Super Responders in Moderate-to-Severe Atopic Dermatitis Under Treatment with Dupilumab: An Explorative Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Exclusion Criteria
- Age ≤ 12 years;
- Dupilumab administration for less than 2 years;
- Clinical follow-up less than 2 years;
- Missing clinical data at 16 or 32 weeks of treatment, or at 1 and 2 years of treatment.
2.3. Outcomes
- -
- Absolute mean value of EASI, PP-NRS, DLQI and POEM at each time point;
- -
- Achievement of EASI ≤ 7, PP-NRS ≤ 4, DLQI ≤ 5, and POEM ≤ 7 at each time-point.
2.4. Definitions
- Responder (Re): achievement of treatment targets EASI ≤ 7, plus PP-NRS ≤ 4. DLQI ≤ 5, and POEM ≤ 7.
- ER: achievement of therapeutic targets (see above) at 16 weeks of treatment (T1) or 32 weeks of treatment (T2).
- SR: ER who maintained treatment targets at 1 year (T3).
- LR: SR who maintained treatment targets at 2 years (T6).
2.5. Endpoints
- (i)
- Description of the trend of outcomes analyzed, patient Re status, and number of patients who were ERs, SRs, or LRs.
- (ii)
- Identification of baseline and clinical characteristics possibly related to being ERs, SRs, or LRs.
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AD | atopic dermatitis |
| SCORAD | scoring atopic dermatitis |
| EASI | eczema area severity index |
| DLQI | dermatology life quality index |
| POEM | patient-oriented eczema measure |
| NRSp | numerical rating scale pruritus |
| ER | early responder |
| SR | super responder |
| LR | long responder |
| N | number |
| SD | standard deviation |
| OR | odds ratio |
| M | male |
| p | p-value |
References
- Mastorino, L.; Duò, V.C.; Vecco, C.; Gelato, F.; Giordano, S.; Roccuzzo, G.; Cavaliere, G.; Avallone, G.; Ortoncelli, M.; Ribero, S.; et al. Impact of comorbidities in the response of atopic patients treated with dupilumab: A real-life study up to 36 weeks. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e1021–e1023. [Google Scholar] [CrossRef]
