 *** |
| Previous KTx, yes, N (%) | 9 (2.5) | 3 (3.7) | 4 (2.3) | 2 (1.98) | p = 0.735 * |
| Charlson Comorbidity Index, median (IQR) | 3 (2–4) | 2 (2–3) | 3 (2–4) | 3 (3–4) | p < 0.05 |
| All Patients n = 353 100% | KDPI < 35% n = 80 22.7% | KDPI 35–85% n = 172 48.7% | KDPI > 85% n = 101 28.6% | p | |
|---|---|---|---|---|---|
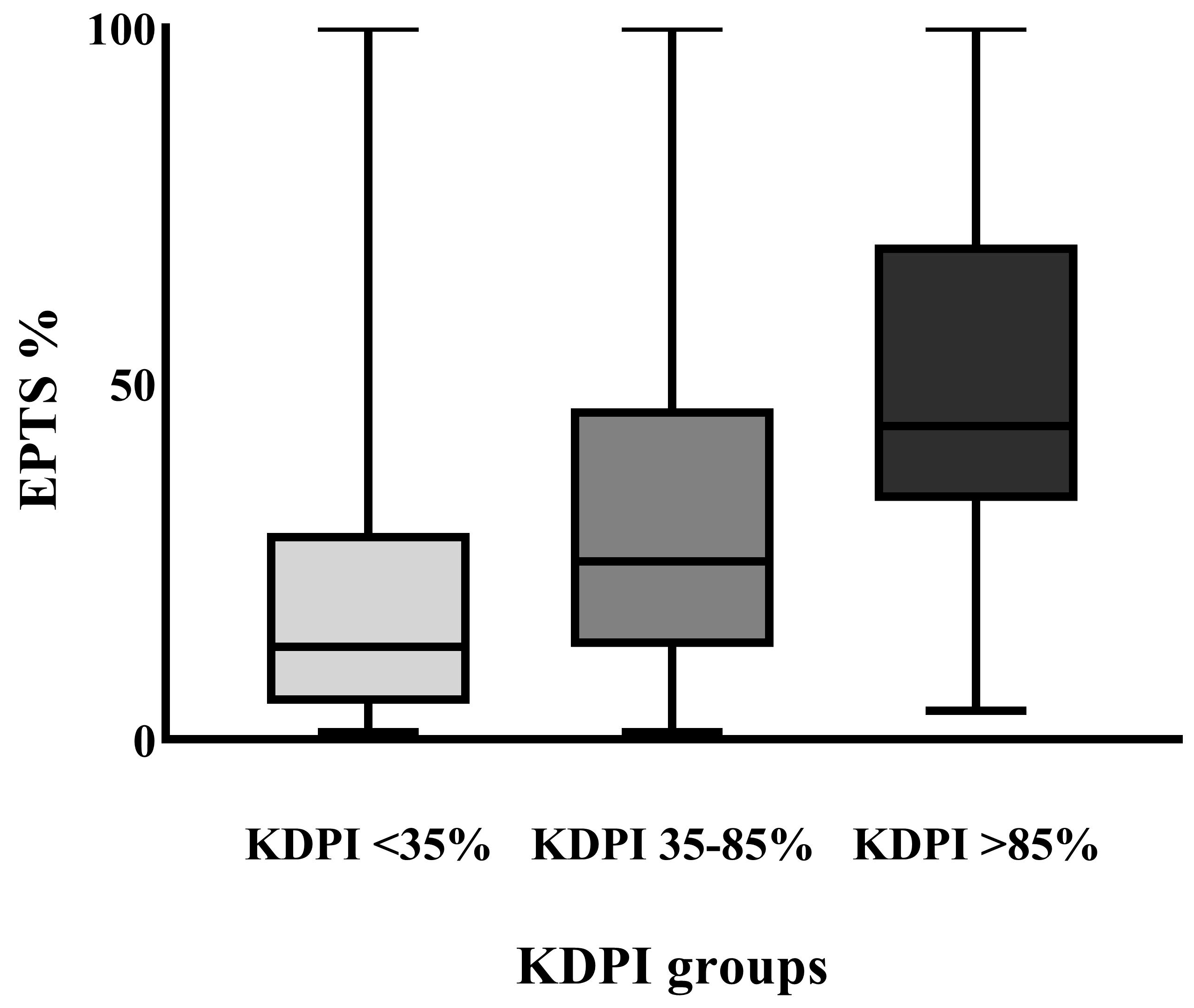
| EPTS score %, median (IQR) | 30 (52–14) | 13.5 (28.75–5) | 25 (46.75–13) | 44 (69.5–33.5) | p < 0.001 * |
| KDPI score %, median (IQR) | 65 (87–38) | 25.5 (31–11.7) | 63 (74–51) | 96 (99–92) | p < 0.001 * |
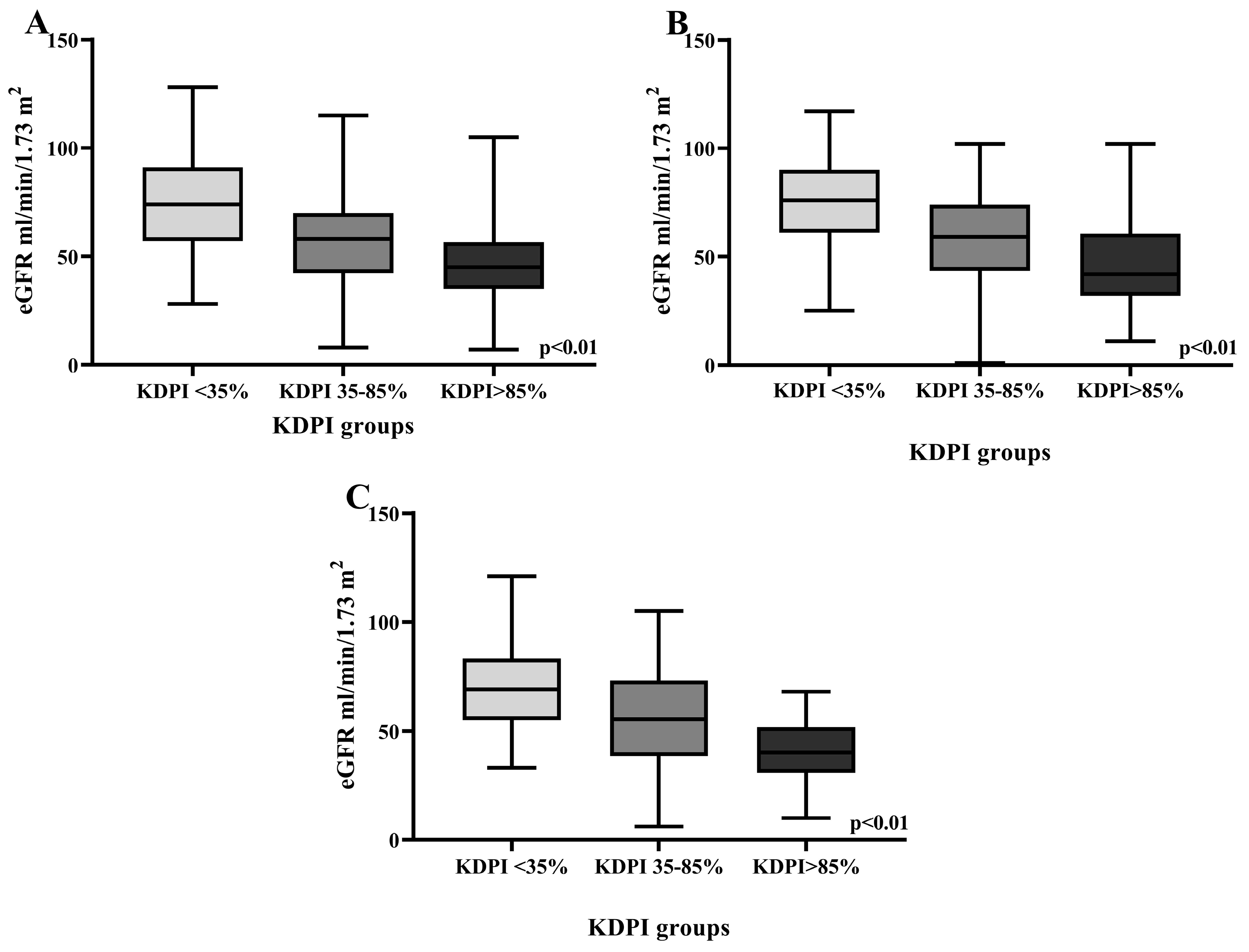
| One-month eGFR mL/min/1.73 m2, median (IQR) | 44.83 (67.25–28.75) | 61.2 (78.6–43.7) | 47 (87.7–29.2) | 35.5 (45.7–22.6) | p < 0.001 ** |
| One-year eGFR mL/min/1.73 m2, mean (SD) | 58.91 (23.59) | 74.6 (22.56) | 58 (21.7) | 47.5 (20.3) | p < 0.001 ** |
| Three-year eGFR mL/min/1.73 m2, mean (SD) | 60.67 (24.18) | 75.9 (21.6) | 57.7 (21.9) | 47.6 (22.6) | p < 0.001 ** |
| Five-year eGFR mL/min/1.73 m2, mean (SD) | 59.15 (23.65) | 69.3 (20.8) | 55 (23.7) | 40.7 (15) | p < 0.001 ** |
| All Patients | KDPI < 35% (80; 22.7) | KDPI 35–85% (172; 48.7) | KDPI > 85% (101; 28.6) | p | |
|---|---|---|---|---|---|
| 5-year estimated survival on waiting list (%), median (IQR) | 67 (79.3–57.6) | 79.3 (84.9–68) | 70.6 (79.7–59.2) | 60.2 (65.3–52.2) | p < 0.001 ** |
