Dulaglutide 1.5 mg Significantly Improves Glycemic Control and Lowers LDL-Cholesterol and Body Weight in Romanian Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. Study Protocol and Assessments
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
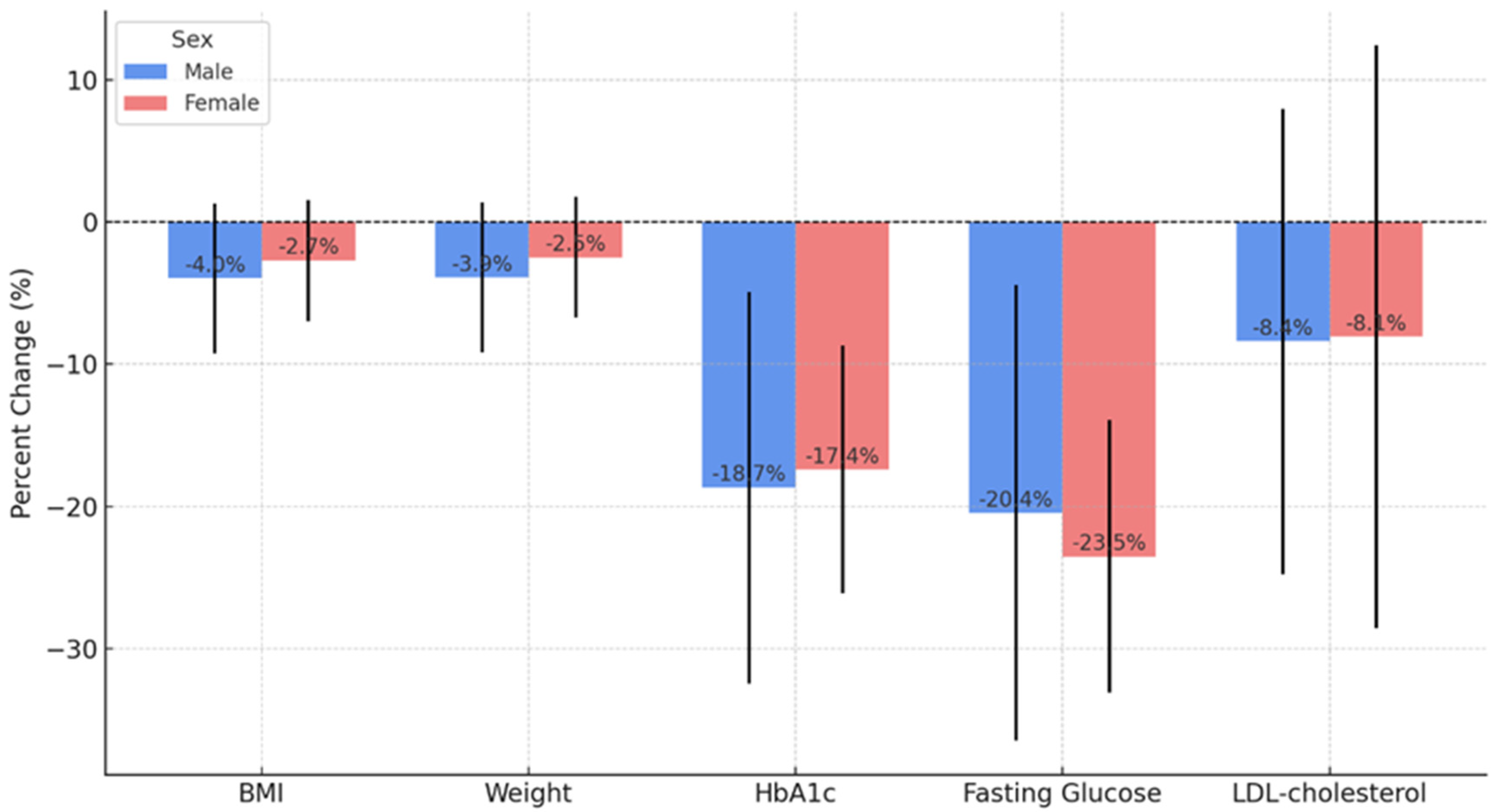
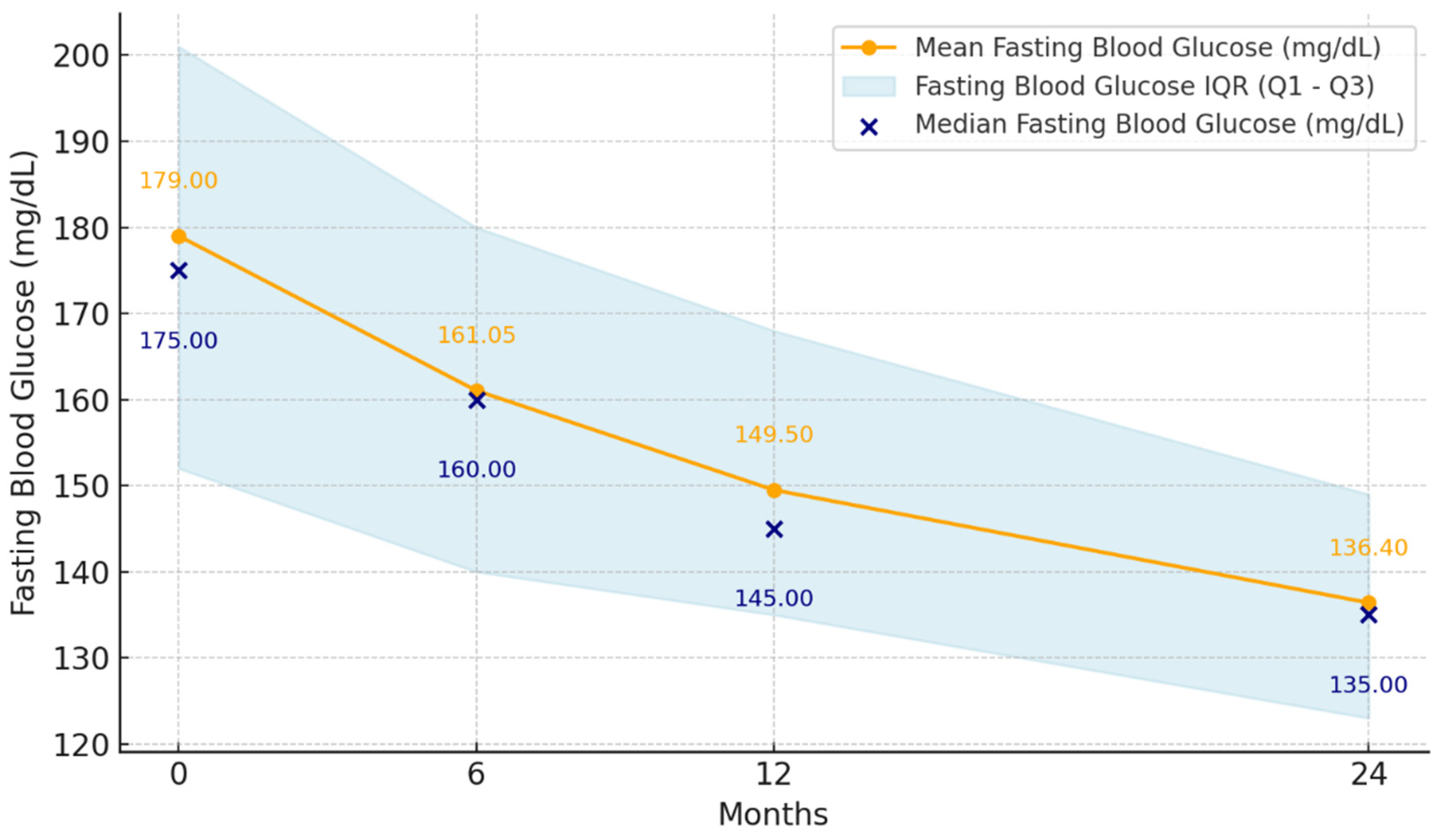
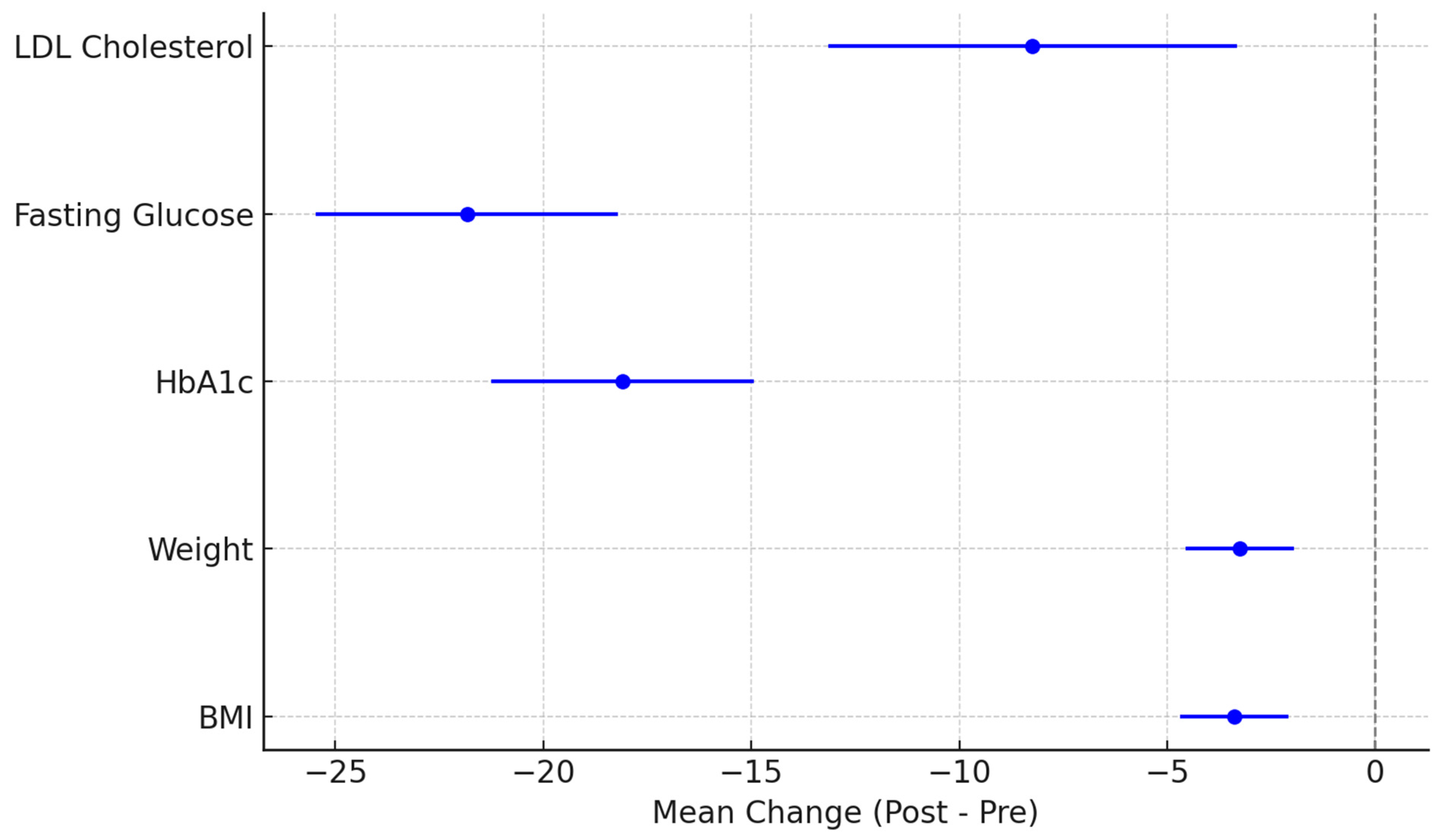
3.2. Glycemic and LDL-Cholesterol Control
3.3. Weight Control
4. Discussion
4.1. The Effect of Dulaglutide on Glycemic Control
4.2. The Impact of Dulaglutide on Lowering LDL-Cholesterol
4.3. The Effects of Dulaglutide on Weight Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas: Sixth Edition 2014 Update. 2014. Available online: https://diabetesatlas.org/ (accessed on 17 April 2025).
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; Davidson, M.B.; Einhorn, D.; Garber, J.R.; et al. AACE/ACE Comprehensive Diabetes Management Algorithm 2015. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2015, 21, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Burness, C.B.; Scott, L.J. Dulaglutide: A Review in Type 2 Diabetes. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2015, 29, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The Incretin System: Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Brown, D.X.; Evans, M. Choosing between GLP-1 Receptor Agonists and DPP-4 Inhibitors: A Pharmacological Perspective. J. Nutr. Metab. 2012, 2012, 381713. [Google Scholar] [CrossRef]
- Trulicity | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/trulicity (accessed on 17 April 2025).
- Umpierrez, G.E.; Blevins, T.; Rosenstock, J.; Cheng, C.; Anderson, J.H.; Bastyr, E.J.; EGO Study Group. The Effects of LY2189265, a Long-Acting Glucagon-like Peptide-1 Analogue, in a Randomized, Placebo-Controlled, Double-Blind Study of Overweight/Obese Patients with Type 2 Diabetes: The EGO Study. Diabetes Obes. Metab. 2011, 13, 418–425. [Google Scholar] [CrossRef]
- Barrington, P.; Chien, J.Y.; Showalter, H.D.H.; Schneck, K.; Cui, S.; Tibaldi, F.; Ellis, B.; Hardy, T.A. A 5-Week Study of the Pharmacokinetics and Pharmacodynamics of LY2189265, a Novel, Long-Acting Glucagon-like Peptide-1 Analogue, in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2011, 13, 426–433. [Google Scholar] [CrossRef]
- Grunberger, G.; Chang, A.; Garcia Soria, G.; Botros, F.T.; Bsharat, R.; Milicevic, Z. Monotherapy with the Once-Weekly GLP-1 Analogue Dulaglutide for 12 Weeks in Patients with Type 2 Diabetes: Dose-Dependent Effects on Glycaemic Control in a Randomized, Double-Blind, Placebo-Controlled Study. Diabet. Med. J. Br. Diabet. Assoc. 2012, 29, 1260–1267. [Google Scholar] [CrossRef]
- Terauchi, Y.; Satoi, Y.; Takeuchi, M.; Imaoka, T. Monotherapy with the Once Weekly GLP-1 Receptor Agonist Dulaglutide for 12 Weeks in Japanese Patients with Type 2 Diabetes: Dose-Dependent Effects on Glycaemic Control in a Randomised, Double-Blind, Placebo-Controlled Study. Endocr. J. 2014, 61, 949–959. [Google Scholar] [CrossRef]
