Machine Learning Based Assessment of Inguinal Lymph Node Metastasis in Patients with Squamous Cell Carcinoma of the Vulva
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayhan, A.; Velipasaoglu, M.; Salman, M.C.; Guven, S.; Gultekin, M.; Bayraktar, O. Prognostic Factors for Recurrence and Survival in Primary Vulvar Squamous Cell Cancer. Acta Obstet. Gynecol. Scand. 2008, 87, 1143–1149. [Google Scholar] [CrossRef]
- Fons, G.; Burger, M.P.; ten Kate, F.J.; van der Velden, J. Identification of Potential Prognostic Markers for Vulvar Cancer Using Immunohistochemical Staining of Tissue Microarrays. Int. J. Gynecol. Pathol. 2007, 26, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Abrao, F.S.; Baracat, E.C.; Marques, A.F.; Abrao, M.S.; Torloni, H.; Coelho, F.R.G.; Alves, A.C.; De Lima, G.R. Carcinoma of the Vulva: Clinicopathologic Factors Involved in Inguinal and Pelvic Lymph Node Metastasis. J. Reprod. Med. Obstet. Gynecol. 1990, 35, 1113–1116. [Google Scholar]
- Zare, S.Y.; Ciscato, A.; Fadare, O. Tumor Budding Activity Is an Independent Prognostic Factor in Squamous Cell Carcinoma of the Vulva. Hum. Pathol. 2022, 126, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dongre, H.N.; Elnour, R.; Tornaas, S.; Fromreide, S.; Thomsen, L.C.V.; Kolseth, I.B.M.; Nginamau, E.S.; Johannessen, A.C.; Vintermyr, O.K.; Costea, D.E.; et al. TP53 Mutation and Human Papilloma Virus Status as Independent Prognostic Factors in a Norwegian Cohort of Vulva Squamous Cell Carcinoma. Acta Obstet. Gynecol. Scand. 2023, 103, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Klamminger, G.G.; Nigdelis, M.P.; Degirmenci, Y.; Hamoud, B.H.; Solomayer, E.F.; Schnöder, L.; Holleczek, B.; Schmidt, M.; Hasenburg, A.; Wagner, M. Histopathological Biomarkers in Squamous Cell Carcinoma of the Vulva: The Prognostic Relevance of Tumor-Infiltrating Lymphocytes (TILs)—A Retrospective Study of 157 Cases. Discov. Oncol. 2025, 16, 572. [Google Scholar] [CrossRef]
- Zapardiel, I.; Iacoponi, S.; Coronado, P.J.; Zalewski, K.; Chen, F.; Fotopoulou, C.; Dursun, P.; Kotsopoulos, I.C.; Jach, R.; Buda, A.; et al. Prognostic Factors in Patients with Vulvar Cancer: The VULCAN Study. Int. J. Gynecol. Cancer 2020, 30, 1285–1291. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Hanozet, F.; Ditto, A.; Solima, E.; Zanaboni, F.; Vecchione, F.; Kusamura, S. Clinical and Pathological Prognostic Factors in Squamous Cell Carcinoma of the Vulva. Gynecol. Oncol. 2006, 102, 333–337. [Google Scholar] [CrossRef]
- Cheng, X.; Zang, R.; Wu, X.; Li, Z.; Cai, S.; Zhang, Z. Recurrence Patterns and Prognostic Factors in Chinese Patients With Squamous Cell Carcinoma of the Vulva Treated With Primary Surgery. Int. J. Gynecol. Cancer 2009, 19, 158–162. [Google Scholar] [CrossRef]
