Impact of Delayed Admission on Treatment Modality and Outcomes of Aneurysmal Subarachnoid Hemorrhage: A Prefecture-Wide, Multicenter Japanese Study
Abstract
1. Introduction
2. Materials and Methods
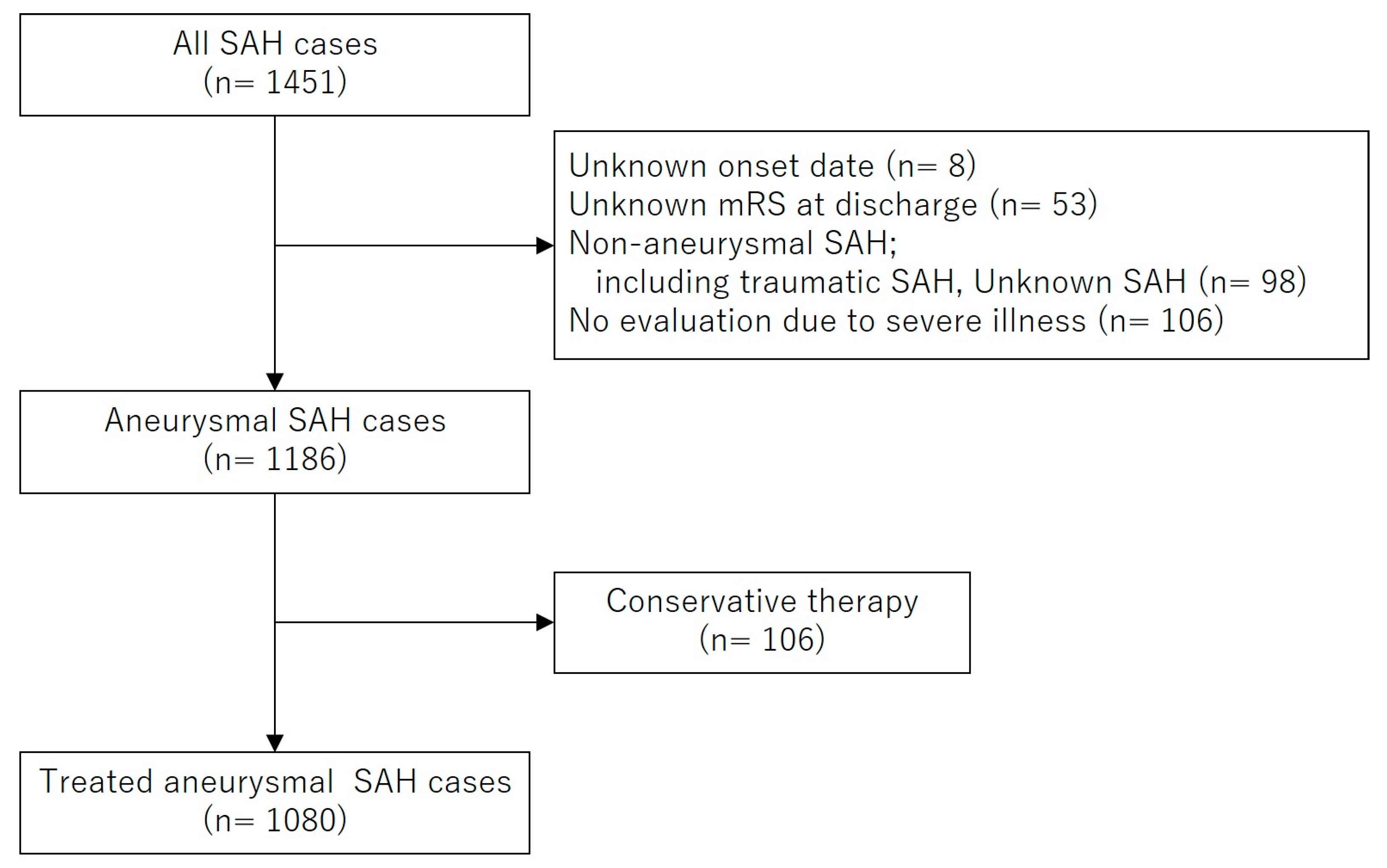
2.1. Patient Selection, Clinical Evaluation, and Study Design
2.2. Date Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAH | Subarachnoid Hemorrhage |
| mRS | Modified Rankin Scale |
| WFNS | World Federation of Neurosurgical Societies |
| ICA | Internal Cerebral Artery |
| ACA | Anterior Cerebral Artery |
| MCA | Middle Cerebral Artery |
| PCQ | Posterior Circulation |
| KATSUO | Kochi Acute Stroke Survey of Onset |
| STROBE | Strengthening the Reporting of Observational Study in Epidemiology |
References
- Huhtakangas, J.; Lehto, H.; Seppä, K.; Kivisaari, R.; Niemelä, M.; Hernesniemi, J.; Lehecka, M. Long-term excess mortality after aneurysmal subarachnoid hemorrhage: Patients with multiple aneurysms at risk. Stroke 2015, 46, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.V. Acute endovascular treatment by coil embolisation of ruptured intracranial aneurysms. Ann. R. Coll. Surg. Engl. 2001, 83, 253–256. [Google Scholar] [PubMed]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society Guideline 2021 for the Treatment of Stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Neil-Dwyer, G.; Lang, D. ‘Brain attack’-aneurysmal subarachnoid haemorrhage: Death due to delayed diagnosis. J. R. Coll. Physicians Lond. 1997, 31, 49–52. [Google Scholar] [CrossRef]
- Inagawa, T. Delayed diagnosis of aneurysmal subarachnoid hemorrhage in patients: A community-based study. J. Neurosurg. 2011, 115, 707–714. [Google Scholar] [CrossRef]
- Kowalski, R.G.; Claassen, J.; Kreiter, K.T.; Bates, J.E.; Ostapkovich, N.D.; Connolly, E.S.; Mayer, S.A. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004, 291, 866–869. [Google Scholar] [CrossRef]
- Goertz, L.; Pflaeging, M.; Hamisch, C.; Kabbasch, C.; Pennig, L.; von Spreckelsen, N.; Laukamp, K.; Timmer, M.; Goldbrunner, R.; Brinker, G.; et al. Delayed hospital admission of patients with aneurysmal subarachnoid hemorrhage: Clinical presentation, treatment strategies, and outcome. J. Neurosurg. 2021, 134, 1182–1189. [Google Scholar] [CrossRef]
- Fukuda, H.; Hyohdoh, Y.; Ninomiya, H.; Ueba, Y.; Ohta, T.; Kawanishi, Y.; Kadota, T.; Hamada, F.; Fukui, N.; Nonaka, M.; et al. Impact of areal socioeconomic status on prehospital delay of acute ischaemic stroke: Retrospective cohort study from a prefecture-wide survey in Japan. BMJ Open 2023, 13, e075612. [Google Scholar] [CrossRef]
- Fukuda, H.; Ninomiya, H.; Ueba, Y.; Ohta, T.; Kaneko, T.; Kadota, T.; Hamada, F.; Fukui, N.; Nonaka, M.; Watari, Y.; et al. Impact of temperature decline from the previous day as a trigger of spontaneous subarachnoid hemorrhage: Case-crossover study of prefectural stroke database. J. Neurosurg. 2020, 133, 374–382. [Google Scholar] [CrossRef]