- Skrivanek, Z.; Gaydos, B.L.; Chien, J.Y.; Geiger, M.J.; Heathman, M.A.; Berry, S.; Anderson, J.H.; Forst, T.; Milicevic, Z.; Berry, D. Dose-Finding Results in an Adaptive, Seamless, Randomized Trial of Once-Weekly Dulaglutide Combined with Metformin in Type 2 Diabetes Patients (AWARD-5). Diabetes Obes. Metab. 2014, 16, 748–756. [Google Scholar] [CrossRef]
- Umpierrez, G.; Tofé Povedano, S.; Pérez Manghi, F.; Shurzinske, L.; Pechtner, V. Efficacy and Safety of Dulaglutide Monotherapy versus Metformin in Type 2 Diabetes in a Randomized Controlled Trial (AWARD-3). Diabetes Care 2014, 37, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Weinstock, R.S.; Umpierrez, G.E.; Guerci, B.; Skrivanek, Z.; Milicevic, Z. Efficacy and Safety of Dulaglutide versus Sitagliptin after 52 Weeks in Type 2 Diabetes in a Randomized Controlled Trial (AWARD-5). Diabetes Care 2014, 37, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Povedano, S.T.; Forst, T.; González, J.G.G.; Atisso, C.; Sealls, W.; Fahrbach, J.L. Once-Weekly Dulaglutide versus Once-Daily Liraglutide in Metformin-Treated Patients with Type 2 Diabetes (AWARD-6): A Randomised, Open-Label, Phase 3, Non-Inferiority Trial. Lancet 2014, 384, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Wysham, C.; Blevins, T.; Arakaki, R.; Colon, G.; Garcia, P.; Atisso, C.; Kuhstoss, D.; Lakshmanan, M. Efficacy and Safety of Dulaglutide Added onto Pioglitazone and Metformin versus Exenatide in Type 2 Diabetes in a Randomized Controlled Trial (AWARD-1). Diabetes Care 2014, 37, 2159–2167. [Google Scholar] [CrossRef]
- Giorgino, F.; Benroubi, M.; Sun, J.-H.; Zimmermann, A.G.; Pechtner, V. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2). Diabetes Care 2015, 38, 2241–2249. [Google Scholar] [CrossRef]
- Blonde, L.; Jendle, J.; Gross, J.; Woo, V.; Jiang, H.; Fahrbach, J.L.; Milicevic, Z. Once-Weekly Dulaglutide versus Bedtime Insulin Glargine, Both in Combination with Prandial Insulin Lispro, in Patients with Type 2 Diabetes (AWARD-4): A Randomised, Open-Label, Phase 3, Non-Inferiority Study. Lancet 2015, 385, 2057–2066. [Google Scholar] [CrossRef]
- Araki, E.; Inagaki, N.; Tanizawa, Y.; Oura, T.; Takeuchi, M.; Imaoka, T. Efficacy and Safety of Once-weekly Dulaglutide in Combination with Sulphonylurea and/or Biguanide Compared with Once-daily Insulin Glargine in Japanese Patients with Type 2 Diabetes: A Randomized, Open-label, Phase III, Non-inferiority Study. Diabetes Obes. Metab. 2015, 17, 994–1002. [Google Scholar] [CrossRef]
- Miyagawa, J.; Odawara, M.; Takamura, T.; Iwamoto, N.; Takita, Y.; Imaoka, T. Once-Weekly Glucagon-like Peptide-1 Receptor Agonist Dulaglutide Is Non-Inferior to Once-Daily Liraglutide and Superior to Placebo in Japanese Patients with Type 2 Diabetes: A 26-Week Randomized Phase III Study. Diabetes Obes. Metab. 2015, 17, 974–983. [Google Scholar] [CrossRef]
