Exploring the Risk: Peripheral Retinal Degenerations in Young Australian Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Study Design
2.3. Data Preparation
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Demographic Comparisons
3.2. Peripheral Retinal Degeneration Prevalence
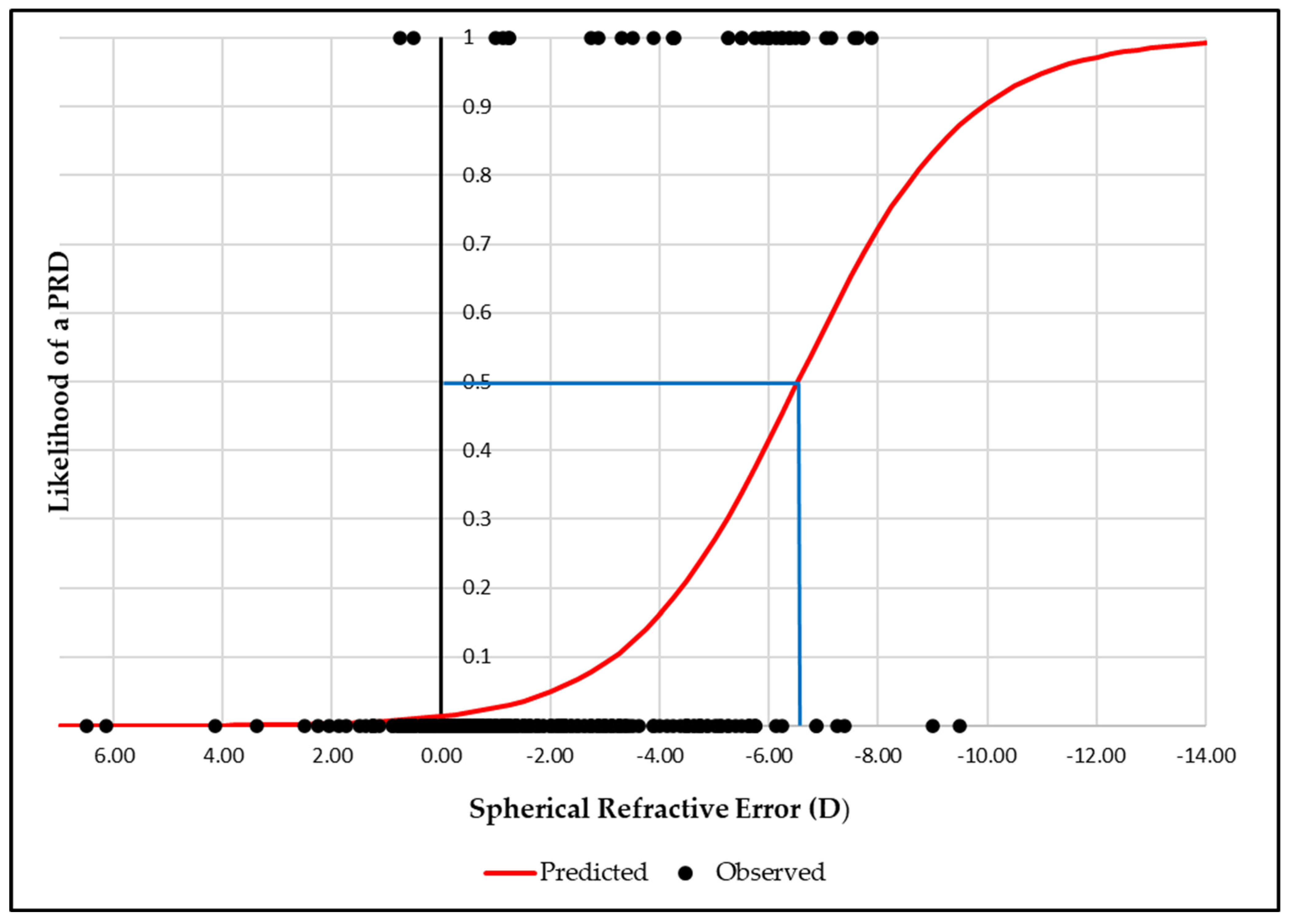
3.3. Association Between Refractive Error and Peripheral Retinal Degenerations
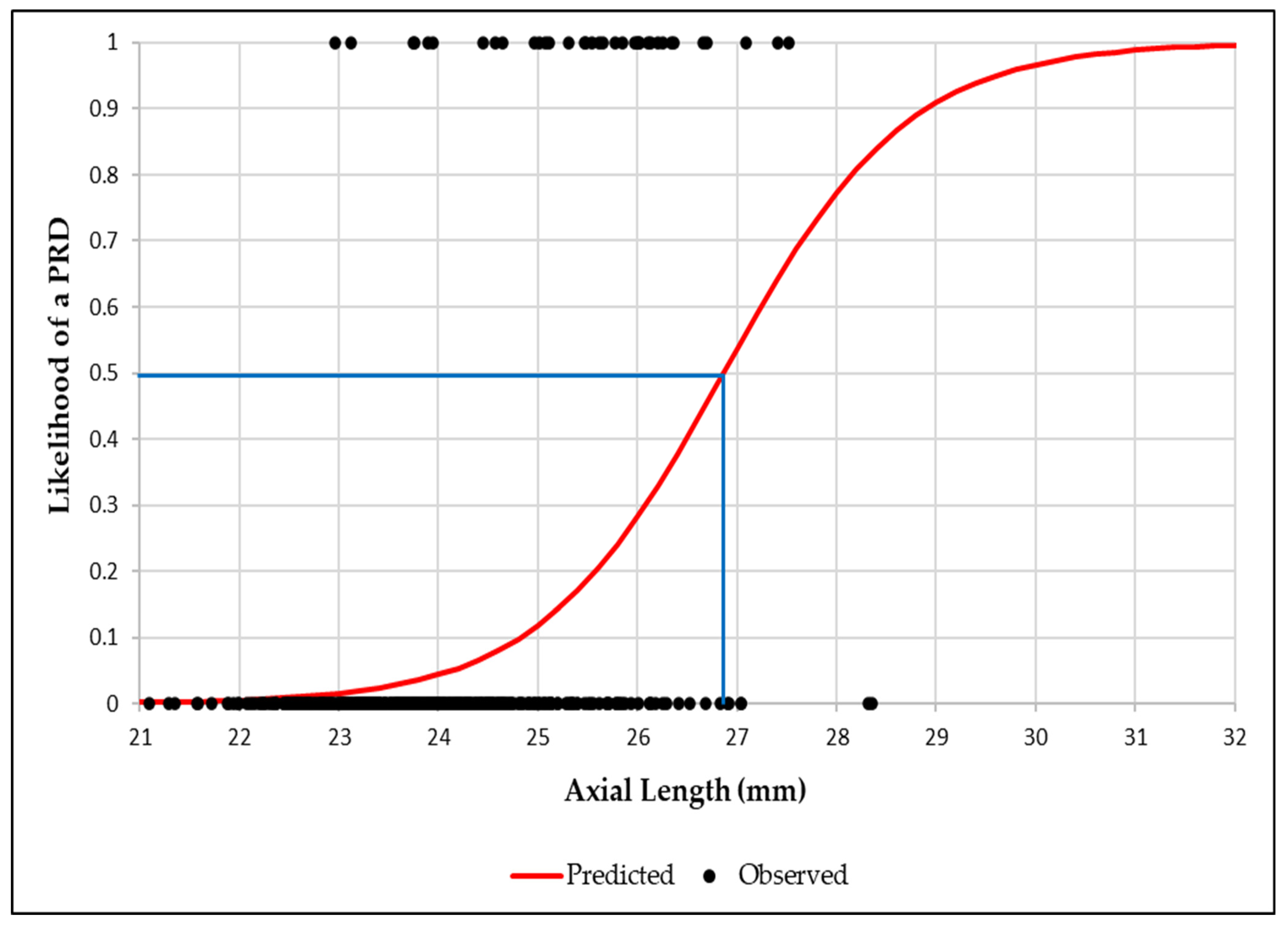
3.4. Association Between Axial Length and Peripheral Retinal Degenerations
3.5. Binary Logistic Regression Analysis Between Axial Length, Spherical Equivalent Refractive Error and Peripheral Retinal Degenerations
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRD | Peripheral Retinal Degeneration |
| RE | Refractive Error |
| AL | Axial Length |
| UWF | Ultra-widefield |
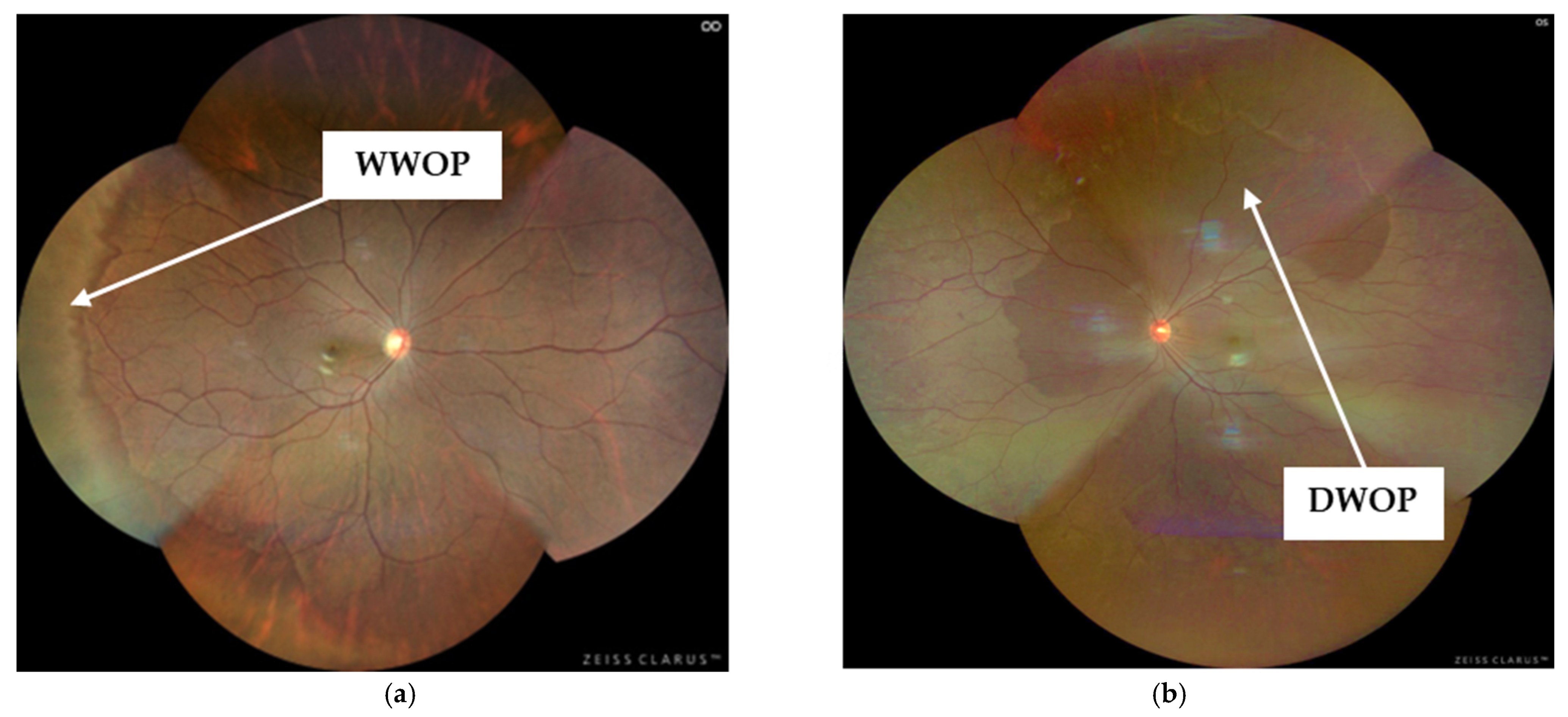
| WWOP | White Without Pressure |
| DWOP | Dark Without Pressure |
| LD | Lattice Degeneration |
| CHRPE | Congenital Hypertrophy of the Retinal Pigment Epithelium |
| OR | Odds Ratio |
| BIO | Binocular Indirect Ophthalmoscopy |
| DFE | Dilated Fundus Examination |
References
- Michaud, L.; Forcier, P. Prevalence of asymptomatic ocular conditions in subjects with refractive-based symptoms. J. Optom. 2014, 7, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Lam, C.S.; Yap, M.K. Prevalence of myopia-related retinal changes among 12–18 year old Hong Kong Chinese high myopes. Ophthalmic Physiol. Opt. 2013, 33, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H. Peripheral retinal degenerations and the risk of retinal detachment. Am. J. Ophthalmol. 2003, 136, 155–160. [Google Scholar] [CrossRef]
- Mrejen, S.; Ledesma-Gil, G.; Engelbert, M. Peripheral Retinal Abnormalities. In Pathologic Myopia; Spaide, R.F., Ohno-Matsui, K., Yannuzzi, L.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 329–346. [Google Scholar]