| 5-year estimated survival if KTx performed (%), median (IQR) | 87.5 (93.7–73.9) | 94.8 (96–92.2) | 89.7 (93.1–83.7) | 78.3 (82.2–69.6) | p < 0.001 ** |
| 5-year estimated survival benefit if KTx performed (%), median (IQR) | 17.5 (22.05–13.5) | 15.7 (23.8–11.5) | 19.6 (24.2–13.7) | 17 (18.9–15.8) | p < 0.001 ** |
| Total | KDPI < 35% | KDPI 35–85% | KDPI > 85% | p | |
|---|---|---|---|---|---|
| 1-year graft survival proportion, Nfunctional/Ntotal, (%) | 339/350 (96.9) | 79/80 (98.8) | 169 (95.9) | 98/101 (97.0) | p = 0.48 |
| 1-year graft survival time, mean (CI) | 11.7 (11.5–11.9) | 11.8 (11.6–12.4) | 11.6 (11.4–11.9) | 11.7 (11.4–12.1) | |
| 1-year patient survival proportion, Nsurvivors/Ntotal (%) | 332/350 (94.9) | 77/80 (96.2) | 163/169 (96.4) | 92/101 (91.1) | p = 0.124 |
| 1-year patient survival time, mean (CI) | 11.6 (11.5–11.8) | 11.7 (11.3–12.1) | 11.8 (11.6–11.9) | 11.4 (10.9–11.8) | |
| 3-year graft survival proportion, Nfunctional/Ntotal, (%) | 216/229 (94.3) | 61/62 (98.4) | 103/111 (92.8) | 52/56 (92.9) | p = 0.278 |
| 3-year graft survival time, mean (CI) | 34.3 (33.4–35.2) | 35.4 (34.3–36.5) | 34.0 (32.6–35.4) | 33.7 (31.6–35.9) | |
| 3-year patient survival proportion, Nsurvivors/Ntotal (%) | 200/229 (87.3) | 57/62 (91.9) | 102/111 (91.9) | 41/56 (73.2) | p < 0.01 |