- The Department of Health Policy in Kochi Prefectural Office. Available online: https://www.pref.kochi.lg.jp/soshiki/130401/ (accessed on 20 September 2019).
- Lawton, M.T.; Vates, G.E. Subarachnoid Hemorrhage. N. Engl. J. Med. 2017, 377, 257–266. [Google Scholar] [CrossRef]
- Haley, E.C., Jr.; Kassell, N.F.; Torner, J.C. The international cooperative study on the timing of aneurysm surgery. The North American experience. Stroke 1992, 23, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Rosengart, A.; Huo, D.; Karrison, T. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2003, 99, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Hayashi, K.; Yoshino, K.; Koyama, T.; Lo, B.; Kurosaki, Y.; Yamagata, S. Impact of aneurysm projection on intraoperative complications during surgical clipping of ruptured posterior communicating artery aneurysms. Neurosurgery 2016, 78, 381–390. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, J.; Rinkel, G.J. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain 2001, 124, 249–278. [Google Scholar] [CrossRef]
- Mayer, P.L.; Awad, I.A.; Todor, R.; Harbaugh, K.; Varnavas, G.; Lansen, T.A.; Dickey, P.; Harbaugh, R.; Hopkins, L.N. Misdiagnosis of symptomatic cerebral aneurysm. Prevalence and correlation with outcome at four institutions. Stroke 1996, 27, 1558–1563. [Google Scholar] [CrossRef]
- Brilstra, E.H.; Rinkel, G.J.; Algra, A.; van Gijn, J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000, 55, 1656–1660. [Google Scholar] [CrossRef]
- Ohkuma, H.; Tsurutani, H.; Suzuki, S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke 2001, 32, 1176–1180. [Google Scholar] [CrossRef]
- Kassell, N.F.; Torner, J.C. Aneurysmal rebleeding: A preliminary report from the Cooperative Aneurysm Study. Neurosurgery 1983, 13, 479–481. [Google Scholar] [CrossRef]
- Suzuki, R.; Masaoka, H.; Hirata, Y.; Marumo, F.; Isotani, E.; Hirakawa, K. The role of endothelin-1 in the origin of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 1992, 77, 96–100. [Google Scholar] [CrossRef]
- Mayberg, M.R.; Okada, T.; Bark, D.H. The role of hemoglobin in arterial narrowing after subarachnoid hemorrhage. J. Neurosurg. 1990, 72, 634–640. [Google Scholar] [CrossRef]
- Wolf, S.; Mielke, D.; Barner, C.; Malinova, V.; Kerz, T.; Wostrack, M.; Czorlich, P.; Salih, F.; Engel, D.C.; Ehlert, A.; et al. Effectiveness of lumbar cerebrospinal fluid drain among patients with aneurysmal subarachnoid hemorrhage: A randomized clinical trial. JAMA Neurol. 2023, 80, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Matsukawa, H.; Kamiyama, H.; Tsuboi, T.; Noda, K.; Hashimoto, A.; Miyazaki, T.; Kinoshita, Y.; Saito, N.; Tokuda, S.; et al. Preventing cerebral vasospasm after aneurysmal subarachnoid hemorrhage with aggressive cisternal clot removal and nicardipine. World Neurosurg. 2017, 107, 630–640. [Google Scholar] [CrossRef]