- Lataifeh, I.; Carraro Nascimento, M.; Nicklin, J.L.; Perrin, L.C.; Crandon, A.J.; Obermair, A. Patterns of Recurrence and Disease-Free Survival in Advanced Squamous Cell Carcinoma of the Vulva. Gynecol. Oncol. 2004, 95, 701–705. [Google Scholar] [CrossRef]
- Woelber, L.; Eulenburg, C.; Choschzick, M.; Kruell, A.; Petersen, C.; Gieseking, F.; Jaenicke, F.; Mahner, S. Prognostic Role of Lymph Node Metastases in Vulvar Cancer and Implications for Adjuvant Treatment. Int. J. Gynecol. Cancer 2012, 22, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.; Stewen, K.; Bührer, T.-L.; Schmidt, M.W.; van der Ven, J.; Anic, K.; Linz, V.C.; Hamoud, B.H.; Brenner, W.; Peters, K.; et al. Current Approaches to the Management of Sentinel Node Procedures in Early Vulvar Cancer in Germany: A Web-Based Nationwide Analysis of Practices. J. Clin. Med. 2023, 12, 2048. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer-Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. Vulvar Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. Natl. Compr. Cancer Netw. 2024, 22, 117–135. [Google Scholar] [CrossRef]
- Asami, Y.; Hiranuma, K.; Takayanagi, D.; Matsuda, M.; Shimada, Y.; Kato, M.K.; Kuno, I.; Murakami, N.; Komatsu, M.; Hamamoto, R.; et al. Predictive Model for the Preoperative Assessment and Prognostic Modeling of Lymph Node Metastasis in Endometrial Cancer. Sci. Rep. 2022, 12, 19004. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, B.; Hong, C.; Yuan, J.; Deng, J.; Chen, Y.; Ye, J.; Li, Y. The Value of Machine Learning in Preoperative Identification of Lymph Node Metastasis Status in Endometrial Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2023, 13, 1289050. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Mao, W.; Tan, L.; Yang, Y.; Liu, S.; Zhang, Y.; Li, B.; Zhao, D. Prediction of Postoperative Pathologic Risk Factors in Cervical Cancer Patients Treated with Radical Hysterectomy by Machine Learning. Curr. Oncol. 2022, 29, 9613–9629. [Google Scholar] [CrossRef] [PubMed]
- Monthatip, K.; Boonnag, C.; Muangmool, T.; Charoenkwan, K. A Machine Learning-Based Prediction Model of Pelvic Lymph Node Metastasis in Women with Early-Stage Cervical Cancer. J. Gynecol. Oncol. 2024, 35, e17. [Google Scholar] [CrossRef]
- Meng, Y.; Yan, X.; Fan, J. Construction of Prediction Model of Lymph Node Metastasis of Early Cervical Cancer Based on Machine Learning Algorithm and Its Application: Experience of 204 Cases in a Single Center. Am. J. Transl. Res. 2023, 15, 1852–1861. [Google Scholar]
