Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
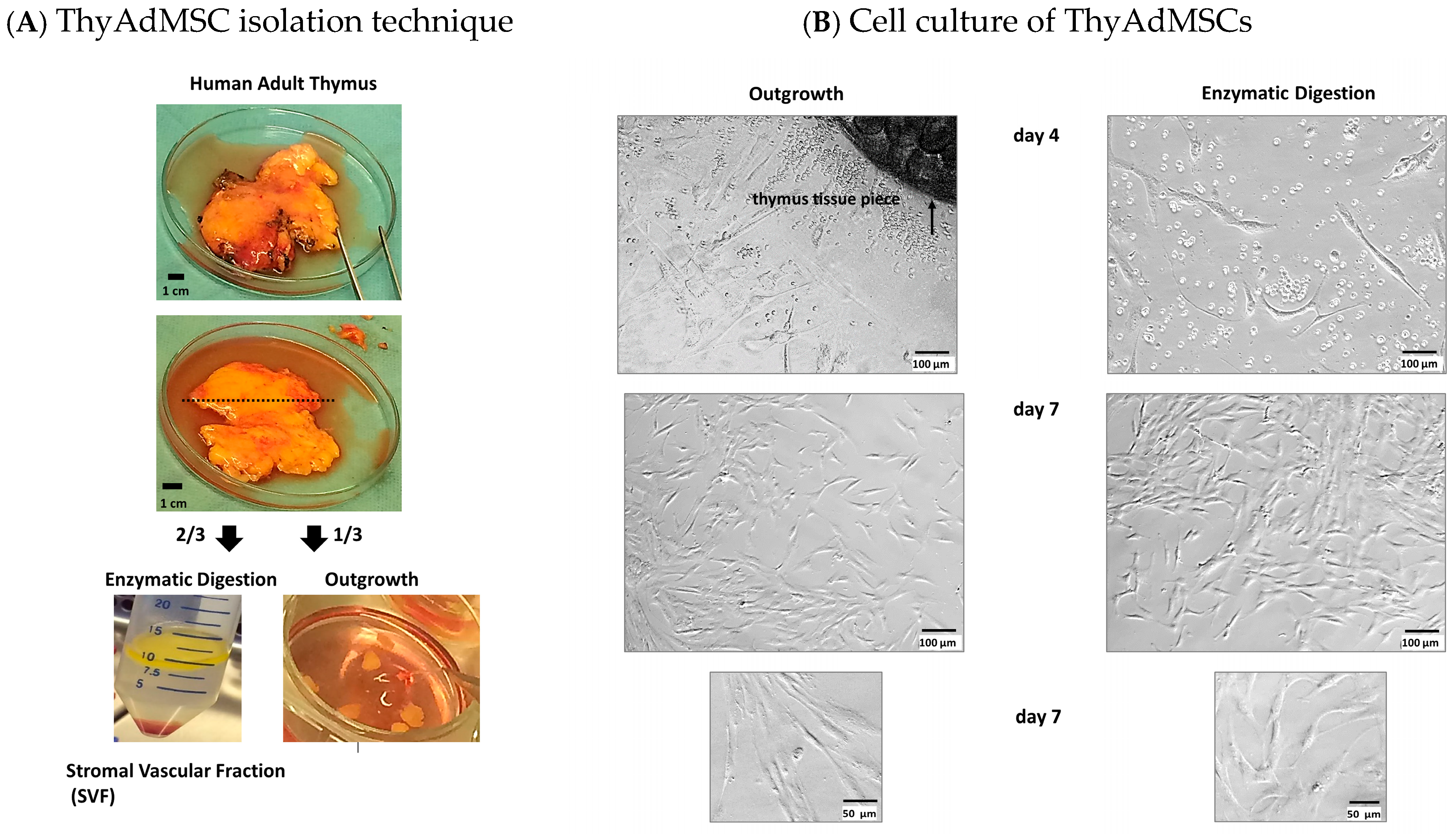
2.2. Isolation of AdMSCs from Adult Thymus
2.3. Animal Component-Free Cell Culture
2.4. Characterisation of AdMSCs from Adult Thymus
2.5. In Vitro Multilineage Differentiation and Histological Verification
2.6. Proliferation Assay
2.7. Mixed Lymphocyte Reaction and Indolamine-2,3-Dioxygenase (IDO) Quantification
2.8. Quantitative Gene Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. Successful Isolation and Cultivation of ThyAdMSCs Under Animal Component-Free Culture Conditions
3.2. Characterization of ThyAdMSCs
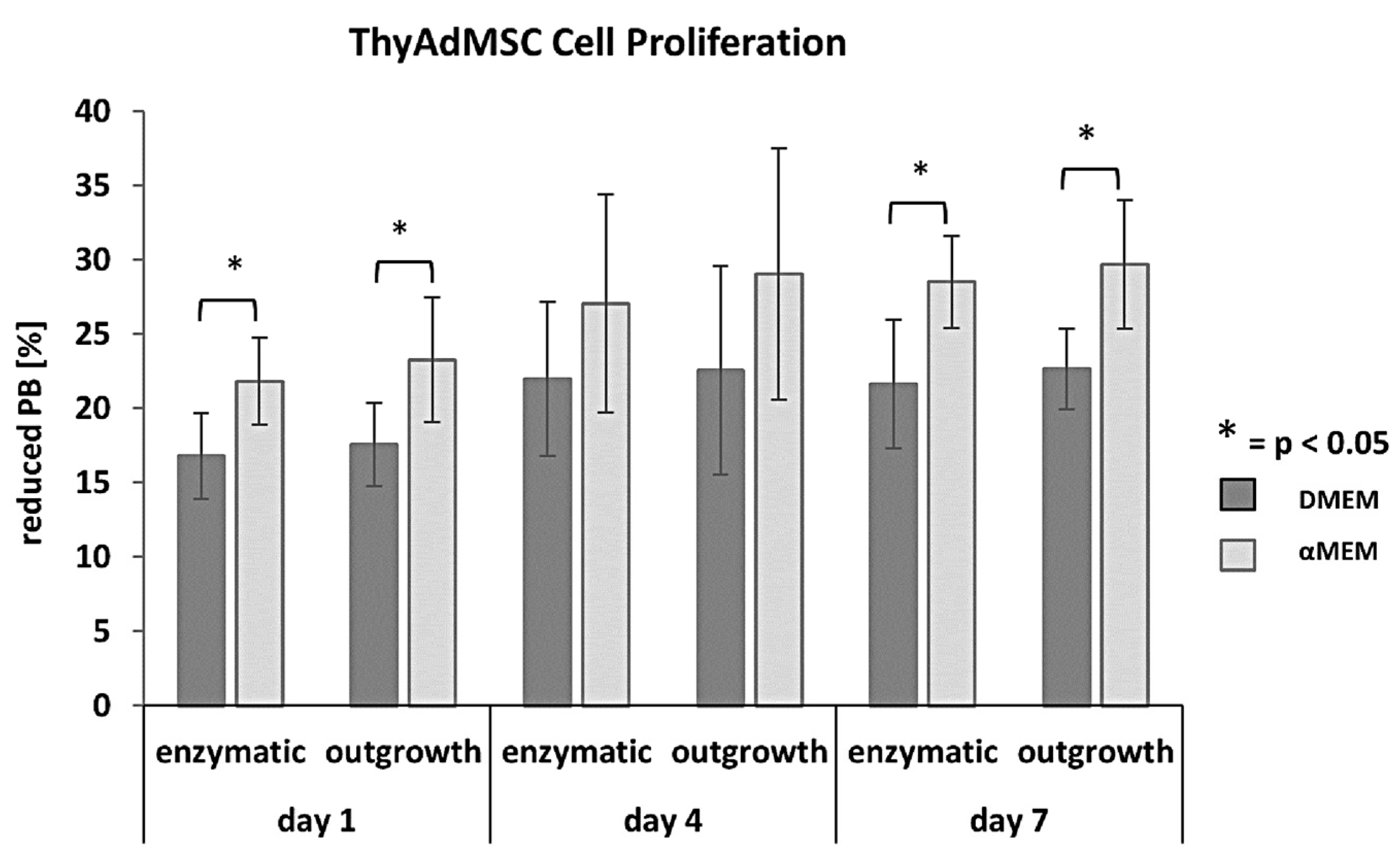
3.3. Proliferation Capacity Depending on the Culture Medium
3.4. ThyAdMSCs Are Capable of Multilineage Differentiation
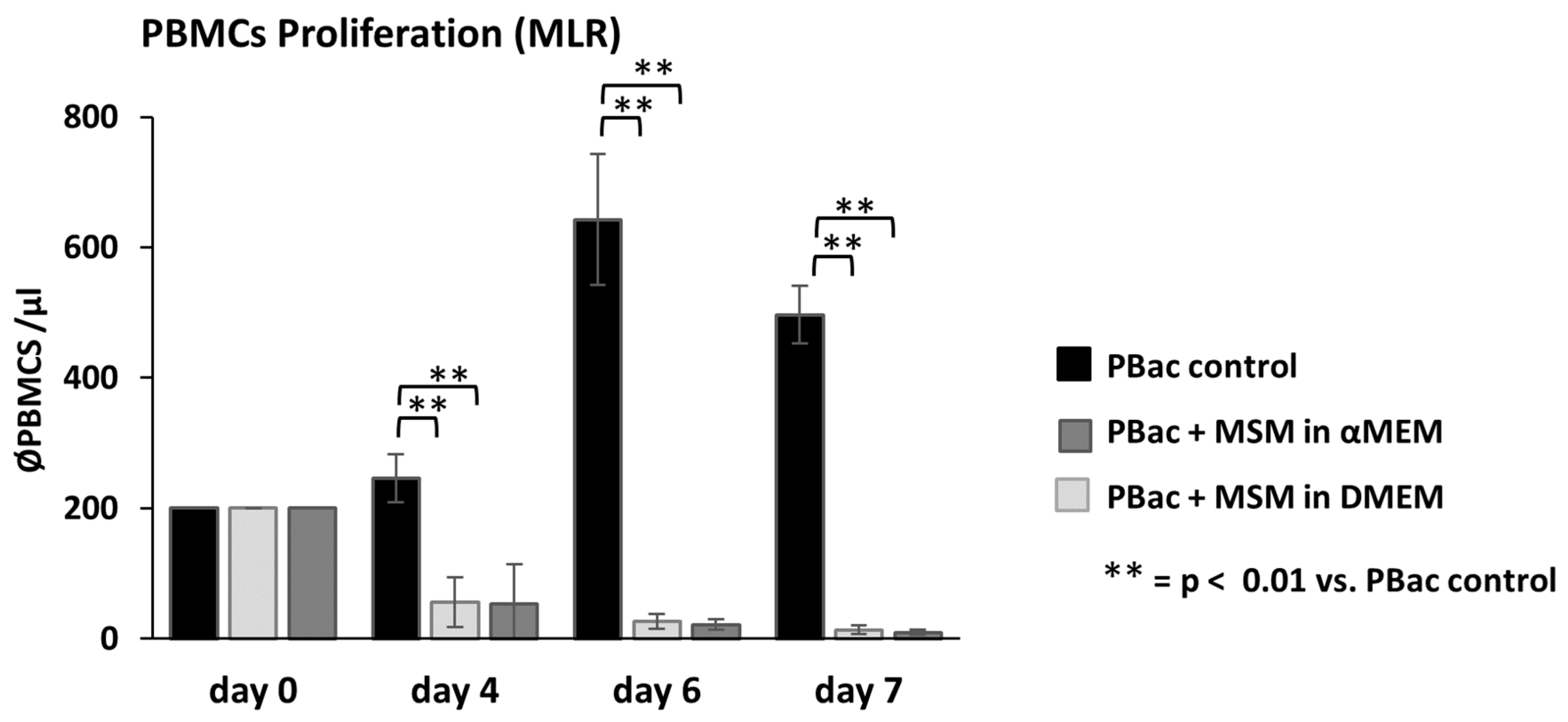
3.5. ThyAdMSCs Inhibit Immune Cell Proliferation in Mixed Lymphocyte Reaction
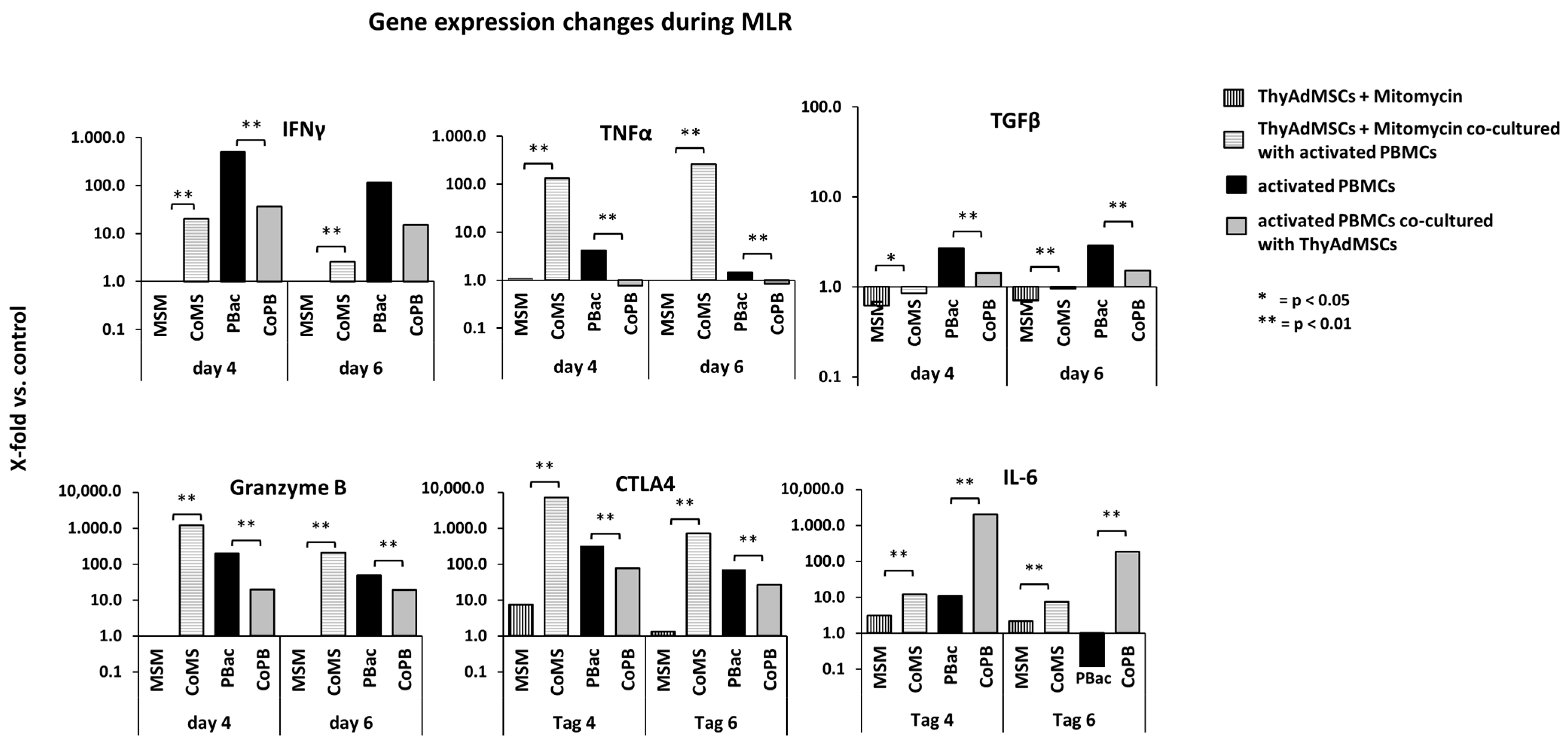
3.6. Altered Gene Expression of Activated PBMCs Co-Cultured with ThyAdMSCs
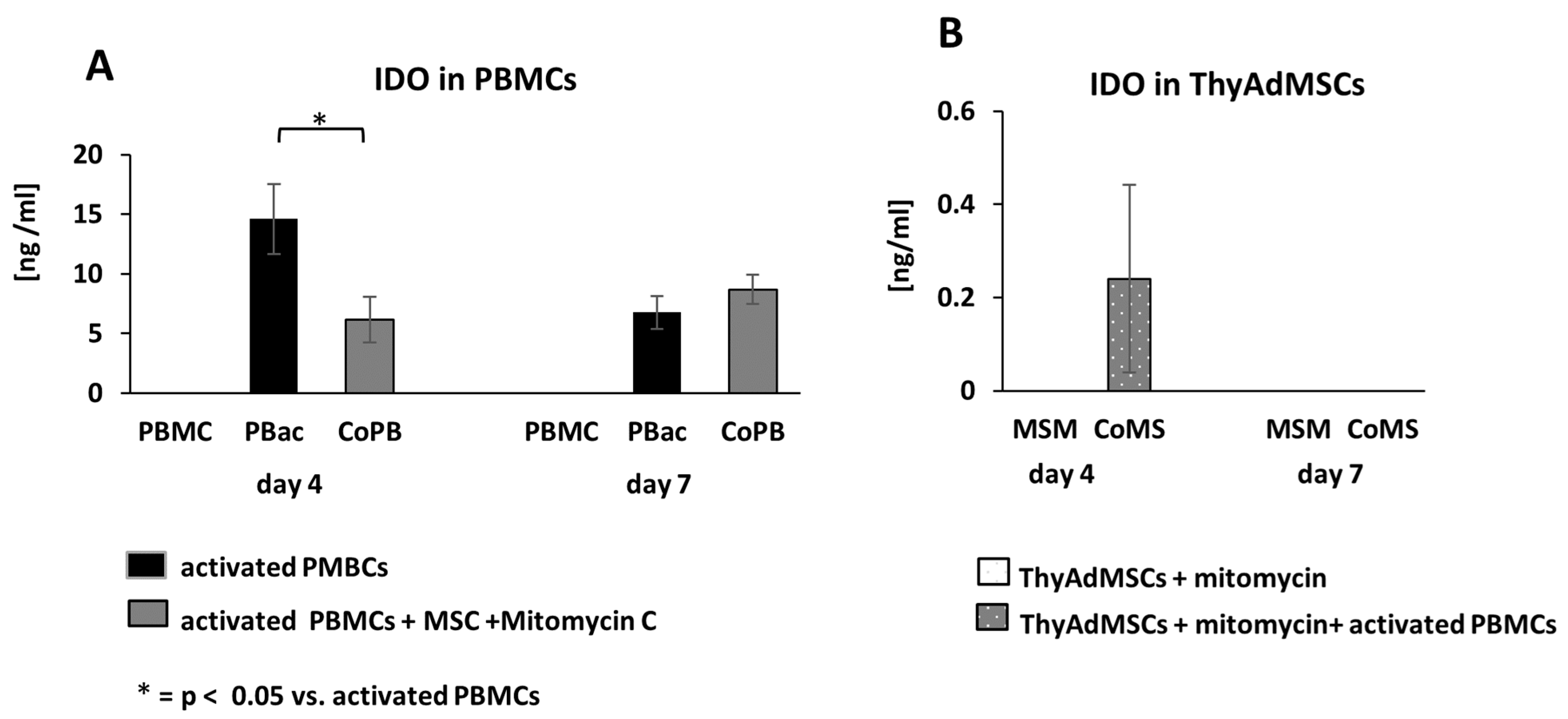
3.7. IDO Activity During MLR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD31 | Platelet endothelial cell adhesion molecule (PECAM-1). |