| 3-year patient survival time, mean (CI) | 33.1 (31.9–34.1) | 33.9 (32.1–35.9) | 34.2 (32.9–35.4) | 29.6 (26.6–32.6) | |
| 5-year graft survival proportion, Nfunctional/Ntotal, (%) | 157/172 (91.3) | 56/58 (96.5) | 75/83 (90.2) | 26/31 (83.5) | p = 0.09 |
| 5-year graft survival time, mean (CI) | 55.9 (53.8–58.9) | 58.5 (56.4–60.7) | 54.9 (51.7–58.3) | 53.1 (46.9–59.4) | |
| 5-year patient survival proportion, Nsurvivors/Ntotal (%) | 143/172 (83.1) | 55/58 (94.8) | 72/83 (86.5) | 16/31 (50.8) | p < 0.001 |
| 5-year patient survival time, mean (CI) | 53.5 (51.8–55.9) | 57.6 (54.9–60.4) | 55.4 (52.7–58.2) | 40.9 (32.8–49.9) |
| Total | EPTS < 20% | EPTS 20–60% | EPTS > 60% | p | |
|---|---|---|---|---|---|
| 1-year graft survival proportion, Nfunctional/Ntotal, (%) | 339/350 (96.9) | 121/124 (97.6) | 152/157 (96.8) | 66/69 (95.7) | p = 0.743 |
| 1-year graft survival time, mean (CI) | 11.7 (11.5–11.9) | 11.9 (11.7–12.1) | 11.7 (11.4–11.9) | 11.5 (10.9–12.1) | |
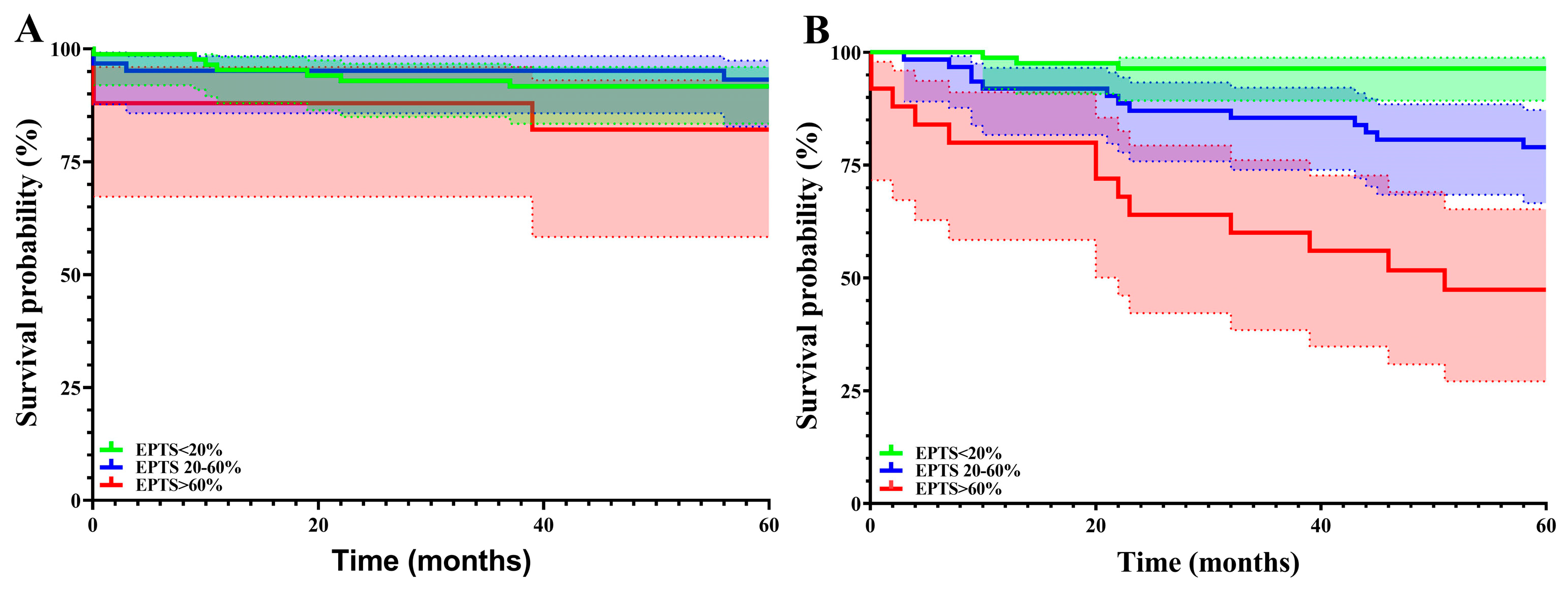
| 1-year patient survival proportion, Nsurvivors/Ntotal (%) | 332/350 (94.9) | 123/124 (99.2) | 148/157 (94.3) | 61/69 (88.4) | p = 0.004 |
| 1-year patient survival time, mean (CI) | 11.6 (11.5–11.8) | 11.9 (11.8–12.1) | 11.7 (11.4–11.9) | 11.0 (10.3–11.7) | |