- Krogue, J.D.; Azizi, S.; Tan, F.; Flament-Auvigne, I.; Brown, T.; Plass, M.; Reihs, R.; Müller, H.; Zatloukal, K.; Richeson, P.; et al. Predicting Lymph Node Metastasis from Primary Tumor Histology and Clinicopathologic Factors in Colorectal Cancer Using Deep Learning. Commun. Med. 2023, 3, 59. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Jia, Z.; Li, Y.; Wu, H.; Qu, S.; Zhu, J.; Liu, X.; Xu, C. Application of Machine Learning Algorithms to Predict Lymph Node Metastasis in Gastric Neuroendocrine Neoplasms. Heliyon 2023, 9, e20928. [Google Scholar] [CrossRef] [PubMed]
- Shahriarirad, R.; Meshkati Yazd, S.M.; Fathian, R.; Fallahi, M.; Ghadiani, Z.; Nafissi, N. Prediction of Sentinel Lymph Node Metastasis in Breast Cancer Patients Based on Preoperative Features: A Deep Machine Learning Approach. Sci. Rep. 2024, 14, 1351. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.; Morley-Bunker, A.; McLauchlan, J.; Glyn, T.; Eglinton, T. Use of Artificial Intelligence for the Prediction of Lymph Node Metastases in Early-Stage Colorectal Cancer: Systematic Review. BJS Open 2024, 8, zrae033. [Google Scholar] [CrossRef]
- Li, Y.; Xie, F.; Xiong, Q.; Lei, H.; Feng, P. Machine Learning for Lymph Node Metastasis Prediction of in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 946038. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Brierley, J.D., Gospodarowicz, M.K., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Akazawa, M.; Hashimoto, K. Artificial Intelligence in Gynecologic Cancers: Current Status and Future Challenges—A Systematic Review. Artif. Intell. Med. 2021, 120, 102164. [Google Scholar] [CrossRef]
- Fiste, O.; Liontos, M.; Zagouri, F.; Stamatakos, G.; Dimopoulos, M.A. Machine Learning Applications in Gynecological Cancer: A Critical Review. Crit. Rev. Oncol. Hematol. 2022, 179, 103808. [Google Scholar] [CrossRef]
- Gandotra, S.; Kumar, Y.; Modi, N.; Choi, J.; Shafi, J.; Ijaz, M.F. Comprehensive Analysis of Artificial Intelligence Techniques for Gynaecological Cancer: Symptoms Identification, Prognosis and Prediction. Artif. Intell. Rev. 2024, 57, 220. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Q.; Xie, Q.; Peng, Y.; Chen, M.; Huang, Z.; Lin, Z.; Yao, T. Preoperative Prediction Model of Lymph Node Metastasis in the Inguinal and Femoral Region Based on Radiomics and Artificial Intelligence. Int. J. Gynecol. Cancer 2024, 34, 1437–1444. [Google Scholar] [CrossRef]