- Miniotti, M.; Ribero, S.; Mastorino, L.; Ortoncelli, M.; Gelato, F.; Bailon, M.; Trioni, J.; Stefan, B.; Quaglino, P.; Leombruni, P. Long-term psychological outcome of patients with moderate-to-severe atopic dermatitis continuously treated with Dupilumab: Data up to 3 years. Exp. Dermatol. 2023, 32, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Viola, R.; Panzone, M.; Avallone, G.; Gallo, G.; Ortoncelli, M.; Cavaliere, G.; Quaglino, P.; Ribero, S. Dupilumab induces a rapid decrease of pruritus in adolescents: A pilot real-life study. Dermatol. Ther. 2021, 34, e15115. [Google Scholar] [CrossRef]
- Jowita, S.T.; Magdalena, T. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar] [PubMed]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2024, 1447, 21–35. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Valle, G.A.; Barbarot, S.; Bieber, T.; Brough, H.; Pinton, P.C.; Christen-Zäch, S.; et al. European guideline (EuroGuiDerm) on atopic eczema: Part I-systemic therapy. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1409–1431. [Google Scholar] [CrossRef]
- Vestergaard, C.; Skovsgaard, C.; Johansen, C.; Deleuran, M.; Thyssen, J.P. Treat-to-Target in Atopic Dermatitis. Am. J. Clin. Dermatol. 2024, 25, 91–98. [Google Scholar] [CrossRef]
- Liao, Q.; Pan, H.; Guo, Y.; Lan, Y.; Huang, Z.; Wu, P. Comparative efficacy and safety of dupilumab versus newly approved biologics and JAKi in pediatric atopic dermatitis: A systematic review and network meta-analysis. PLoS ONE 2025, 20, e0319400. [Google Scholar] [CrossRef]
- Bruin-Weller, M.; Biedermann, T.; Bissonnette, R.; Deleuran, M.; Foley, P.; Girolomoni, G.; Hercogová, J.; Hong, C.; Katoh, N.; Pink, A.; et al. Treat-to-Target in Atopic Dermatitis: An International Consensus on a Set of Core Decision Points for Systemic Therapies. Acta Derm. Venereol. 2021, 101, adv00402. