The Critical Role of Iron in Pregnancy, Puerperium, and Fetal Development
Abstract
1. Introduction
2. Epidemiology
3. Physiological Distribution of Iron—A Starting Point for Understanding Deficiency
4. Normal Pregnancy Development and Iron Deficiency
4.1. Iron Deficiency and Reproductive Outcomes: Implantation, Infertility, Miscarriage, and Ectopic Pregnancy
4.2. Iron Deficiency and the Risk of Congenital Heart Defects (CHD)
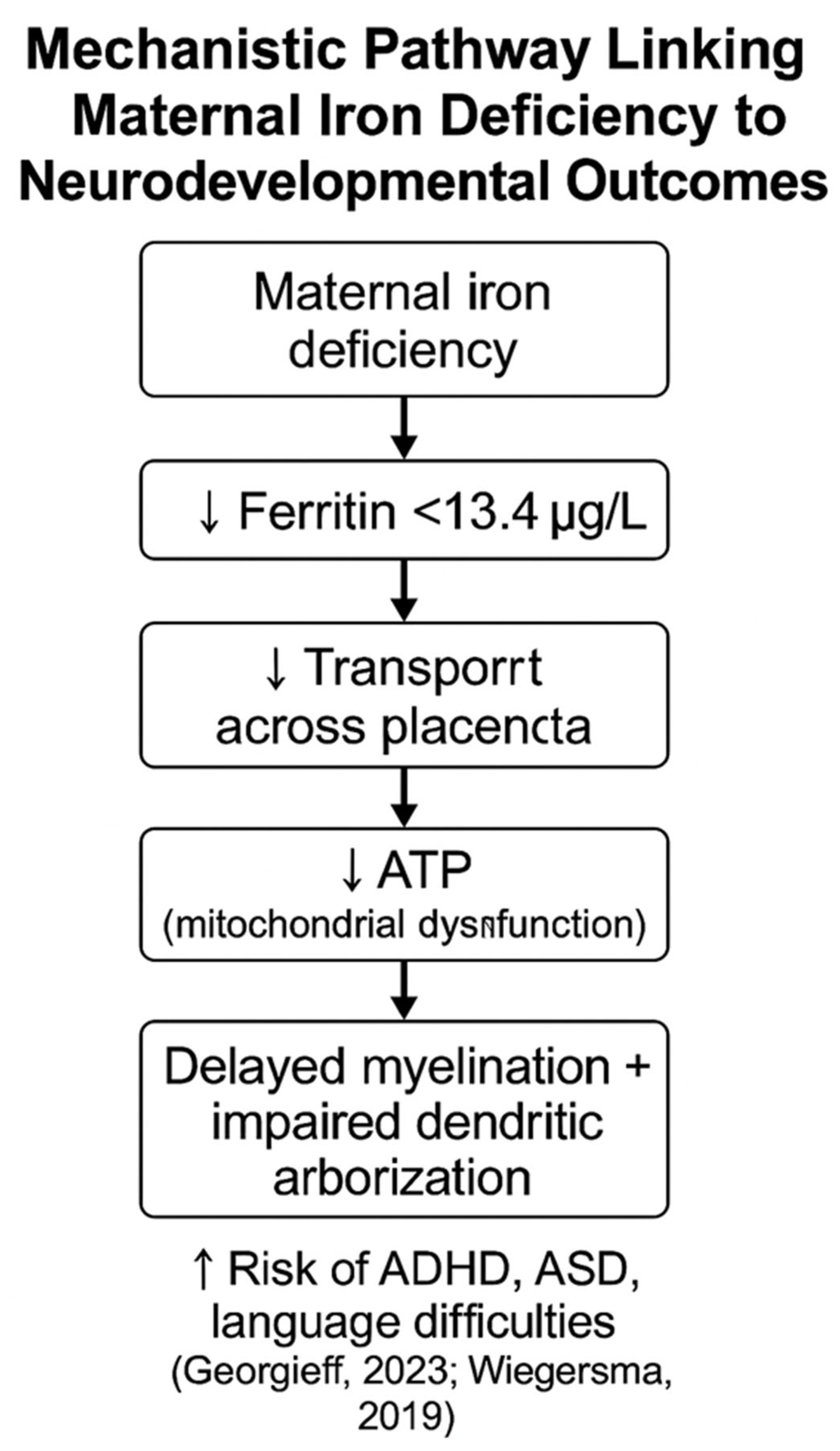
4.3. Neurodevelopmental and Perinatal Effects of Maternal Iron Deficiency
4.4. Restless Legs Syndrome and Iron-Related Neurological Changes in Pregnancy
4.5. Cardiac Function and ECG Changes in Pregnant Women with Iron Deficiency
4.6. Immune Suppression and Maternal Outcomes in Iron Deficiency Anemia
4.7. Perinatal Mental Health and Iron Status
4.8. Impact of Iron Deficiency on Breastfeeding
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brannon, P.M.; Taylor, C.L. Iron Supplementation during Pregnancy and Infancy: Uncertainties and Implications for Research and Policy. Nutrients 2017, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, X.; Zhang, M.; Yang, C.; Lv, S.; Li, S.; Zhong, C.; Geng, S. Adverse effects of iron deficiency anemia on pregnancy outcome and offspring development and intervention of three iron supplements. Sci. Rep. 2021, 11, 1347. [Google Scholar] [CrossRef]
- Díaz-Torres, S.; Díaz-López, A.; Arija, V. Effect of Prenatal Iron Supplementation Adapted to Hemoglobin Levels in Early Pregnancy on Fetal and Neonatal Growth—ECLIPSES Study. Nutrients 2024, 16, 437. [Google Scholar] [CrossRef]
- Garzon, S.; Cacciato, P.M.; Certelli, C.; Salvaggio, C.; Magliarditi, M.; Rizzo, G. Iron Deficiency Anemia in Pregnancy: Novel Approaches for an Old Problem. Oman Med. J. 2020, 35, e166. [Google Scholar] [CrossRef] [PubMed]
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin. Hematol. 2015, 52, 339. [Google Scholar] [CrossRef]
- Davidson, E.M.; Scoullar, M.J.L.; Peach, E.; Morgan, C.; Melepia, P.; Opi, D.H.; Supsup, H.; Hezeri, P.; Philip, W.P.; Kabiu, D.; et al. Quantifying differences in iron deficiency-attributable anemia during pregnancy and postpartum. Cell Rep. Med. 2023, 4, 101097. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ouf, N.M.; Jan, M.M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 2015, 36, 146. [Google Scholar] [CrossRef]
- Ministry of Health and Family Welfare. National Family Health Survey (NFHS-5), 2019–2021: India, Phase-II; Government of India: New Delhi, India, 2021. Available online: https://mohfw.gov.in/sites/default/files/NFHS-5_Phase-II_0.pdf (accessed on 13 May 2025).
- (1 C.E.). Available online: https://www.ceicdata.com/en/poland/health-statistics/pl-prevalence-of-anemia-among-pregnant-women- (accessed on 13 May 2025).