- Reilly, C.; Amidei, C.; Tolentino, J.; Jahromi, B.S.; Macdonald, R.L. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2004, 101, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Moore, J.M.; Griessenauer, C.J.; Xu, J.; Teng, I.; Dmytriw, A.A.; Chiu, A.H.; Ogilvy, C.S.; Thomas, A. Ultra-early angiographic vasospasm after aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. World Neurosurg. 2017, 102, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Shibuya, M.; Sato, S.; Sugiyama, H.; Seto, M.; Takakura, K. Safety and efficacy of fasudil monotherapy and fasudil-ozagrel combination therapy in patients with subarachnoid hemorrhage: Sub-analysis of the post-marketing surveillance study. Neurol. Med. Chir. 2008, 48, 241–247. [Google Scholar] [CrossRef][Green Version]
- Mahaney, K.K.; Todd, M.M.; Torner, J.C.; IHAST Investigators. Variation of patient characteristics, management, and outcome with timing of surgery for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2011, 114, 1045–1053. [Google Scholar] [CrossRef]
| Variable | Value | Missing Value |
|---|---|---|
| Mean age (years) | 67 [55–78] | |
| Woman | 778 (72.0) | |
| Hypertension | 620 (57.4) | 41 (3.8) |
| Smoking | 369 (34.2) | 65 (6.0) |
| Premorbid mRS 0 or 1 | 983 (91.0) | 2 (0.2) |
| Delayed admission | 69 (6.4) | |
| Location of aneurysm | ||
| ICA | 340 (31.5) | |
| ACA | 363 (33.6) | |
| MCA | 219 (20.3) | |
| PCQ | 158 (14.6) | |
| Aneurysm size in mm | 5.4 [3.9–7.5] | 53 (4.9) |
| WFNS grade | 5 (0.5) | |
| I | 362 (33.5) | |
| II | 268 (24.8) | |
| III | 38 (3.5) | |
| IV | 189 (17.5) | |
| V | 218 (20.2) | |
| Fisher group | 3 (0.3) | |
| 1 | 14 (10.6) | |
| 2 | 126 (11.7) | |
| 3 | 878 (81.5) | |
| 4 | 59 (5.6) | |
| Endovascular therapy | 533 (49.4) | |
| Procedure-related complications | 230 (21.3) | 8 (0.7) |
| Ischemic | 204 (19.0) | |
| Hemorrhagic | 40 (3.2) | |
| Symptomatic vasospasm | 134 (12.5) | 5 (0.5) |
| Chronic hydrocephalus | 239 (22.1) | 4 (0.4) |
| Poor functional outcomes (mRS 3-6) | 593 (54.9) |
| Variable | Early Admission | Delayed Admission | p Value |
|---|---|---|---|
| Age | 66 [55–78] | 71 [58–78] | 0.19 |
| Woman | 731 (72.3) | 47 (68.1) | 0.45 |
| Hypertension | 583 (57.7) | 37 (53.6) | 0.54 |
| Smoking | 348 (34.4) | 21 (30.4) | 0.60 |
| Premorbid mRS 0 or 1 | 920 (90.1) | 63 (94.0) | 0.40 |
| Location of aneurysm | 0.041 | ||
| ICA | 310 (30.7) | 30 (43.5) | |
| ACA | 338 (33.4) | 25 (36.2) | |
| MCA | 212 (21.0) | 7 (10.1) | |
| PCQ | 151 (14.9) | 7 (10.1) | |
| Aneurysm size | 5.4 [4.0–7.5] | 5.8 [3.8–7.0] | 0.80 |
| WFNS grade | <0.001 | ||
| I | 311 (30.8) | 51 (73.9) | |