| CD73 | Ecto-5′-nucleotidase (NT5E). |
| CD90 | Thymocyte differentiation antigen 1 (Thy-1). |
| CD105 | Endoglin (ENG). |
| CD271 | Low affinity nerve growth factor receptor (LNGFR). |
| αMEM | Alpha Minimal Essential Medium. |
| DMEM | Dulbecco’s Modified Eagle’s Medium. |
| IDO | Indolamine-2:3-dioxygenase. |
| pPL | Pooled human platelet lysate. |
| SSEA4 | Stage specific embryonal antigen 4. |
| ThyAdMSC | Thymic adipose-derived mesenchymal stromal cell. |
References
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 628767. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2022, 18, 23–36. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Cotogni, P.; Barbero, C.; Rinaldi, M. Deep sternal wound infection after cardiac surgery: Evidences and controversies. World J. Crit. Care Med. 2015, 4, 265–273. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Nishino, M.; Ashiku, S.K.; Kocher, O.N.; Thurer, R.L.; Boiselle, P.M.; Hatabu, H. The thymus: A comprehensive review. Radiographics 2006, 26, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger-Gleixner, M.; Spriewald, B.M.; Tandler, R.; Kondruweit, M.; Amann, K.; Weyand, M.; Ensminger, S.M. Increased transcript levels of TNF-alpha, TGF-beta, and granzyme B in endomyocardial biopsies correlate with allograft rejection. Exp. Clin. Transpl. 