- Benson, A.E.; Shatzel, J.J.; Ryan, K.S.; Hedges, M.A.; Martens, K.L.; Aslan, J.E.; Lo, J.O. The incidence, complications, and treatment of iron deficiency in pregnancy. Eur. J. Haematol. 2022, 109, 633. [Google Scholar] [CrossRef]
- McCarthy, E.K.; Schneck, D.; Basu, S.; Xenopoulos-Oddsson, A.; McCarthy, F.P.; Kiely, M.; Georgieff, M. Longitudinal evaluation of iron status during pregnancy: A prospective cohort study in a high-resource setting. Am. J. Clin. Nutr. 2024, 120, 1259. [Google Scholar] [CrossRef]
- Stewart, T.; Lambourne, J.; Thorp-Jones, D.; Thomas, W. Implementation of early management of iron deficiency in pregnancy during the SARS-CoV-2 pandemic. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 258, 60. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; Gimeno, M.; Coronel, P.; Caspersen, I.H.; Basora, J.; Arija, V. Maternal factors associated with iron deficiency without anaemia in early pregnancy: ECLIPSES study. Ann. Hematol. 2023, 102, 741. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.E.; Lo, J.O.; Caughey, A.B. Iron Deficiency and Iron Deficiency Anemia During Pregnancy. JAMA Netw. Open 2024, 7, e2429151. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2822546 (accessed on 13 May 2025). [CrossRef]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.K.; Qasem, W.; Cai, C. Iron and the Breastfed Infant. Antioxidants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Qasem, W.; Friel, J.K. An Overview of Iron in Term Breast-Fed Infants. Clin. Med. Insights Pediatr. 2015, 9, 79–84. [Google Scholar] [CrossRef]
- Georgieff, M. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516. [Google Scholar] [CrossRef]
- Burke, R.M.; León, J.S.; Suchdev, P.S. Identification, Prevention and Treatment of Iron Deficiency during the First 1000 Days. Nutrients 2014, 6, 4093. [Google Scholar] [CrossRef]
- Georgieff, M. The importance of iron deficiency in pregnancy on fetal, neonatal, and infant neurodevelopmental outcomes. Int. J. Gynecol. Obstet. 2023, 162, 83. [Google Scholar] [CrossRef]
- Alwan, N.A.; Cade, J.E.; McArdle, H.J.; Greenwood, D.C.; Hayes, H.E.; Simpson, N.A.B. Maternal iron status in early pregnancy and birth outcomes: Insights from the Baby’s Vascular health and Iron in Pregnancy study. Br. J. Nutr. 2015, 113, 1985–1992. [Google Scholar] [CrossRef]
- Wiegersma, A.M.; Dalman, C.; Lee, B.K.; Karlsson, H.; Gardner, R.M. Association of Prenatal Maternal Anemia with Neurodevelopmental Disorders. JAMA Psychiatry 2019, 76, 1294. [Google Scholar] [CrossRef]
- Simon, A.; Laufer, N. Assessment and treatment of repeated implantation failure (RIF). J. Assist. Reprod. Genet. 2012, 29, 1227. [Google Scholar] [CrossRef] [PubMed]
- Watters, M.; Martínez-Aguilar, R.; Maybin, J.A. The Menstrual Endometrium: From Physiology to Future Treatments. Front. Reprod. Health 2022, 3, 794352. [Google Scholar] [CrossRef]