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Simpson, E.L.; Boguniewicz, M.; De Bruin-Weller, M.S.; Foley, P.; Kataoka, Y.; Bégo-Le-Bagousse, G.; Chen, Z.; Shumel, B.; Chao, J.; et al. Dupilumab Provides Rapid and Sustained Clinically Meaningful Responses in Adults with Moderate-to-severe Atopic Dermatitis. Acta Derm. Venereol. 2021, 101, adv00585. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Susca, S.; Cariti, C.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Dapavo, P.; Ribero, S.; Quaglino, P. Superresponders at biologic treatment for psoriasis: A comparative study among IL17 and IL23 inhibitors. Exp. Dermatol. 2023, 32, 2187–2188. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villaverde, R.; Domínguez-Cruz, J.; Navarro-Triviño, F.J.; Galán-Gutiérrez, M.; Armario-Hita, J.C.; Pereyra-Rodriguez, J.J. Atopic Dermatitis: Background, Objectives and Future Perspectives (Superresponders). Life 2022, 12, 1192. [Google Scholar] [CrossRef]
- Ariëns, L.F.; van der Schaft, J.; Spekhorst, L.S.; Bakker, D.S.; Romeijn, G.L.; Kouwenhoven, T.A.; Kamsteeg, M.; Voorberg, A.N.; Oosting, A.J.; de Ridder, I.; et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-Week results from the Dutch BioDay registry. J. Am. Acad. Dermatol. 2021, 84, 1000–1009. [Google Scholar] [CrossRef]
- Nettis, E.; Ferrucci, S.M.; Pellacani, G.; Di Leo, E.; Argenziano, G.; Foti, C.; Rongioletti, F.; Patruno, C.; Ortoncelli, M.; Macchia, L.; et al. Dupilumab in atopic dermatitis: Predictors of treatment outcome and time to response. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e896–e898. [Google Scholar] [CrossRef]
- Alegre-Bailo, A.; Sánchez-Gilo, A.; Román Mendoza, N.M.; Mateos-Rico, J.J.; Vicente-Martín, F.J. Tralokinumab treatment in atopic dermatitis: Depicting super-responders. J. Dermatol. 2023, 50, 1650–1652. [Google Scholar] [CrossRef]
- Limão, R.; Brás, R.; Pedro, E.; Silva, S.L.; Lopes, A. Dupilumab in patients with atopic dermatitis: Assessing treatment response, clinical features and potential biomarkers in real-life. Eur. Ann. Allergy Clin. Immunol. 2024. [Google Scholar] [CrossRef]