| II | 257 (25.4) | 11 (15.9) | |
| III | 36 (3.6) | 2 (2.9) | |
| IV | 187 (18.5) | 2 (2.9) | |
| V | 217 (21.5) | 1 (1.4) | |
| Fisher group | <0.001 | ||
| 1 | 6 (0.6) | 8 (11.6) | |
| 2 | 98 (9.7) | 28 (40.6) | |
| 3 | 847 (83.8) | 31 (44.9) | |
| 4 | 57 (5.6) | 2 (2.9) |
| Variable | Early Admission n = 1011 | Delayed Admission n = 69 | p Value |
|---|---|---|---|
| Endovascular therapy | 496 (49.1) | 37 (53.6) | 0.46 |
| Procedure-related complications | 217 (21.6) | 13 (18.8) | 0.58 |
| Symptomatic vasospasm | 120 (11.9) | 14 (20.3) | 0.042 |
| Poor functional outcomes (mRS 3-6) | 489 (53.2) | 18 (28.6) | <0.001 |
| Variable | Symptomatic Vasospasm n = 134 | No symptomatic Vasospasm n = 941 | Univariate Analysis OR (95% CI) | p Value | Multivariable Analysis OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Age | 66 [55–77] | 68 [58–78] | 1.01 (0.99–1.03) | 0.21 | 1.01 (0.99–1.03) | 0.29 |
| Woman | 92 (68.7) | 681 (72.4) | 0.86 (0.57–1.24) | 0.37 | 0.68 (0.42–1.11) | 0.13 |
| Hypertension | 79 (59.0) | 540 (57.4) | 1.07 (0.73–1.56) | 0.73 | 1.01 (0.67–1.51) | 0.97 |
| Smoking | 43 (32.1) | 325 (34.5) | 0.89 (0.60–1.32) | 0.57 | 0.79 (0.47–1.33) | 0.37 |
| Location of aneurysm | ||||||
| ICA | 41 (30.6) | 296 (31.5) | Ref | Ref | ||
| ACA | 50 (37.3) | 312 (33.2) | 1.16 (0.74–1.80) | 0.52 | 1.09 (0.68–1.76) | 0.71 |
| MCA | 32 (23.9) | 186 (19.8) | 1.24 (0.76–20.4) | 0.39 | 0.85 (0.50–1.45) | 0.56 |
| PCQ | 11 (8.2) | 147 (15.6) | 0.54 (0.27–1.08) | 0.082 | 0.82 (0.39–1.71) | 0.59 |
| Aneurysm size | 5.4 [3.9–7.5] | 5.6 [4.2–7.5] | 1.00 (0.95–1.06) | 0.91 | 0.99 (0.94–1.05) | 0.89 |
| WFNS grades IV–V | 58 (43.3) | 345 (36.8) | 1.30 (0.90–1.88) | 0.16 | 1.57 (1.05–2.33) | 0.027 |
| Fisher group 3 | 110 (82.1) | 764 (81.2) | 1.04 (0.65–1.67) | 0.86 | 1.38 (0.82–2.30) | 0.23 |
| Delayed admission | 14 (10.4) | 55 (5.8) | 1.88 (1.01–3.48) | 0.045 | 2.51 (1.26–5.00) | 0.009 |
| Endovascular therapy | 38 (28.4) | 493 (52.4) | 0.36 (0.24–0.54) | <0.001 | 0.32 (0.21–0.50) | <0.001 |
| Favorable Outcomes n = 476 | Poor Outcomes n = 507 | Univariate Analysis OR (95% CI) | p Value | Multivariable Analysis OR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Age | 60 [49–68.75] | 70.0 [59–79] | 1.05 (1.04–1.06) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| Female sex | 316 (66.4) | 376 (74.2) | 1.45 (1.10–1.91) | 0.008 | 0.97 (0.66–1.45) | 0.90 |
| Hypertension | 242 (50.8) | 313 (61.7) | 1.66 (1.28–2.16) | <0.001 | 1.28 (0.92–1.77) | 0.14 |
| Smoking | 211 (44.3) | 148 (29.2) | 0.51 (0.39–0.67) | <0.001 | 0.67 (0.45–0.98) | 0.04 |
| Location of aneurysm | ||||||
| ICA | 153 (32.1) | 145 (28.6) | Ref | Ref | ||
| ACA | 165 (34.7) | 175 (34.5) | 1.12 (0.82–1.53) | 0.48 | 1.42 (0.96–2.11) | 0.08 |
| MCA | 93 (19.5) | 108 (21.3) | 1.23 (0.86–1.75) | 0.27 | 1.10 (0.69–1.76) | 0.69 |