- Birkenfeld, A.; Goldfarb, A.; Rachmilewitz, E.A.; Schenker, J.G.; Okon, E. Endometrial glandular haemosiderosis in homozygous beta-thalassaemia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989, 31, 173. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611. [Google Scholar] [CrossRef] [PubMed]
- Holzer, I.; Ott, J.; Beitl, K.; Mayrhofer, D.; Heinzl, F.; Ebenbauer, J.; Parry, J.P. Iron status in women with infertility and controls: A case-control study. Front. Endocrinol. 2023, 14, 1173100. [Google Scholar] [CrossRef] [PubMed]
- Silfvast, A.T.; Simberg, N. P-416 Low serum ferritin level might be associated with an increased risk of miscarriages in infertility patietns. Hum. Reprod. 2022, 37, deac107.393. [Google Scholar] [CrossRef]
- Wu, N.; Ye, E.; Ba, Y.; Caikai, S.; Ba, B.; Li, L.; Zhu, Q. The global burden of maternal disorders attributable to iron deficiency related sub-disorders in 204 countries and territories: An analysis for the Global Burden of Disease study. Front. Public Health 2024, 12, 1406549. [Google Scholar] [CrossRef]
- Chundakkadan, M.S.; Chandramathy, K.; Selvest, N. Clinical presentation and outcome of ectopic pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 2021, 10, 3301. [Google Scholar] [CrossRef]
- El-Shirif, A.; Sadiq, S. A Case of Ectopic Pregnancy—An Unusual Diagnostic Challenge and Lesson Learnt. Open J. Obstet. Gynecol. 2015, 5, 192. [Google Scholar] [CrossRef]
- Kalisch-Smith, J.I.; Ved, N.; Szumska, D.; Munro, J.E.; Troup, M.; Harris, S.; Rodriguez-Caro, H.; Jacquemot, A.; Miller, J.J.; Stuart, E.M.; et al. Maternal iron deficiency perturbs embryonic cardiovascular development in mice. Nat. Commun. 2021, 12, 3447. [Google Scholar] [CrossRef]
- Yang, J.; Kang, Y.; Cheng, Y.; Zeng, L.; Shen, Y.L.; Shi, G.; Liu, Y.; Qu, P.; Zhang, R.; Yan, H.; et al. Iron intake and iron status during pregnancy and risk of congenital heart defects: A case-control study. Int. J. Cardiol. 2019, 301, 74. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Solenthaler, F.; Albrecht, C. Materno-fetal iron transfer and the emerging role of ferroptosis pathways. Biochem. Pharmacol. 2022, 202, 115141. [Google Scholar] [CrossRef]
- Georgieff, M. The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008, 36, 1267. [Google Scholar] [CrossRef]
- Özyurt, R.; Bulutlar, E. Effect of Iron Deficiency Anemia on Fetal and Maternal Morbidity. Bagcilar Med. Bull. 2024, 9, 87–93. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Krakowiak, P.; Hansen, R.; Ozonoff, S. Maternal Intake of Supplemental Iron and Risk of Autism Spectrum Disorder. Am. J. Epidemiol. 2014, 180, 890. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, C.; Schramm, J.; Strathmann, F.G.; Lee, D.L.; Gelein, R.; Luebke, A.E.; Mayer-Pröschel, M. Identifying a Window of Vulnerability during Fetal Development in a Maternal Iron Restriction Model. PLoS ONE 2011, 6, e17483. [Google Scholar] [CrossRef]