- Weinstock, R.S.; Guerci, B.; Umpierrez, G.; Nauck, M.A.; Skrivanek, Z.; Milicevic, Z. Safety and Efficacy of Once-Weekly Dulaglutide versus Sitagliptin after 2 Years in Metformin-Treated Patients with Type 2 Diabetes (AWARD-5): A Randomized, Phase III Study. Diabetes Obes. Metab. 2015, 17, 849–858. [Google Scholar] [CrossRef]
- Edwards, K.L.; Minze, M.G. Dulaglutide: An Evidence-Based Review of Its Potential in the Treatment of Type 2 Diabetes. Core Evid. 2015, 10, 11–21. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Hart, R.; Colhoun, H.M.; Diaz, R.; Lakshmanan, M.; Botros, F.T.; Probstfield, J.; Riddle, M.C.; Rydén, L.; Atisso, C.M.; et al. The Effect of Dulaglutide on Stroke: An Exploratory Analysis of the REWIND Trial. Lancet Diabetes Endocrinol. 2020, 8, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Branch, K.R.H.; Dagenais, G.R.; Avezum, A.; Basile, J.; Conget, I.; Cushman, W.C.; Jansky, P.; Lakshmanan, M.; Lanas, F.; Leiter, L.A.; et al. Dulaglutide and Cardiovascular and Heart Failure Outcomes in Patients with and without Heart Failure: A Post-Hoc Analysis from the REWIND Randomized Trial. Eur. J. Heart Fail. 2022, 24, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Cirrincione, A.; Casuccio, A.; Del Cuore, A.; Daidone, M.; Di Chiara, T.; Di Raimondo, D.; Corte, V.D.; Maida, C.; Simonetta, I.; et al. Efficacy of Dulaglutide on Vascular Health Indexes in Subjects with Type 2 Diabetes: A Randomized Trial. Cardiovasc. Diabetol. 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, Y.; Xu, M.; Zhao, X.; Chen, M. Effects of Dulaglutide on Endothelial Progenitor Cells and Arterial Elasticity in Patients with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2022, 21, 200. [Google Scholar] [CrossRef]
- Au, H.C.T.; Zheng, Y.J.; Le, G.H.; Wong, S.; Phan, L.; Teopiz, K.M.; Kwan, A.T.H.; Rhee, T.G.; Rosenblat, J.D.; Ho, R.; et al. A Systematic Review in Effects of Glucagon-like Peptide-1 (GLP-1) Mono-Agonists on Functional Connectivity: Target Engagement and Rationale for the Development in Mental Disorders. J. Affect. Disord. 2025, 370, 321–327. [Google Scholar] [CrossRef]
- Menon, T.; Lee, S.; Gong, X.Y.; Wong, S.; Le, G.H.; Kwan, A.T.H.; Teopiz, K.M.; Ho, R.; Cao, B.; Rhee, T.G.; et al. A Systematic Review on the Efficacy of GLP-1 Receptor Agonists in Mitigating Psychotropic Drug-Related Weight Gain. CNS Spectr. 2024, 29, 347–353. [Google Scholar] [CrossRef]
- Trott, M.; Arnautovska, U.; Siskind, D. GLP-1 Receptor Agonists and Weight Loss in Schizophrenia—Past, Present, and Future. Curr. Opin. Psychiatry 2024, 37, 363–369. [Google Scholar] [CrossRef]
- Bak, M.; Campforts, B.; Domen, P.; van Amelsvoort, T.; Drukker, M. Glucagon-like Peptide Agonists for Weight Management in Antipsychotic-Induced Weight Gain: A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2024, 150, 516–529. [Google Scholar] [CrossRef]
- Aoun, L.; Almardini, S.; Saliba, F.; Haddadin, F.; Mourad, O.; Jdaidani, J.; Morcos, Z.; Al Saidi, I.; Bou Sanayeh, E.; Saliba, S.; et al. GLP-1 Receptor Agonists: A Novel Pharmacotherapy for Binge Eating (Binge Eating Disorder and Bulimia Nervosa)? A Systematic Review. J. Clin. Transl. Endocrinol. 2024, 35, 100333. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Kwan, A.T.H.; Rosenblat, J.D.; Teopiz, K.M.; Mansur, R.B. Psychotropic Drug-Related Weight Gain and Its Treatment. Am. J. Psychiatry 2024, 181, 26–38. [Google Scholar] [CrossRef]