- Akbani, M.I.; Reddy, K.R.K.; Vishwanath, K.; Saleem, H. Association of Posterior Pole Degeneration with Axial Length in the Cases of Myopia- A Cross Sectional Study. Indian J. Public Health Res. Dev. 2014, 5, 64–67. [Google Scholar] [CrossRef]
- Chen, D.Z.; Koh, V.; Tan, M.; Tan, C.S.; Nah, G.; Shen, L.; Bhargava, M.; Cheng, C.Y.; Zhao, P.; Wong, T.Y.; et al. Peripheral retinal changes in highly myopic young Asian eyes. Acta Ophthalmol. 2018, 96, e846–e851. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Luo, H.; Zhang, X.; Sun, J.; Zhong, Z.; Sun, X. Analysis of White and Dark without Pressure in a Young Myopic Group Based on Ultra-Wide Swept-Source Optical Coherence Tomography Angiography. J. Clin. Med. 2022, 11, 4830. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Y.T.; Huang, W.B.; Liu, R.J.; Zuo, Y.J.; He, L.W.; Zhong, L.T.; Zhang, S.C. Prevalence and characteristics of peripheral myopic retinopathy in Guangzhou office workers. Int. J. Ophthalmol. 2018, 11, 1390–1395. [Google Scholar] [CrossRef]
- Nilagiri, V.K.; Lee, S.S.-Y.; Lingham, G.; Charng, J.; Yazar, S.; Hewitt, A.W.; Griffiths, L.R.; Sanfilippo, P.G.; Tsai, T.-H.; Mackey, D.A. Distribution of Axial Length in Australians of Different Age Groups, Ethnicities, and Refractive Errors. Transl. Vis. Sci. Technol. 2023, 12, 14. [Google Scholar] [CrossRef]
- Lam, D.S.; Fan, D.S.; Chan, W.M.; Tam, B.S.; Kwok, A.K.; Leung, A.T.; Parsons, H. Prevalence and characteristics of peripheral retinal degeneration in Chinese adults with high myopia: A cross-sectional prevalence survey. Optom. Vis. Sci. 2005, 82, 235–238. [Google Scholar] [CrossRef]
- Bowling, B. Kanski’s Clinical Ophthalmology: A Systematic Approach, 8th ed.; W.B. Saunders: Philadelphia, PA, USA, 2016. [Google Scholar]
- Byer, N.E. Clinical Study of Senile Retinoschisis. Arch. Ophthalmol. 1968, 79, 36–44. [Google Scholar] [CrossRef]
- Patel, S.N.; Shi, A.; Wibbelsman, T.D.; Klufas, M.A. Ultra-widefield retinal imaging: An update on recent advances. Ther. Adv. Ophthalmol. 2020, 12, 2515841419899495. [Google Scholar] [CrossRef] [PubMed]