- Kemppinen, L.; Mattila, M.; Ekholm, E.; Pallasmaa, N.; Törmä, A.; Varakas, L.; Mäkikallio, K. Gestational iron deficiency anemia is associated with preterm birth, fetal growth restriction, and postpartum infections. J. Perinat. Med. 2020, 49, 431. [Google Scholar] [CrossRef] [PubMed]
- Hensley, J.G. Leg Cramps and Restless Legs Syndrome During Pregnancy. J. Midwifery Women’s Health 2009, 54, 211. [Google Scholar] [CrossRef]
- Srivanitchapoom, P.; Pandey, S.; Hallett, M. Restless legs syndrome and pregnancy: A review. Park. Relat. Disord. 2014, 20, 716. [Google Scholar] [CrossRef]
- Watson, S. Pregnancy and RLS. 2022. Available online: https://www.webmd.com/baby/pregnancy-and-rls-restless-legs-syndrome (accessed on 13 May 2025).
- Dixon, H. Handling Restless Leg Syndrome During Pregnancy. 2023. Available online: https://pregnantchicken.com/restless-leg-syndrome-during-pregnancy/ (accessed on 13 May 2025).
- Sifakis, S.; Pharmakides, G. Anemia in Pregnancy. Ann. N. Y. Acad. Sci. 2000, 900, 125. [Google Scholar] [CrossRef]
- Goshtasebi, A.; Alizadeh, M.; Behboudi-Gandevani, S. Association between Maternal Anaemia and Postpartum Depression in an Urban Sample of Pregnant Women in Iran. J. Health Popul. Nutr. 2013, 31, 398. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, S.A.; Etaati, Z.; Mirani, S.; Saberi, P.; Mahnaz, S.; Salmasian, H.; Naderi, N. The association between iron status and some immunological factors in the pregnancy. DOAJ Dir. Open Access J. 2011, 9, 251. [Google Scholar]
- Prema, K.; Ramalakshmi, B.A.; Madhavapeddi, R.; Babu, S. Immune status of anaemic pregnant women. BJOG Int. J. Obstet. Gynaecol. 1982, 89, 222. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, K.; Li, S.; Zhao, R.; Zhang, Q. The association between the CD4/CD8 ratio and surgical site infection risk among HIV-positive adults: Insights from a China hospital. Front. Immunol. 2023, 14, 1135725. [Google Scholar] [CrossRef]
- Igbinosa, I.; Leonard, S.A.; Noelette, F.; Davies-Balch, S.; Carmichael, S.L.; Main, E.K.; Lyell, D.J. Racial and Ethnic Disparities in Anemia and Severe Maternal Morbidity. Obstet. Gynecol. 2023, 142, 845–854. [Google Scholar] [CrossRef]
- Weintraub, M.J.; Schneck, C.D.; Walshaw, P.D.; Chang, K.D.; Singh, M.K.; Axelson, D.; Birmaher, B.; Miklowitz, D.J. Characteristics of youth at high risk for bipolar disorder compared to youth with bipolar I or II disorder. J. Psychiatr. Res. 2020, 123, 48. [Google Scholar] [CrossRef]
- Could Iron Deficiency Be Harming Your Milk Supply? 2025. Available online: https://www.motherlove.com/blogs/all/could-iron-deficiency-be-harming-your-milk-supply (accessed on 13 May 2025).