- Eyerich, K.; Weisenseel, P.; Pinter, A.; Schäkel, K.; Asadullah, K.; Wegner, S.; Muñoz-Elias, E.J.; Bartz, H.; Taut, F.J.H.; Reich, K. IL-23 blockade with guselkumab potentially modifies psoriasis pathogenesis: Rationale and study protocol of a phase 3b, randomised, double-blind, multicentre study in participants with moderate-to-severe plaque-type psoriasis (GUIDE). BMJ Open 2021, 11, e049822. [Google Scholar] [CrossRef]
- Canonica, G.W.; Bourdin, A.; Peters, A.T.; Desrosiers, M.; Bachert, C.; Weidinger, S.; Simpson, E.L.; Daizadeh, N.; Chen, Z.; Kamat, S.; et al. Dupilumab Demonstrates Rapid Onset of Response Across Three Type 2 Inflammatory Diseases. J. Allergy Clin. Immunol. Pract. 2022, 10, 1515–1526. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gooderham, M.; Katoh, N.; Aoki, V.; Pink, A.E.; Binamer, Y.; Rademaker, M.; Fomina, D.; Gutermuth, J.; Ahn, J.; et al. Combining treat-to-target principles and shared decision-making: International expert consensus-based recommendations with a novel concept for minimal disease activity criteria in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
| Patients, N | 171 |
| Mean age (SD) | 41.13 (16.52) |
| Mean BMI (SD) | 23.85 (3.99) |
| Mean age of onset (SD) | 14.09 (19.98) |
| Sex (male) % | 55.6% |
| Smoking habit % | |
| no | 56% |
| ex | 11.9% |
| yes | 6.6% |
| Alcohol (%) | |
| No | 42.3% |
| Sometimes | 51.2% |
| Yes | 6.6% |
| Pediatric onset (%) | 63.9% |
| Prurigo-like lesions (%) | 7% |
| Family history (%) | 37% |
| Predisposition to allergic conjunctivitis (%) | 24.4% |
| Previous topical steroids (%) | 98.8% |
| Previous topical calcineurin inhibitor (%) | 45.9% |
| Previous phototerapy (%) | 8.8% |
| Previous systemic steroids (%) | 95.3% |
| Previous cyclosporine (%) | 82.9% |
| Mean IgE T0 (SD) | 4170.2 (6149.73) |
| Base Line | T1 | T2 | T3 | T4 | T5 | T6 | |