- Nixon, T.R.W.; Davie, R.L.; Snead, M.P. Posterior vitreous detachment and retinal tear—A prospective study of community referrals. Eye 2024, 38, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Abadia, B.; Desco, M.C.; Mataix, J.; Palacios, E.; Navea, A.; Calvo, P.; Ferreras, A. Non-Mydriatic Ultra-Wide Field Imaging Versus Dilated Fundus Exam and Intraoperative Findings for Assessment of Rhegmatogenous Retinal Detachment. Brain Sci. 2020, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Klimczuk, A. The SAGE Encyclopedia of Theory in Psychology; SAGE Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Thiagalingam, S.; Wang, J.J.; Mitchell, P. Absence of Change in Choroidal Nevi Across 5 Years in an Older Population. Arch. Ophthalmol. 2004, 122, 89–93. [Google Scholar] [CrossRef]
- Chien, J.L.; Sioufi, K.; Surakiatchanukul, T.; Shields, J.A.; Shields, C.L. Choroidal nevus: A review of prevalence, features, genetics, risks, and outcomes. Curr. Opin. Ophthalmol. 2017, 28, 228–237. [Google Scholar] [CrossRef]
- Liu, Y.; Moore, A.T. Congenital focal abnormalities of the retina and retinal pigment epithelium. Eye 2020, 34, 1973–1988. [Google Scholar] [CrossRef]
- Karlin, D.B.; Curtin, B.J. Peripheral chorioretinal lesions and axial length of the myopic eye. Am. J. Ophthalmol. 1976, 81, 625–635. [Google Scholar] [CrossRef]
- Dhull, V.; Manisha, N.; Sundan, S.; Rachana, G. A Study of White Without Pressure Peripheral Retinal Lesions in Emmetropia, Myopia and Hypermetropia. Saudi J. Med. Pharm. Sci. 2019, 5, 503–511. [Google Scholar]
- Celorio, J.M.; Pruett, R.C. Prevalence of lattice degeneration and its relation to axial length in severe myopia. Am. J. Ophthalmol. 1991, 111, 20–23. [Google Scholar] [CrossRef]
- Galvis, V.; Tello, A.; Otero, J.; Serrano, A.; Gómez, L.; Camacho Lopez, P.; Lopez-Jaramillo, P. Prevalence of refractive errors in Colombia: MIOPUR study. Br. J. Ophthalmol. 2018, 102, 1320–1323. [Google Scholar] [CrossRef]