- Griffin, I.J.; Abrams, S.A. Iron and Breastfeeding. Pediatr. Clin. N. Am. 2001, 48, 401. [Google Scholar] [CrossRef]
- Iron Deficient Anemia and Milk Supply. 2024. Available online: https://internationalbreastfeedinginstitute.com/2024/09/iron-deficient-anemia-and-milk-supply-what-cbcs-need-to-know/ (accessed on 13 May 2025).
| Health Domain | Impact of Iron Deficiency | Source |
|---|---|---|
| Mother | Anemia and fatigue | Benson et al., 2022 [10] |
| Mother | Cardiac rhythm disturbances (QTc, tachycardia) | Sifakis and Pharmakides, 2000 [44] |
| Mother | Restless legs syndrome (RLS) | Srivanitchapoom et al., 2014 [41] |
| Mother | Prenatal and postpartum depression | Goshtasebi et al., 2013 [45] |
| Mother | Impaired immunity, increased risk of infections | Sobhani et al., 2011 [46] |
| Mother | Lactation difficulties—reduced milk volume and iron content | Benson et al., 2022 [10] |
| Mother | Increased risk of perinatal complications (hemorrhage, infections, prolonged hospitalization) | Kemppinen et al., 2020 [39] |
| Mother | Higher risk of cardiovascular complications (cardiac hypertrophy) | Sifakis and Pharmakides, 2000 [44] |
| Fetus/ Newborn | Low birth weight | Alwan et al., 2015 [21] |
| Fetus/ Newborn | Fetal growth restriction (FGR) | Kemppinen et al., 2020 [39] |
| Fetus/ Newborn | Increased risk of congenital heart defects | Kalisch-Smith et al., 2021 [32] |
| Fetus/ Newborn | Neurodevelopmental disorders (e.g., autism, ADHD, intellectual disability) | Wiegersma et al., 2019 [22] |
| Fetus/ Newborn | Disrupted myelination and dendritic development | Georgieff, 2023 [20] |
| Fetus/ Newborn | Higher risk of miscarriage and implantation failure | Silfvast and Simberg, 2022 [28] |
| Fetus/ Newborn | Increased risk of ectopic pregnancy | Wu et al., 2024 [29] |
| Fetus/ Newborn | Impaired energy metabolism in the fetal brain | Zaugg et al., 2022 [34] |
| Infant | Risk of anemia by 9 months of age | Georgieff, 2020 [18] |
| Infant | Attention, working memory and language deficits | Özyurt and Bulutlar, 2024 [36] |
| Infant | Shortened breastfeeding duration | Benson et al., 2022 [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych-Krekora, K.; Sylwestrzak, O.; Krekora, M. The Critical Role of Iron in Pregnancy, Puerperium, and Fetal Development. J. Clin. Med. 2025, 14, 3482. https://doi.org/10.3390/jcm14103482
Zych-Krekora K, Sylwestrzak O, Krekora M. The Critical Role of Iron in Pregnancy, Puerperium, and Fetal Development. Journal of Clinical Medicine. 2025; 14(10):3482. https://doi.org/10.3390/jcm14103482
Chicago/Turabian StyleZych-Krekora, Katarzyna, Oskar Sylwestrzak, and Michał Krekora. 2025. "The Critical Role of Iron in Pregnancy, Puerperium, and Fetal Development" Journal of Clinical Medicine 14, no. 10: 3482. https://doi.org/10.3390/jcm14103482
APA StyleZych-Krekora, K., Sylwestrzak, O., & Krekora, M. (2025). The Critical Role of Iron in Pregnancy, Puerperium, and Fetal Development. Journal of Clinical Medicine, 14(10), 3482. https://doi.org/10.3390/jcm14103482