|---|---|---|---|---|---|---|---|
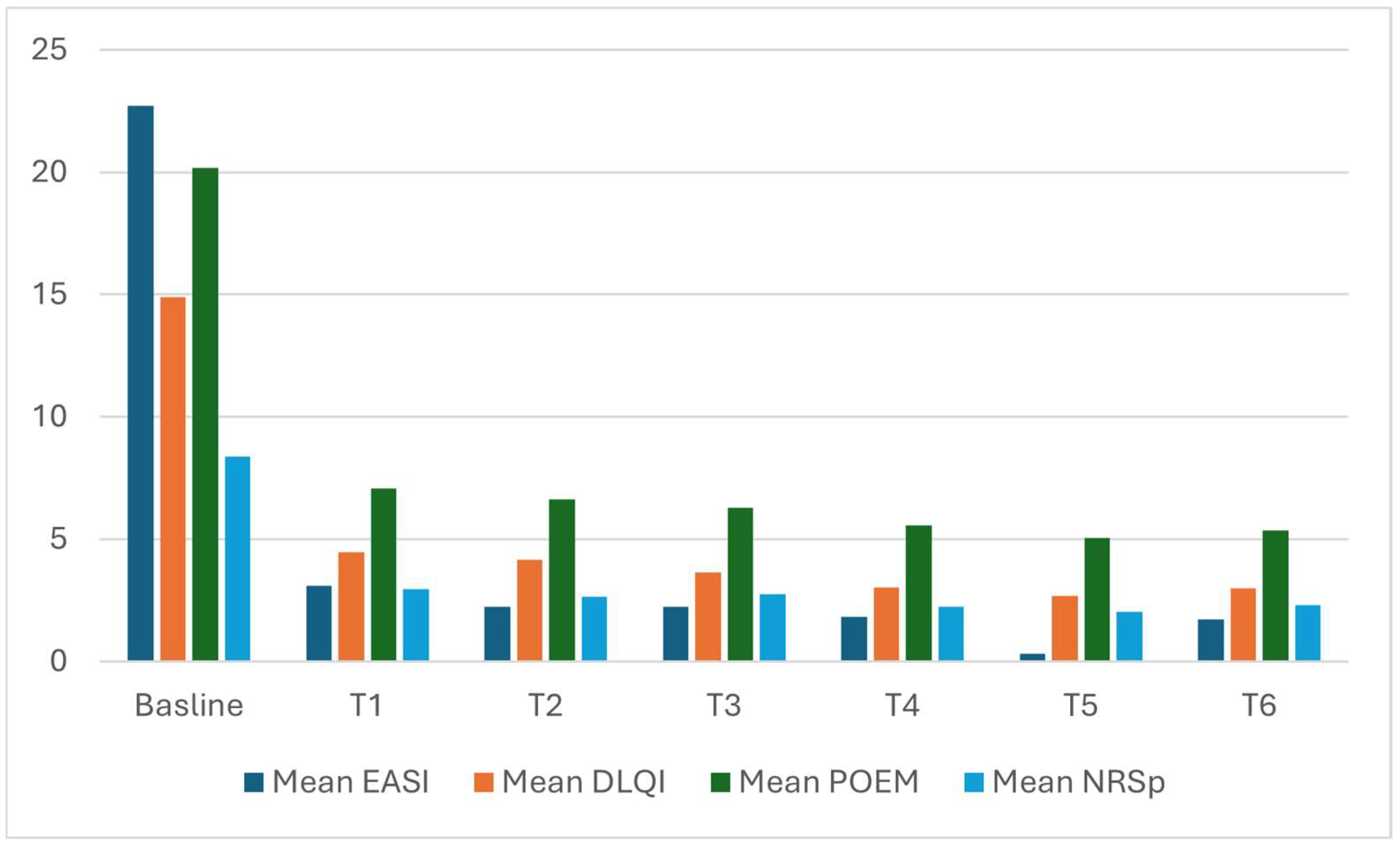
| Mean EASI (SD) | 22.73 (11.1) | 3.09 (4.07) | 2.23 (2.73) | 2.22 (2.73) | 1.81 (1.98) | 0.3 (0.8) | 1.73 (2.38) |
| Median (Q1–Q3) | 24 (15.3–28) | 2 (0–4) | 1 (0–3) | 1.35 (0–3) | 1 (0–3) | 0 (0–0) | 1 (0–3) |
| Mean DLQI (SD) | 14.9 (7.1) | 4.45 (4.94) | 4.15 (5.2) | 3.64 (4.36) | 3.04 (3.9) | 2.69 (3.16) | 2.98 (4.34) |
| Median (Q1–Q3) | 15 (10–20) | 3 (1–6) | 2 (1–6.25) | 2 (1–6) | 1 (0–5) | 1 (0–4) | 1 (0–4) |
| Mean POEM (SD) | 20.18 (7.1) | 7.08 (5.7) | 6.61 (6.12) | 6.29 (5.57) | 5.58 (5.46) | 5.04 (5.08) | 5.35 (5.66) |
| Median (Q1–Q3) | 22 (16–26) | 6 (2.25–10.75) | 5 (2–10) | 5 (2–9) | 4 (1–8) | 4 (1–8) | 4 (1–8) |
| Mean NRSp (SD) | 8.36 (1.88) | 2.96 (2.58) | 2.66 (2.4) | 2.74 (2.37) | 2.25 (2.3) | 2.04 (2.04) | 2.29(2.37) |
| Median (Q1–Q3) | 9 (7–19) | 2 (1–5) | 2 (1–4.5) | 2 (1–4) | 2 (0–3) | 2 (0–3) | 2 (0–3) |
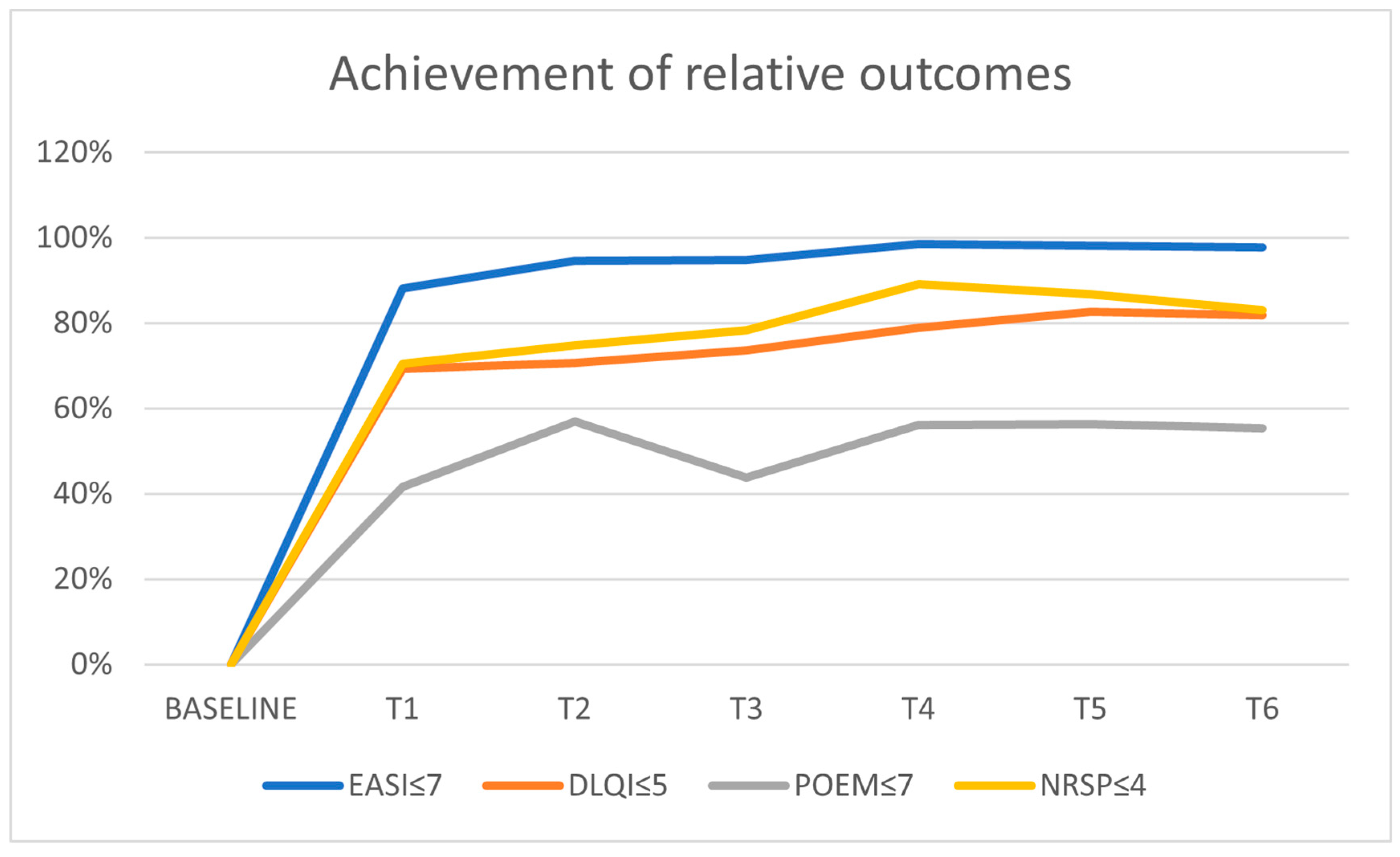
| EASI ≤ 7 | 0% | 88.2% | 94.6% | 94.7% | 98.6% | 98.2% | 97.7% |
| DLQI ≤ 5 | 0% | 69.4% | 70.7% | 73.7% | 79% | 82.6% | 81.9% |
| POEM ≤ 7 | 0% | 57.8% | 66.5% | 66.7% | 69.7% | 73.1% | 72.3% |
| NRSP ≤ 4 | 0% | 70.4% | 74.9% | 78.4% | 89.1% | 86.8% | 83% |
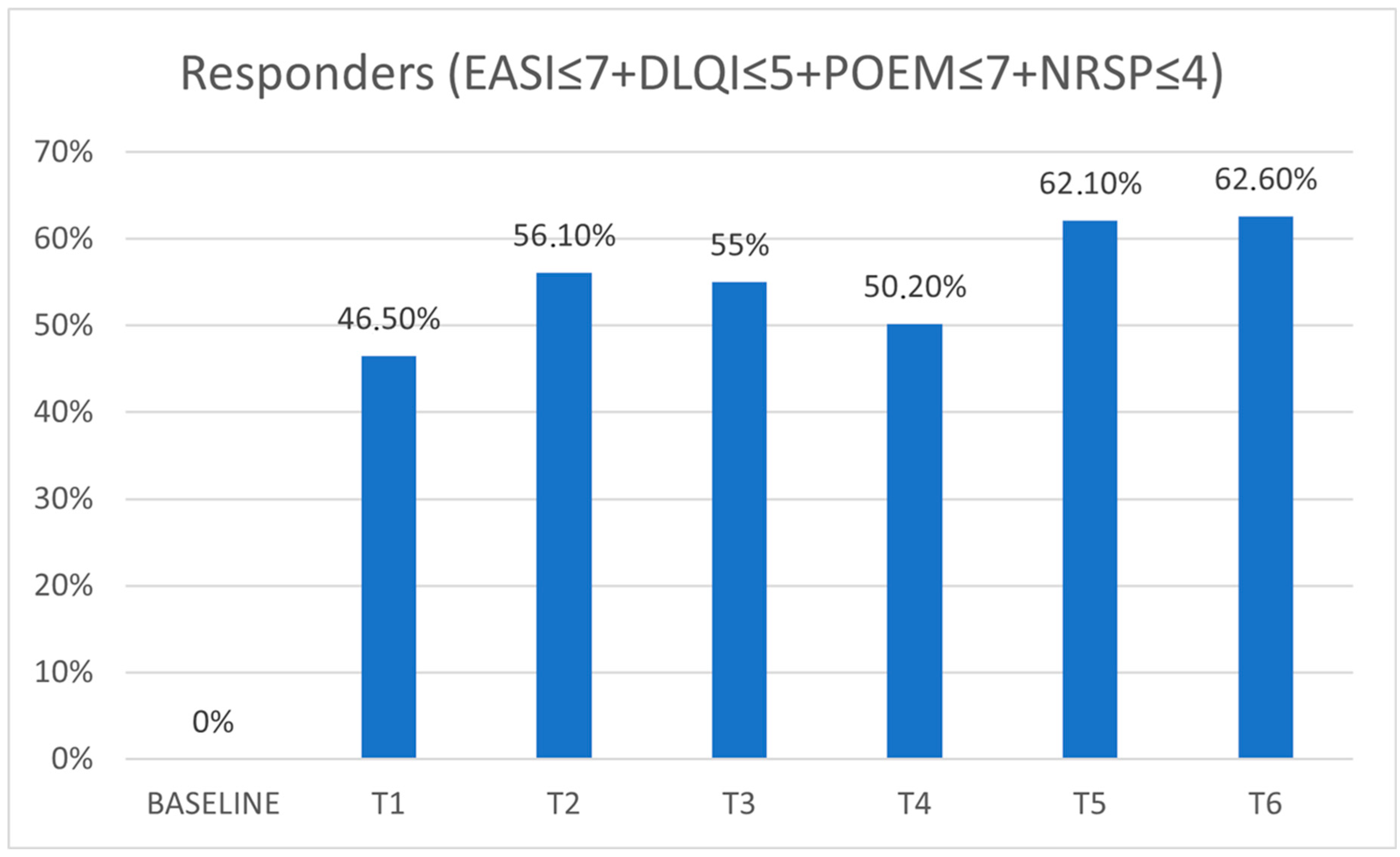
| Re | 0% | 46.5% | 56.1% | 55% | 50.2% | 62.1% | 62.6% |
| ER | 76.6% | ||||||
| SR | 49.1% | ||||||
| LR | 40.4% | ||||||
| ER | Non-ER | p-Value | Multivariate Analysis (ER) | SR | Non-SR | p-Value | Multivariate analysis (SR) | LR | Non-LR | p-Value | Multivariate Analysis (LR) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age (SD) | 41.75 (16.22) | 39.13 (17.51) | 0.381 | 41.75 (16.29) | 40.54 (16.61) | 0.633 | 41.64 (15.79) | 40.79 (17.06) | 0.744 | |||
| Mean BMI (SD) | 24.1 (3.92) | 23.04 (4.18) | 0.149 | OR 1.04 (0.96–1.14), p = 0.474 | 24.04 (4.02) | 23.67 (3.98) | 0.549 | 24 (4.02) | 23.75 (3.99) | 0.687 | ||