- Ruda, A.I.; Ciobanu, D.M.; Inceu, G.; Rusu, A.; Roman, G. The Effect of Dulaglutide on Glycemic and Weight Control in Patients with Type 2 Diabetes. Med. Pharm. Rep. 2023, 96, 52–57. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Body Mass Index (BMI). Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 23 April 2025).
- Frias, J.P.; Bonora, E.; Nevarez Ruiz, L.; Li, Y.G.; Yu, Z.; Milicevic, Z.; Malik, R.; Bethel, M.A.; Cox, D.A. Efficacy and Safety of Dulaglutide 3.0 Mg and 4.5 Mg Versus Dulaglutide 1.5 Mg in Metformin-Treated Patients with Type 2 Diabetes in a Randomized Controlled Trial (AWARD-11). Diabetes Care 2021, 44, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B.; Dagogo-Jack, S.; Thieu, V.; Garcia-Perez, L.-E.; Pavo, I.; Yu, M.; Robertson, K.E.; Zhang, N.; Giorgino, F. Effect of Once-Weekly Dulaglutide on Glycated Haemoglobin (HbA1c) and Fasting Blood Glucose in Patient Subpopulations by Gender, Duration of Diabetes and Baseline HbA1c. Diabetes Obes. Metab. 2018, 20, 409–418. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Robinson, S.; Boye, K.S.; Mody, R.; Strizek, A.A.; Konig, M.; Malik, R.E.; Kennedy-Martin, T. Real-World Effectiveness of Dulaglutide in Patients with Type 2 Diabetes Mellitus: A Literature Review. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2020, 11, 1437–1466. [Google Scholar] [CrossRef]
- Yoon, J.H.; Hong, A.R.; Choi, W.; Park, J.Y.; Kim, H.K.; Kang, H.-C. Real-World Efficacy and Safety of Dulaglutide in Korean Patients with Type 2 Diabetes Mellitus: A Retrospective Study in a Tertiary Referral Center. Chonnam Med. J. 2021, 57, 211–218. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.K.; Kim, H.S.; Jung, C.H.; Park, J.-Y.; Lee, W.J. Dulaglutide as an Add-on to Insulin in Type 2 Diabetes; Clinical Efficacy and Parameters Affecting the Response in Real-World Practice. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2745–2753. [Google Scholar] [CrossRef]
- Dungan, K.M.; Weitgasser, R.; Perez Manghi, F.; Pintilei, E.; Fahrbach, J.L.; Jiang, H.H.; Shell, J.; Robertson, K.E. A 24-Week Study to Evaluate the Efficacy and Safety of Once-Weekly Dulaglutide Added on to Glimepiride in Type 2 Diabetes (AWARD-8). Diabetes Obes. Metab. 2016, 18, 475–482. [Google Scholar] [CrossRef]
- Toeller, M.; Buyken, A.E.; Heitkamp, G.; Berg, G.; Scherbaum, W.A. Prevalence of Chronic Complications, Metabolic Control and Nutritional Intake in Type 1 Diabetes: Comparison between Different European Regions. EURODIAB Complications Study Group. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 1999, 31, 680–685. [Google Scholar] [CrossRef]