2011, 9, 387–392. [Google Scholar]
- Terness, P.; Bauer, T.M.; Röse, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002, 196, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef]
- Klein, C.; Strobel, J.; Zingsem, J.; Richter, R.H.; Goecke, T.W.; Beckmann, M.W.; Eckstein, R.; Weisbach, V. Ex vivo expansion of hematopoietic stem- and progenitor cells from cord blood in coculture with mesenchymal stroma cells from amnion, chorion, Wharton’s jelly, amniotic fluid, cord blood, and bone marrow. Tissue Eng. Part A 2013, 19, 2577–2585. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Frias, A.M.; Pirraco, R.P.; Rada, T.; Reis, R.L.; Gomes, M.E.; Marques, A.P. Human adipose tissue-derived SSEA-4 subpopulation multi-differentiation potential towards the endothelial and osteogenic lineages. Tissue Eng. Part A 2013, 19, 235–246. [Google Scholar] [CrossRef]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A J. Int. Soc. Anal. Cytol. 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Yoon, J.H.; Roh, E.Y.; Shin, S.; Jung, N.H.; Song, E.Y.; Chang, J.Y.; Kim, B.J.; Jeon, H.W. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. BioMed Res. Int. 2013, 2013, 428726. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, I.; Mishra, R.; Radhakrishnan, H.; Sankaran, R.; Garikipati, V.N.; Marappagounder, D. Human adult stem cells maintain a constant phenotype profile irrespective of their origin, Basal media, and long term cultures. Stem Cells Int. 2015, 2015, 146051. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Fekete, N.; Gadelorge, M.; Furst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailander, V.; Lotfi, R.; Ignatius, A.; Sensebe, L.; et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2012, 14, 540–554. [Google Scholar] [CrossRef]
- Laner-Plamberger, S.; Lener, T.; Schmid, D.; Streif, D.A.; Salzer, T.; Oller, M.; Hauser-Kronberger, C.; Fischer, T.; Jacobs, V.R.; Schallmoser, K.; et al. Mechanical fibrinogen-depletion supports heparin-free mesenchymal stem cell propagation in human platelet lysate. J. Transl. Med. 2015, 13, 354. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Hemeda, H.; Kalz, J.; Walenda, G.; Lohmann, M.; Wagner, W. Heparin concentration is critical for cell culture with human platelet lysate. Cytotherapy 2013, 15, 1174–1181. [Google Scholar] [CrossRef]