| 3-year graft survival proportion, Nfunctional/Ntotal, (%) | 216/229 (94.3) | 92/98 (93.9) | 87/91 (95.6) | 37/40 (92.5) | p = 0.728 |
| 3-year graft survival time, mean (CI) | 34.3 (33.4–35.2) | 34.5 (33.3–35.7) | 34.6 (33.2–35.9) | 33.3 (30.4–36.2) | |
| 3-year patient survival proportion, Nsurvivors/Ntotal (%) | 200/229 (87.3) | 95/98 (96.9) | 78/91 (85.7) | 27/40 (67.5) | p < 0.001 |
| 3-year patient survival time, mean (CI) | 33.1 (31.9–34.1) | 35.5 (34.6–36.1) | 32.6 (30.8–34.5) | 28.2 (24.2–32.1) | |
| 5-year graft survival proportion, Nfunctional/Ntotal, (%) | 157/172 (91.3) | 78/85 (91.8) | 58/62 (93.5) | 21/25 (84) | p = 0.245 |
| 5-year graft survival time, mean (CI) | 55.9 (53.8–58.0) | 56.3 (53.6–59.0) | 57.1 (53.9–60.2) | 51.6 (43.7–59.4) | |
| 5-year patient survival proportion, Nsurvivors/Ntotal (%) | 143/172 (83.1) | 82/85 (96.5) | 49/62 (79) | 12/25 (48) | p < 0.001 |
| 5-year patient survival time, mean (CI) | 53.5 (51.2–55.9) | 58.4 (56.6–60.2) | 52.7 (48.6–56.7) | 39.7 (30.3–48.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elec, F.I.; Moisoiu, T.; Negrut, M.F.; Simon, R.; Elec, A.D.; Muntean, A.M.; Horciag, G.; Sitaru, A.M.; Rachisan, A.L.; Oniscu, G.; et al. Validation of Kidney Donor Profile Index and Estimated Post-Transplant Survival Scores in an Eastern European Transplantation Center—A Seven-Year Retrospective Observational Study. J. Clin. Med. 2025, 14, 3540. https://doi.org/10.3390/jcm14103540