- Cokolic, M.; Lalic, N.M.; Micic, D.; Mirosevic, G.; Klobucar Majanovic, S.; Lefterov, I.N.; Graur, M. Patterns of Diabetes Care in Slovenia, Croatia, Serbia, Bulgaria and Romania: An Observational, Non-Interventional, Cross-Sectional Study. Wien. Klin. Wochenschr. 2017, 129, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Salmen, T.; Pietrosel, V.-A.; Reurean-Pintilei, D.; Iancu, M.A.; Cimpeanu, R.C.; Bica, I.-C.; Dumitriu-Stan, R.-I.; Potcovaru, C.-G.; Salmen, B.-M.; Diaconu, C.-C.; et al. Assessing Cardiovascular Target Attainment in Type 2 Diabetes Mellitus Patients in Tertiary Diabetes Center in Romania. Pharmaceuticals 2024, 17, 1249. [Google Scholar] [CrossRef] [PubMed]
- Reurean-Pintilei, D.; Potcovaru, C.-G.; Salmen, T.; Mititelu-Tartau, L.; Cinteză, D.; Lazăr, S.; Pantea Stoian, A.; Timar, R.; Timar, B. Assessment of Cardiovascular Risk Categories and Achievement of Therapeutic Targets in European Patients with Type 2 Diabetes. J. Clin. Med. 2024, 13, 2196. [Google Scholar] [CrossRef] [PubMed]
- Zugravu, C.; Patrascu, D.; Pantea Stoian, A. Diabetics’ Adherence to Medical Treatment/Self-Monitoring Program—A Pilot Study. Int. J. Collab. Res. Intern. Med. Public Health 2014, 6, 276. [Google Scholar]
- Gherbon, A.; Frandes, M.; Dîrpeş, D.; Timar, R.; Timar, B. Impact of SGLT-2 Inhibitors on Modifiable Cardiovascular Risk Factors in Romanian Patients with Type 2 Diabetes Mellitus. Diabetol. Metab. Syndr. 2024, 16, 85. [Google Scholar] [CrossRef]
- Serafinceanu, C.; Elian, V.I.; Catrinoiu, D.; Guja, C.; Mihai, B.; Mota, M.; Roman, G.; Timar, R. Clinical and therapeutic characteristics of patients with type 2 diabetes mellitus in Romania—Mentor study. Romanian J. Diabetes Nutr. Metab. Dis. 2018, 25, 409–418. [Google Scholar] [CrossRef]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; Ceccarelli, E.; Tarquini, R.; Gennari, L.; Gonnelli, S.; Caffarelli, C. Adiponectin May Play a Crucial Role in the Metabolic Effects of GLP-1RAs Treatment in Patients with Type 2 Diabetes Mellitus: A Preliminary Longitudinal Study. Endocrine 2025, 87, 951–958. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Gardner, M.S.; Kusovschi, J.D.; Parks, B.A.; Schieltz, D.M.; Bareja, A.; McGarrah, R.W., III; Kraus, W.E.; Kuklenyik, Z.; Pirkle, J.L.; et al. Inaccurately Reported Statin Use Affects the Assessing of Lipid Profile Measures and Their Association with Coronary Artery Disease Risk. Clin. Chem. 2024, 70, 528–537. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nelson, A.J. Achieving More Optimal Lipid Control with Non-Statin Lipid Lowering Therapy. Curr. Atheroscler. Rep. 2025, 27, 32. [Google Scholar] [CrossRef]
- Giorgino, F.; Guerci, B.; Füchtenbusch, M.; Lebrec, J.; Boye, K.; Orsini Federici, M.; Heitmann, E.; Dib, A.; Yu, M.; Sapin, H.; et al. The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide and Liraglutide in Patients with Type 2 Diabetes (TROPHIES): Final, 24-Month Analysis of Time to First Significant Treatment Change, Treatment Persistence and Clinical Outcomes. Diabetes Obes. Metab. 2023, 25, 3465–3477. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cho, Y.K.; Kim, M.J.; Jung, C.H.; Park, J.