- Garganese, G.; Fragomeni, S.M.; Pasciuto, T.; Leombroni, M.; Moro, F.; Evangelista, M.T.; Bove, S.; Gentileschi, S.; Tagliaferri, L.; Paris, I.; et al. Ultrasound Morphometric and Cytologic Preoperative Assessment of Inguinal Lymph-node Status in Women with Vulvar Cancer: MorphoNode Study. Ultrasound Obstet. Gynecol. 2020, 55, 401–410. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Moro, F.; Palluzzi, F.; Mascilini, F.; Rufini, V.; Collarino, A.; Inzani, F.; Giacò, L.; Scambia, G.; Testa, A.C.; et al. Evaluating the Risk of Inguinal Lymph Node Metastases before Surgery Using the Morphonode Predictive Model: A Prospective Diagnostic Study in Vulvar Cancer Patients. Cancers 2023, 15, 1121. [Google Scholar] [CrossRef] [PubMed]
- Suman, S. An Integrative Network Analysis to Reveal Network Biomarkers for Vulvar Cancer. Hum. Gene 2023, 37, 201194. [Google Scholar] [CrossRef]
- Hertz, A.M.; Hertz, N.M.; Johnsen, N.V. Identifying Bladder Rupture Following Traumatic Pelvic Fracture: A Machine Learning Approach. Injury 2020, 51, 334–339. [Google Scholar] [CrossRef]
- Klamminger, G.G.; Gérardy, J.-J.; Jelke, F.; Mirizzi, G.; Slimani, R.; Klein, K.; Husch, A.; Hertel, F.; Mittelbronn, M.; Kleine-Borgmann, F.B. Application of Raman Spectroscopy for Detection of Histologically Distinct Areas in Formalin-Fixed Paraffin-Embedded Glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab077. [Google Scholar] [CrossRef] [PubMed]
- HARRELL, F.E.; LEE, K.L.; MARK, D.B. Multivariable Prognostic Models: Issues In Developing Models, Evaluating Assumptions And Adequacy, And Measuring And Reducing Errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Hassanzade, M.; Attaran, M.; Treglia, G.; Yousefi, Z.; Sadeghi, R. Lymphatic Mapping and Sentinel Node Biopsy in Squamous Cell Carcinoma of the Vulva: Systematic Review and Meta-Analysis of the Literature. Gynecol. Oncol. 2013, 130, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, A.G.J.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel Node Dissection Is Safe in the Treatment of Early-Stage Vulvar Cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef]
- Hacker, N.F.; Berek, J.S.; Lagasse, L.D.; Leuchter, R.S.; Moore, J.G. Management of Regional Lymph Nodes and Their Prognostic Influence in Vulvar Cancer. Obstet. Gynecol. 1983, 61, 408–412. [Google Scholar]
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Yordan, E.; Berek, J.S.; Jahshan, A.; Mortel, R. Assessment of Current International Federation of Gynecology and Obstetrics Staging of Vulvar Carcinoma Relative to Prognostic Factors for Survival (a Gynecologic Oncology Group Study). Am. J. Obstet. Gynecol. 1991, 164, 997–1003; discussion 1003–1004. [Google Scholar] [CrossRef]