| Age of onset: Mean (SD) | 13.18 (19.22) | 17.08 (22.26) | 0.282 | 13.95 (20.08) | 14.23 (20) | 0.928 | 13.81 (20.01) | 14.29 (20.06) | 0.879 | |||
| Sex (M) % | 56.5% | 52.5% | 0.657 | 60.7% | 50.5% | 0.182 | OR 0.65 (0.31–1.37), p = 0.255) | 59.4% | 52.9% | 0.403 | ||
| Smoking habits % | 0.322 | 0.783 | 0.627 | |||||||||
| no | 54.3% | 61.6% | 58.1% | 53.7% | 59% | 51.5% | ||||||
| ex | 13.9% | 5.1% | 10.5% | 13.4% | 11% | 13.2% | ||||||
| yes | 31.8% | 33.3 | 31.4% | 32.9% | 30% | 35.3% | ||||||
| Alcohol % | 0.556 | 0.692 | 0.629 | |||||||||
| No | 40.3% | 48.7% | 42.7% | 41.9% | 43.5% | 41.4% | ||||||
| Sometimes | 53.5% | 43.6% | 52.4% | 50% | 52.2% | 50.5% | ||||||
| Yes | 6.2% | 7.7% | 4.9% | 8.1% | 4.3% | 8.1% | ||||||
| Pediatric onset % | 65.9% | 57.5% | 0.334 | 63.1% | 64.7% | 0.827 | 62.3% | 65% | 0.721 | |||
| Prurigo-like lesions % | 8.2% | 3.1% | 0.295 | 8.5% | 7.1% | 0.936 | 8.3% | 6.2% | 0.447 | |||
| Familiarity % | 39.7% | 28.2% | 0.194 | OR 0.89 (0.45–1.76), p = 0.735 | 24.4% | 24.4% | 0.285 | 39.8% | 32.8% | 0.363 | ||
| Predisposition to allergic conjunctivitis % | 24.2% | 25% | 0.92 | 24.4% | 24.4% | 0.997 | 23.8% | 25.4% | 0.812 | |||
| Previous topical steroids % | 99.2% | 97.5% | 0.416 | 100% | 97.7% | 0.497 | 100% | 98% | 0.517 | |||
| Previous topical calcineurin inhibitors % | 43.8% | 52.5% | 0.337 | 38.6% | 52.9% | 0.061 | OR 1.47 (0.71–3.01), p = 0.297 | 38.2% | 51% | 0.102 | OR 1.25 (0.66–2.39), p = 0.497 | |
| Previous phototerapy % | 9.2% | 7.5% | 0.512 | 9.6% | 8% | 0.791 | 11.8% | 6.9% | 0.283 | |||
| Previous systemic steroids % | 94.6% | 97.5% | 0.889 | 92.8% | 97.7% | 0.161 | OR 1.69 (0.26–10.81), p = 0.581 | 92.6% | 97.1% | 0.169 | OR 2,81 (0.63–12.41), p = 0.174 | |
| Previous cyclosporine % | 81.5% | 87.5% | 0.87 | 80.7% | 85.1% | 0.453 | 79.4% | 85.3% | 0.318 | |||
| Mean IgE (SD) T0 | 4481.56 (7316.1) | 4412.02 (5698.9) | 0.961 | 3434.22 (4605.5) | 4992.06 (7434.5) | 0.155 | OR 1 (1–1), p = 0.326 | 3881.86 (5708.75) | 4629.2 (6829.75) | 0.507 | ||