-Y.; Lee, W.J. Durability of Glucose-Lowering Effect of Dulaglutide in Patients with Type 2 Diabetes Mellitus: A Real-World Data Study. Front. Endocrinol. 2022, 13, 1032793. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Cho, Y.K.; Lee, J.; Kim, H.S.; Kang, Y.M.; Jung, C.H.; Park, J.-Y.; Lee, W.J. Clinical Efficacy and Parameters Affecting the Response to Dulaglutide Treatment in Patients with Type 2 Diabetes: A Retrospective, Real-World Data Study. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2019, 10, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of Diabetes Mellitus and Prediabetes in the Adult Romanian Population: PREDATORR Study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Lin, C.; Cai, X.; Wang, J.; Wang, Y.; Lv, F.; Yang, W.; Ji, L. Characterizing Body Composition Modifying Effects of a Glucagon-like Peptide 1 Receptor-Based Agonist: A Meta-Analysis. Diabetes Obes. Metab. 2025, 27, 259–267. [Google Scholar] [CrossRef]
- Neeland, I.J.; Linge, J.; Birkenfeld, A.L. Changes in Lean Body Mass with Glucagon-like Peptide-1-Based Therapies and Mitigation Strategies. Diabetes Obes. Metab. 2024, 26 (Suppl. 4), 16–27. [Google Scholar] [CrossRef]
- Xiang, J.; Qin, L.; Zhong, J.; Xia, N.; Liang, Y. GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy via SIRT1 Pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 2433–2446. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, S.; Chen, X.; Niu, S.; Yue, L.; Pan, X.; Li, Z.; Chen, X. An Effective Glucagon-Like Peptide-1 Receptor Agonists, Semaglutide, Improves Sarcopenic Obesity in Obese Mice by Modulating Skeletal Muscle Metabolism. Drug Des. Devel. Ther. 2022, 16, 3723–3735. [Google Scholar] [CrossRef]
- Xiang, J.; Ding, X.-Y.; Zhang, W.; Zhang, J.; Zhang, Y.-S.; Li, Z.-M.; Xia, N.; Liang, Y.-Z. Clinical Effectiveness of Semaglutide on Weight Loss, Body Composition, and Muscle Strength in Chinese Adults. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9908–9915. [Google Scholar] [CrossRef]
- Al-Badri, M.; Almasih Barbar Askar, A.; Khater, A.; Salah, T.; Dhaver, S.E.; Al-Roomi, F.; Mottalib, A.; Hamdy, O. 14-PUB: The Effect of Structured Intensive Lifestyle Intervention on Muscle Mass in Patients with Type 2 Diabetes Receiving GLP-1 Receptor Agonists. Diabetes 2024, 73, 14-PUB. [Google Scholar] [CrossRef]
- Kakegawa, T.; Sugimoto, K.; Saito, K.; Yunaiyama, D.; Araki, Y.; Wada, T.; Takahashi, H.; Yoshimasu, Y.; Takeuchi, H.; Itoi, T. Favorable Liver and Skeletal Muscle Changes in Patients with MASLD and T2DM Receiving Glucagon-like Peptide-1 Receptor Agonist: A Prospective Cohort Study. Medicine 2024, 103, e38444. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y.; Masaki, T.; Kamata, A.; Miyamoto, S.; Yoshida, Y.; Okamoto, M.; Gotoh, K.; Shibata, H. The Effectiveness of GLP-1 Receptor Agonist Semaglutide on Body Composition in Elderly Obese Diabetic Patients: A Pilot Study. Medicines 2022, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, Z.; Nagy, A.; Jahner, K.; Szabados, E. Impact of Selected Glucagon-like Peptide-1 Receptor Agonists on Serum Lipids, Adipose Tissue, and Muscle Metabolism-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 8214. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Jin, Y.; Chen, X.; Song, Q.; Wei, G.; Li, L. Assessment of Changes in Body Composition After 3 Months of Dulaglutide Treatment. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 1301–1308. [Google Scholar] [CrossRef]