- Crim, N.; Esposito, E.; Monti, R.; Correa, L.J.; Serra, H.M.; Urrets-Zavalia, J.A. Myopia as a risk factor for subsequent retinal tears in the course of a symptomatic posterior vitreous detachment. BMC Ophthalmol. 2017, 17, 226. [Google Scholar] [CrossRef] [PubMed]
- Wajuihian, S.; Hansraj, R. Refractive Error in a Sample of Black High School Children in South Africa. Optom. Vis. Sci. 2017, 94, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Fang, Y.; Zhou, Z.; Chen, W.; Hu, J.; Jin, S.; Liu, X.; Wang, L.; Feng, J.; Tong, H.; et al. The Impact of Parental Myopia and High Myopia on the Hyperopia Reserve of Preschool Children. Ophthalmic Res. 2023, 67, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Cheung, C.Y.-l.; Lin, X.; Mitchell, P.; Wong, T.Y.; Mei-Saw, S. Influence of Refractive Error and Axial Length on Retinal Vessel Geometric Characteristics. Investig. Ophthalmol. Vis. Sci. 2011, 52, 669–678. [Google Scholar] [CrossRef]
- Foster, P.J.; Broadway, D.C.; Hayat, S.; Luben, R.; Dalzell, N.; Bingham, S.; Wareham, N.J.; Khaw, K.-T. Refractive error, axial length and anterior chamber depth of the eye in British adults: The EPIC-Norfolk Eye Study. Br. J. Ophthalmol. 2010, 94, 827–830. [Google Scholar] [CrossRef]
- Khan, M.H.; Lam, A.K.C.; Armitage, J.A.; Hanna, L.; To, C.H.; Gentle, A. Impact of Axial Eye Size on Retinal Microvasculature Density in the Macular Region. J. Clin. Med. 2020, 9, 2539. [Google Scholar] [CrossRef]
- Beaujot, R.; McDonald, P. Introduction: Comparative demography of Australia and Canada. Can. Stud. Popul. 2016, 43, 1–4. [Google Scholar] [CrossRef]
- Lai, T.Y.; Fan, D.S.; Lai, W.W.; Lam, D.S. Peripheral and posterior pole retinal lesions in association with high myopia: A cross-sectional community-based study in Hong Kong. Eye 2008, 22, 209–213. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Chen, S.; Shi, T. Peripheral and posterior pole retinal changes in highly myopic Chinese children and adolescents. BMC Ophthalmol. 2024, 24, 65. [Google Scholar] [CrossRef]