- Bianchetti, A.; Chinello, C.; Guindani, M.; Braga, S.; Neva, A.; Verardi, R.; Piovani, G.; Pagani, L.; Lisignoli, G.; Magni, F.; et al. A Blood Bank Standardized Production of Human Platelet Lysate for Mesenchymal Stromal Cell Expansion: Proteomic Characterization and Biological Effects. Front. Cell Dev. Biol. 2021, 9, 650490. [Google Scholar] [CrossRef]
- Laner-Plamberger, S.; Oeller, M.; Poupardin, R.; Krisch, L.; Hochmann, S.; Kalathur, R.; Pachler, K.; Kreutzer, C.; Erdmann, G.; Rohde, E.; et al. Heparin Differentially Impacts Gene Expression of Stromal Cells from Various Tissues. Sci. Rep. 2019, 9, 7258. [Google Scholar] [CrossRef]
- Delabie, W.; De Bleser, D.; Vandewalle, V.; Vandekerckhove, P.; Compernolle, V.; Feys, H.B. Single step method for high yield human platelet lysate production. Transfusion 2023, 63, 373–383. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.L.; Lundy, D.J.; Chuang, E.Y.; Tseng, C.L.; Goubran, H. Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery. J. Biomed. Sci. 2023, 30, 79. [Google Scholar] [CrossRef] [PubMed]