- Dilaver, R.G.; Afsar, R.E.; Crescenzi, R.; Gamboa, J.; Ikizler, T.A. Effects of Dulaglutide on Ectopic Fat Deposition in Chronic Kidney Disease (CKD): A Pilot and Feasibility Study (GLIMP). medRxiv 2025. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.-S. Amelioration of Muscle Wasting by Glucagon-like Peptide-1 Receptor Agonist in Muscle Atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of glucagon-like peptide-1 receptor agonists and co-agonists on body composition: Systematic review and network meta-analysis. Metabolism 2025, 164, 156113. [Google Scholar] [CrossRef]
- Ayoub, M.; Chela, H.; Amin, N.; Hunter, R.; Anwar, J.; Tahan, V.; Daglilar, E. Pancreatitis Risk Associated with GLP-1 Receptor Agonists, Considered as a Single Class, in a Comorbidity-Free Subgroup of Type 2 Diabetes Patients in the United States: A Propensity Score-Matched Analysis. J. Clin. Med. 2025, 14, 944. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Fisher, L.; Guzman, S.; Villa-Caballero, L.; Edelman, S.V. Psychological insulin resistance in patients with type 2 diabetes: The scope of the problem. Diabetes Care 2005, 28, 2543–2545. [Google Scholar] [CrossRef]
- Nunes, A.P.; Iglay, K.; Radican, L.; Engel, S.S.; Yang, J.; Doherty, M.C.; Dore, D.D. Hypoglycaemia Seriousness and Weight Gain as Determinants of Cardiovascular Disease Outcomes among Sulfonylurea Users. Diabetes Obes. Metab. 2017, 19, 1425–1435. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Khan, R. Insulin-Associated Weight Gain in Diabetes--Causes, Effects and Coping Strategies. Diabetes Obes. Metab. 2007, 9, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]

| Variables | N = 55 |
|---|---|
| Age, years | 58.2 (average 33–77) years |
| Men, n (%) | 30 (54.5%) |
| Diabetes duration (years) | 9.5 (3–19) years |
| Arterial hypertension | 29 (52.7%) |
| Complications | |
| Diabetic peripheral polyneuropathy | 15 (27.3%) |
| Diabetic retinopathy | 9 (16.4%) |
| Diabetic nephropathy | 10 (18.1%) |
| Type 2 diabetes treatment | |
| Metformin | 13 (23.6%) |
| Metformin + Sulfonylureas | 34 (61.9%) |
| Metformin + DPP − 4 inhibitors | 5 (9.1%) |
| Sulfonylureas | 3 (5.5%) |
| Non-insulin treated patients | 48 (87.3%) |
| Parameter | Baseline | After 6 Months | After 12 Months | After 24 Months | p-Value |
|---|---|---|---|---|---|
| Fasting plasma glucose (mg/dL) | 179 IQR = [152, 201] | 161 IQR = [140, 180] | 149.5 IQR = [135, 168] | 136.4 IQR = [123, 149] | p < 0.001 * |
| Glycated hemoglobin (HbA1c) (%) | 8.4 IQR = [7.43, 9.3] | 7.7 IQR = [7.2, 8.2] | 7.2 IQR = [6.6, 7.9] | 6.8 IQR = [6.2, 7.1] | p < 0.001 * |