- Bansal, A.S.; Hubbard, G.B., 3rd. Peripheral retinal findings in highly myopic children < or =10 years of age. Retina 2010, 30, S15–S19. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Hoang, T.T.; Pham, C.M.; Nguyen, T.M.; Dang, T.M.; Fricke, T.R. Prevalence and related factors of myopic retinopathy—A hospital-based cross-section study in Vietnam. Clin. Exp. Optom. 2023, 106, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Pierro, L.; Camesasca, F.I.; Mischi, M.; Brancato, R. Peripheral retinal changes and axial myopia. Retina 1992, 12, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Tideman, J.W.L.; Snabel, M.C.C.; Tedja, M.S.; van Rijn, G.A.; Wong, K.T.; Kuijpers, R.W.A.M.; Vingerling, J.R.; Hofman, A.; Buitendijk, G.H.S.; Keunen, J.E.E.; et al. Association of Axial Length with Risk of Uncorrectable Visual Impairment for Europeans with Myopia. JAMA Ophthalmol. 2016, 134, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S. Comparing the prevalence of lattice degeneration and requirement of barrage laser in different grades of myopia. Kerala J. Ophthalmol. 2024, 36, 53–56. [Google Scholar] [CrossRef]
- Burton, T.C. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans. Am. Ophthalmol. Soc. 1989, 87, 143–155. [Google Scholar]
- Midena, E.; Marchione, G.; Di Giorgio, S.; Rotondi, G.; Longhin, E.; Frizziero, L.; Pilotto, E.; Parrozzani, R.; Midena, G. Ultra-wide-field fundus photography compared to ophthalmoscopy in diagnosing and classifying major retinal diseases. Sci. Rep. 2022, 12, 19287. [Google Scholar] [CrossRef]
- Karatepe Hashas, A.S.; Popovic, Z.; Abu-Ishkheidem, E.; Bond-Taylor, M.; Svedberg, K.; Jarar, D.; Zetterberg, M. A new diagnostic method for retinal breaks in patients with posterior vitreous detachment: Ultra-wide-field imaging with the Zeiss Clarus 700. Acta Ophthalmol. 2023, 101, 627–635. [Google Scholar] [CrossRef]
- Matsui, Y.; Ichio, A.; Sugawara, A.; Uchiyama, E.; Suimon, H.; Matsubara, H.; Sugimoto, M.; Ikesugi, K.; Kondo, M. Comparisons of Effective Fields of Two Ultra-Widefield Ophthalmoscopes, Optos 200Tx and Clarus 500. BioMed. Res. Int. 2019, 2019, 7436293. [Google Scholar] [CrossRef]