- Klamminger, G.G.; Nigdelis, M.; Bitterlich, A.; Hamoud, B.H.; Solomayer, E.F.; Hasenburg, A.; Wagner, M. Machine Learning Based Assessment of Inguinal Lymph Node Metastasis in Patients with Squamous Cell Carcinoma of the Vulva. Int. J. Gynecol. Cancer 2025, 35, 101525. [Google Scholar] [CrossRef]

| Variables of Interest | N = 157 |
|---|---|
| Age, years | 66 (median, IQR: 53–79) |
| T1a | 30 (19.1%) |
| T1b | 108 (68.8%) |
| T2 | 19 (12.1%) |
| T3 | - |
| N0 | 124 (79.0%) |
| positive groin lymph node affection (Nmic/N1a to N2c) | 33 (21.0%) |
| L0 | 132 (84.1%) |
| L1 | 25 (15.9%) |
| V0 | 146 (93.0%) |
| V1 | 11 (7.0%) |
| Pn0 | 144 (91.7%) |
| Pn1 | 13 (8.3%) |
| infiltration depth (in cm) | 0.7134 (mean), 0.8120 (std. deviation) |
| R0 | 129 (82.2%) |
| R1 | 28 (17.8%) |
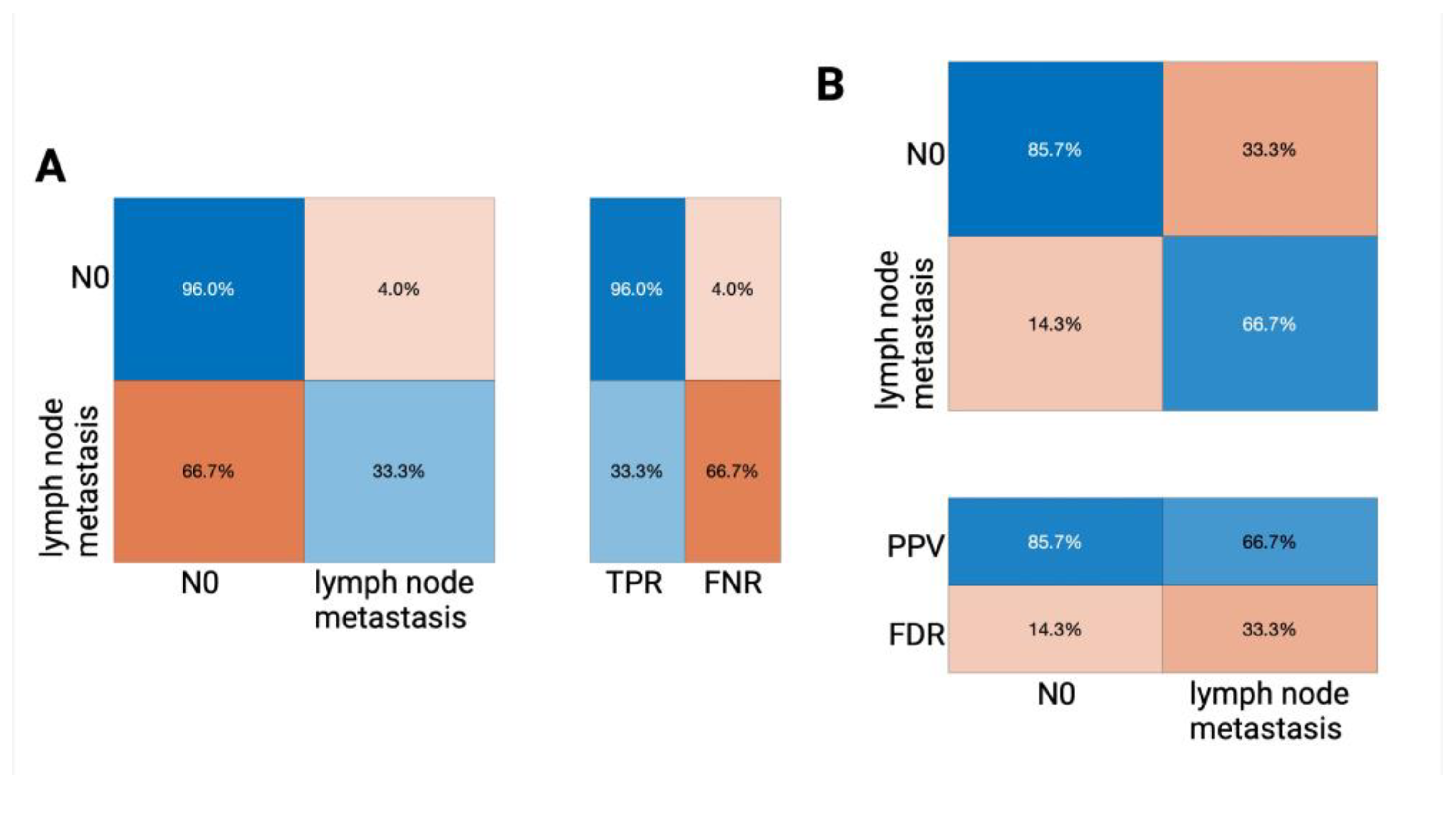
| Key Performance Indicators of Our Tree Classifier Performance (Internal Classifier Validation) | Overall Accuracy = 79.4% |
|---|---|
| no lymph node affection (N0): | |
| TPR (true positive rate) | 85.9% |
| FNR (false negative rate) | 14.1% |
| PPV (positive predictive value) | 87.6% |
| FDR (false discovery rate) | 12.4% |
| positive groin lymph node affection: | |
| TPR (true positive rate) | 55.6% |
| FNR (false negative rate) | 44.4% |
| PPV (positive predictive value) | 51.7% |
| FDR (false discovery rate) | 48.3% |
| AUROC value | 0.6433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klamminger, G.G.; Nigdelis, M.P.; Bitterlich, A.; Haj Hamoud, B.; Solomayer, E.-F.; Hasenburg, A.; Wagner, M. Machine Learning Based Assessment of Inguinal Lymph Node Metastasis in Patients with Squamous Cell Carcinoma of the Vulva. J. Clin. Med. 2025, 14, 3510. https://doi.org/10.3390/jcm14103510
Klamminger GG, Nigdelis MP, Bitterlich A, Haj Hamoud B, Solomayer E-F, Hasenburg A, Wagner M. Machine Learning Based Assessment of Inguinal Lymph Node Metastasis in Patients with Squamous Cell Carcinoma of the Vulva. Journal of Clinical Medicine. 2025; 14(10):3510. https://doi.org/10.3390/jcm14103510
Chicago/Turabian StyleKlamminger, Gilbert Georg, Meletios P. Nigdelis, Annick Bitterlich, Bashar Haj Hamoud, Erich-Franz Solomayer, Annette Hasenburg, and Mathias Wagner. 2025. "Machine Learning Based Assessment of Inguinal Lymph Node Metastasis in Patients with Squamous Cell Carcinoma of the Vulva" Journal of Clinical Medicine 14, no. 10: 3510. https://doi.org/10.3390/jcm14103510
APA StyleKlamminger, G. G., Nigdelis, M. P., Bitterlich, A., Haj Hamoud, B., Solomayer, E.-F., Hasenburg, A., & Wagner, M. (2025). Machine Learning Based Assessment of Inguinal Lymph Node Metastasis in Patients with Squamous Cell Carcinoma of the Vulva. Journal of Clinical Medicine, 14(10), 3510. https://doi.org/10.3390/jcm14103510