| Body weight (kg) | 95.2 IQR = [83.5, 102] | - | - | 91.8 IQR = [80, 100] | p < 0.001 * |
| Body mass index (kg/m2) | 33.4 IQR = [28.6, 37.7] | - | - | 32.2 IQR = [27.72, 35.79] | p < 0.001 * |
| LDL-cholesterol | 112.9 | - | - | 99.2 | p < 0.001 * |
| Parameter Comparison | Test Used | Test Statistic | p-Value | Interpretation |
|---|---|---|---|---|
| BMI baseline vs. 24 months | t-test | t = 4.592 | p < 0.001 | Significant reduction in BMI |
| Body weight baseline vs. 24 months | Wilcoxon | Z = −4.450 | p < 0.001 | Significant reduction in weight |
| HbA1c baseline vs. 6 months | t-test | t = 7.422 | p < 0.001 | Significant improvement in HbA1c |
| HbA1c 6 months vs. 12 months | Wilcoxon | Z = −5.168 | p < 0.001 | Continued improvement in HbA1c |
| HbA1c 12 months vs. 24 months | Wilcoxon | Z = −5.108 | p < 0.001 | Sustained improvement in HbA1c |
| FPG baseline vs. 6 months | Wilcoxon | Z = −4.509 | p < 0.001 | Significant reduction in fasting glucose |
| FPG 6 months vs. 12 months | Wilcoxon | Z = −4.310 | p < 0.001 | Further decrease in fasting glucose |
| FPG 12 months vs. 24 months | Wilcoxon | Z = −4.974 | p < 0.001 | Continued improvement in fasting glucose |
| LDL baseline vs. 24 months | Wilcoxon | Z = −3.427 | p < 0.001 | Significant reduction in LDL-cholesterol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobu, A.M.; Turliuc, S.; Cucu, A.I.; Onofriescu, A.; Dascalu, C.G.; Costea, C.F.; Patrascanu, E.; Morosan, A.P.; Haisan, A.; Filip, C.N.; et al. Dulaglutide 1.5 mg Significantly Improves Glycemic Control and Lowers LDL-Cholesterol and Body Weight in Romanian Patients with Type 2 Diabetes. J. Clin. Med. 2025, 14, 3536. https://doi.org/10.3390/jcm14103536
Bobu AM, Turliuc S, Cucu AI, Onofriescu A, Dascalu CG, Costea CF, Patrascanu E, Morosan AP, Haisan A, Filip CN, et al. Dulaglutide 1.5 mg Significantly Improves Glycemic Control and Lowers LDL-Cholesterol and Body Weight in Romanian Patients with Type 2 Diabetes. Journal of Clinical Medicine. 2025; 14(10):3536. https://doi.org/10.3390/jcm14103536
Chicago/Turabian StyleBobu, Amelian Madalin, Serban Turliuc, Andrei Ionut Cucu, Alina Onofriescu, Cristina Gena Dascalu, Claudia Florida Costea, Emilia Patrascanu, Anca Petruta Morosan, Anca Haisan, Carmen Nicoleta Filip, and et al. 2025. "Dulaglutide 1.5 mg Significantly Improves Glycemic Control and Lowers LDL-Cholesterol and Body Weight in Romanian Patients with Type 2 Diabetes" Journal of Clinical Medicine 14, no. 10: 3536. https://doi.org/10.3390/jcm14103536
APA StyleBobu, A. M., Turliuc, S., Cucu, A. I., Onofriescu, A., Dascalu, C. G., Costea, C. F., Patrascanu, E., Morosan, A. P., Haisan, A., Filip, C. N., Covali, R., Buzduga, C. M., Botnariu, G., & Enache, I. I. C. (2025). Dulaglutide 1.5 mg Significantly Improves Glycemic Control and Lowers LDL-Cholesterol and Body Weight in Romanian Patients with Type 2 Diabetes. Journal of Clinical Medicine, 14(10), 3536. https://doi.org/10.3390/jcm14103536