- Stino, H.; Riessland, S.; Sedova, A.; Datlinger, F.; Sacu, S.; Schmidt-Erfurth, U.; Pollreisz, A. Comparison of two ultra-widefield color-fundus imaging devices for visualization of retinal periphery and microvascular lesions in patients with early diabetic retinopathy. Sci. Rep. 2022, 12, 17449. [Google Scholar] [CrossRef]
- Chen, A.; Dang, S.; Chung, M.M.; Ramchandran, R.S.; Bessette, A.P.; DiLoreto, D.A.; Kleinman, D.M.; Sridhar, J.; Wykoff, C.C.; Kuriyan, A.E. Quantitative Comparison of Fundus Images by 2 Ultra-Widefield Fundus Cameras. Ophthalmol. Retin. 2021, 5, 450–457. [Google Scholar] [CrossRef]
- Sharma, P.; Shareef, I.; Kalaw, F.G.P.; Kako, R.N.; Lin, A.; Alex, V.; Nudleman, E.; Walker, E.H.; Borooah, S. Prevalence of peripheral retinal findings in retinal patients using ultra-widefield pseudocolor fundus imaging. Sci. Rep. 2023, 13, 20515. [Google Scholar] [CrossRef]
- Mirshahi, A.; Ponto, K.A.; Hoehn, R.; Zwiener, I.; Zeller, T.; Lackner, K.; Beutel, M.E.; Pfeiffer, N. Myopia and level of education: Results from the Gutenberg Health Study. Ophthalmology 2014, 121, 2047–2052. [Google Scholar] [CrossRef]
| RE Classification * | Values (D) |
|---|---|
| Hyperopia | >+0.50 |
| Emmetropia | +0.50 to −0.50 |
| Mild myopia | >−0.50 to ≤−3.00 |
| Moderate myopia | >−3.00 to ≤−6.00 |
| High myopia | >−6.00 |
| AL (mm) | Classification * |
|---|---|
| <23.50 | Hyperopia |
| ≥23.50 to ≤24.50 | Emmetropia |
| >24.50 to ≤25.50 | Mild Myopia |
| >25.50 to ≤26.49 | Moderate Myopia |
| ≥26.50 | High Myopia |
| Parameters | No of Eyes (%) | Mean Age in Years | Mean Age with No PRD in Years | Mean Age with PRD in Years |
|---|---|---|---|---|
| Gender Male Female | 150 (33.9) 292 (66.1) | 22.43 ± 11 22.52 ± 3.17 22.38 ± 3.10 | 22.43 ± 3.15 | 22.39 ± 2.74 |
| Peripheral Retinal Degeneration | No. of Eyes (n) | Percentage (%) |
|---|---|---|