| Mean EASI (SD) T0 | 22.58 (11.26) | 23.23 (10.61) | 0.561 | 22.81 (11.69) | 22.66 (10.54) | 0.645 | 22.49 (11.53) | 22.89 (10.83) | 0.718 | |||
| Mean DLQI (SD) T0 | 14.63 (7.14) | 15.76 (6.88) | 0.401 | 14.26 (7.35) | 15.51 (6.79) | 0.38 | 14.79 (7.7) | 14.97 (6.67) | 0.887 | |||
| Mean POEM (SD) T0 | 19.53 (7.04) | 22.2 (4.95) | 0.061 | OR 0.93 (0.88–0.98), p = 0.006 | 19.47 (7.29) | 20.86 (6) | 0.344 | 19.95 (7.45) | 20.34 (6.14) | 0.911 | ||
| Mean NRSp (SD) T0 | 8.31 (1.91) | 8.53 (1.77) | 0.619 | 8.35 (1.89) | 8.37 (1.87( | 0.998 | 8.38 (1.76) | 8.35 (1.96) | 0.884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastorino, L.; Mendes-Bastos, P.; Cavaliere, G.; Siliquini, N.; Ortoncelli, M.; Quaglino, P.; Ribero, S. Super Responders in Moderate-to-Severe Atopic Dermatitis Under Treatment with Dupilumab: An Explorative Real-World Study. J. Clin. Med. 2025, 14, 3480. https://doi.org/10.3390/jcm14103480
Mastorino L, Mendes-Bastos P, Cavaliere G, Siliquini N, Ortoncelli M, Quaglino P, Ribero S. Super Responders in Moderate-to-Severe Atopic Dermatitis Under Treatment with Dupilumab: An Explorative Real-World Study. Journal of Clinical Medicine. 2025; 14(10):3480. https://doi.org/10.3390/jcm14103480
Chicago/Turabian StyleMastorino, Luca, Pedro Mendes-Bastos, Giovanni Cavaliere, Niccolo Siliquini, Michela Ortoncelli, Pietro Quaglino, and Simone Ribero. 2025. "Super Responders in Moderate-to-Severe Atopic Dermatitis Under Treatment with Dupilumab: An Explorative Real-World Study" Journal of Clinical Medicine 14, no. 10: 3480. https://doi.org/10.3390/jcm14103480
APA StyleMastorino, L., Mendes-Bastos, P., Cavaliere, G., Siliquini, N., Ortoncelli, M., Quaglino, P., & Ribero, S. (2025). Super Responders in Moderate-to-Severe Atopic Dermatitis Under Treatment with Dupilumab: An Explorative Real-World Study. Journal of Clinical Medicine, 14(10), 3480. https://doi.org/10.3390/jcm14103480