Elec FI, Moisoiu T, Negrut MF, Simon R, Elec AD, Muntean AM, Horciag G, Sitaru AM, Rachisan AL, Oniscu G, et al. Validation of Kidney Donor Profile Index and Estimated Post-Transplant Survival Scores in an Eastern European Transplantation Center—A Seven-Year Retrospective Observational Study. Journal of Clinical Medicine. 2025; 14(10):3540. https://doi.org/10.3390/jcm14103540
Chicago/Turabian StyleElec, Florin Ioan, Tudor Moisoiu, Matei Florin Negrut, Robert Simon, Alina Daciana Elec, Adriana Milena Muntean, Georgeta Horciag, Ana Maria Sitaru, Andreea Liana Rachisan, Gabriel Oniscu, and et al. 2025. "Validation of Kidney Donor Profile Index and Estimated Post-Transplant Survival Scores in an Eastern European Transplantation Center—A Seven-Year Retrospective Observational Study" Journal of Clinical Medicine 14, no. 10: 3540. https://doi.org/10.3390/jcm14103540
APA StyleElec, F. I., Moisoiu, T., Negrut, M. F., Simon, R., Elec, A. D., Muntean, A. M., Horciag, G., Sitaru, A. M., Rachisan, A. L., Oniscu, G., & Antal, O. (2025). Validation of Kidney Donor Profile Index and Estimated Post-Transplant Survival Scores in an Eastern European Transplantation Center—A Seven-Year Retrospective Observational Study. Journal of Clinical Medicine, 14(10), 3540. https://doi.org/10.3390/jcm14103540