| White Without Pressure | 16 | 3.39 |
| Dark Without Pressure | 16 | 3.39 |
| Lattice Degeneration | 3 | 0.69 |
| Retinal Hole | 2 | 0.45 |
| Parameters | PRD-No. of Eyes (n) | Percentage (%) |
|---|---|---|
| Emmetropia | 1 | 0.61 |
| Hyperopia | 1 | 2.86 |
| Myopia (all) | 34 | 14.05 |
| Mild Myopia | 6 | 3.95 |
| Moderate Myopia | 15 | 22.06 |
| High Myopia | 13 | 59.09 |
| AL (>24.02 mm) | 30 | 15.23 |
| Parameters | Eyes with No PRD | Eyes with PRD | p a |
|---|---|---|---|
| RE (D) | −1.13 ± 1.94 | −4.78 ± 2.33 | <0.001 |
| AL (mm) | 23.89 ± 1.08 | 25.46 ± 1.13 | <0.001 |
| Parameters | OR | 95%CI | p |
|---|---|---|---|
| RE | |||
| Emmetropia | Reference | ||
| Hyperopia | 1.57 | −1.22–4.37 | 0.27 |
| Mild myopia | 1.91 | −0.22–4.04 | 0.079 |
| Moderate myopia | 3.84 | 1.79–5.86 | <0.001 a |
| High myopia | 5.47 | 3.33–7.61 | <0.001 a |
| AL | |||
| Emmetropia | Reference | ||
| Hyperopia | −0.95 | −2.60–0.71 | 0.261 |
| Mild myopia | 1.48 | 0.37–2.59 | 0.009 a |
| Moderate myopia | 3.00 | 1.88–4.12 | <0.001 a |
| High myopia | 2.82 | 1.41–4.23 | <0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watt, N.A.; Hockley, N.; Armitage, J.A. Exploring the Risk: Peripheral Retinal Degenerations in Young Australian Adults. J. Clin. Med. 2025, 14, 3501. https://doi.org/10.3390/jcm14103501
Watt NA, Hockley N, Armitage JA. Exploring the Risk: Peripheral Retinal Degenerations in Young Australian Adults. Journal of Clinical Medicine. 2025; 14(10):3501. https://doi.org/10.3390/jcm14103501
Chicago/Turabian StyleWatt, Natalie Ann, Nicholas Hockley, and James Andrew Armitage. 2025. "Exploring the Risk: Peripheral Retinal Degenerations in Young Australian Adults" Journal of Clinical Medicine 14, no. 10: 3501. https://doi.org/10.3390/jcm14103501
APA StyleWatt, N. A., Hockley, N., & Armitage, J. A. (2025). Exploring the Risk: Peripheral Retinal Degenerations in Young Australian Adults. Journal of Clinical Medicine, 14(10), 3501. https://doi.org/10.3390/jcm14103501