| PCQ | 65 (13.7) | 79 (15.6) | 1.28 (0.86–1.91) | 0.22 | 1.33 (0.80–2.20) | 0.28 |
| Aneurysm size | 5.0 [3.7–6.8] | 5.8 [4.1–8.2] | 1.09 (1.05–1.14) | <0.001 | 1.08 (1.03–1.13) | 0.002 |
| WFNS grade 4 or 5 | 71 (14.9) | 282 (55.6) | 7.15 (5.26–9.72) | <0.001 | 8.43 (5.88–12.1) | <0.001 |
| Fisher group 3 | 368 (77.3) | 439 (86.6) | 1.98 (1.41–2.78) | <0.001 | 1.58 (1.03–2.43) | 0.038 |
| Delayed admission | 45 (9.5) | 18 (3.6) | 0.35 (0.20–0.62) | <0.001 | 0.53 (0.28–1.02) | 0.059 |
| Endovascular therapy | 215 (45.2) | 259 (51.1) | 1.27 (0.99–1.63) | 0.06 | 1.01 (0.71–1.42) | 0.96 |
| Endovascular Therapy n = 37 | Direct Surgery n = 32 | p Value | |
|---|---|---|---|
| Interval from admission to treatment | 0 [0–1] | 1 [1–8] | 0.007 |
| Treatment within 4 days after admission | 34 (91.9) | 22 (68.8) | 0.028 |
| Procedure-related complications | 7 (18.9) | 6 (18.8) | 0.99 |
| Poor functional outcomes | 14 (37.8) | 8 (25.0) | 0.38 |
| Angiographical vasospasm on admission | 8 (21.6) | 6 (18.8) | 0.77 |
| Location of aneurysm | |||
| ICA | 17 (45.9) | 12 (37.5) | |
| ACA | 11 (29.7) | 14 (43.8) | |
| MCA | 2 (5.4) | 6 (18.8) | |
| PCQ | 7 (18.9) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosokawa, Y.; Fukuda, H.; Hyohdoh, Y.; Kawamura, T.; Shinno, K.; Yanase, Y.; Yokodani, M.; Hoashi, Y.; Moriki, A.; Bando, K.; et al. Impact of Delayed Admission on Treatment Modality and Outcomes of Aneurysmal Subarachnoid Hemorrhage: A Prefecture-Wide, Multicenter Japanese Study. J. Clin. Med. 2025, 14, 3537. https://doi.org/10.3390/jcm14103537
Hosokawa Y, Fukuda H, Hyohdoh Y, Kawamura T, Shinno K, Yanase Y, Yokodani M, Hoashi Y, Moriki A, Bando K, et al. Impact of Delayed Admission on Treatment Modality and Outcomes of Aneurysmal Subarachnoid Hemorrhage: A Prefecture-Wide, Multicenter Japanese Study. Journal of Clinical Medicine. 2025; 14(10):3537. https://doi.org/10.3390/jcm14103537
Chicago/Turabian StyleHosokawa, Yuma, Hitoshi Fukuda, Yuki Hyohdoh, Takako Kawamura, Ken Shinno, Yongran Yanase, Masaki Yokodani, Yu Hoashi, Akihito Moriki, Koji Bando, and et al. 2025. "Impact of Delayed Admission on Treatment Modality and Outcomes of Aneurysmal Subarachnoid Hemorrhage: A Prefecture-Wide, Multicenter Japanese Study" Journal of Clinical Medicine 14, no. 10: 3537. https://doi.org/10.3390/jcm14103537
APA StyleHosokawa, Y., Fukuda, H., Hyohdoh, Y., Kawamura, T., Shinno, K., Yanase, Y., Yokodani, M., Hoashi, Y., Moriki, A., Bando, K., Matsushita, N., Hamada, F., Kawanishi, Y., Ueba, Y., Fukui, N., Masahira, N., Nishimoto, Y., & Ueba, T. (2025). Impact of Delayed Admission on Treatment Modality and Outcomes of Aneurysmal Subarachnoid Hemorrhage: A Prefecture-Wide, Multicenter Japanese Study. Journal of Clinical Medicine, 14(10), 3537. https://doi.org/10.3390/jcm14103537