- Cuerquis, J.; Romieu-Mourez, R.; Francois, M.; Routy, J.P.; Young, Y.K.; Zhao, J.; Eliopoulos, N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: Effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy 2014, 16, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Herzig, M.C.; Christy, B.A.; Montgomery, R.K.; Delavan, C.P.; Jensen, K.J.; Lovelace, S.E.; Cantu, C.; Salgado, C.L.; Cap, A.P.; Bynum, J.A. Interactions of human mesenchymal stromal cells with peripheral blood mononuclear cells in a Mitogenic proliferation assay. J. Immunol. Methods 2021, 492, 113000. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Gobel, U.; Daubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef]
- Durand, N.; Zubair, A.C. Autologous versus allogeneic mesenchymal stem cell therapy: The pros and cons. Surgery 2022, 171, 1440–1442. [Google Scholar] [CrossRef]
- Daneste, H.; Mohammadzadeh Boukani, L.; Ramezani, N.; Asadi, F.; Zaidan, H.K.; Sadeghzade, A.; Ehsannia, M.; Azarashk, A.; Gholizadeh, N. Combination therapy along with mesenchymal stem cells in wound healing; the state of the art. Adv. Med. Sci. 2023, 68, 441–449. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Mabotuwana, N.S.; Rech, L.; Lim, J.; Hardy, S.A.; Murtha, L.A.; Rainer, P.P.; Boyle, A.J. Paracrine Factors Released by Stem Cells of Mesenchymal Origin and their Effects in Cardiovascular Disease: A Systematic Review of Pre-clinical Studies. Stem Cell Rev. Rep. 2022, 18, 2606–2628. [Google Scholar] [CrossRef]
- Yan, W.; Xia, Y.; Zhao, H.; Xu, X.; Ma, X.; Tao, L. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J. Mol. Cell. Cardiol. 2024, 188, 1–14. [Google Scholar] [CrossRef]
- Baer, P.C.; Overath, J.M.; Urbschat, A.; Schubert, R.; Koch, B.; Bohn, A.A.; Geiger, H. Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 1719. [Google Scholar] [CrossRef]
- Hermann, M.; Peddi, A.; Gerhards, A.; Schmid, R.; Schmitz, D.; Arkudas, A.; Weisbach, V.; Horch, R.E.; Kengelbach-Weigand, A. Secretome of Adipose-Derived Stem Cells Cultured in Platelet Lysate Improves Migration and Viability of Keratinocytes. Int. J. Mol. Sci. 2023, 24, 3522. [Google Scholar] [CrossRef]


| Name | RRID * | Source |
|---|---|---|
| CD105-PerCP-Cy5.5 | AB_2033933 | BD Bioscience |
| CD90-FITC | AB_395969 | BD Bioscience |
| CD73-APC | AB_10612019 | BD Bioscience |
| CD31-APC-Cy7 | AB_2738350 | BD Bioscience |
| SSEA4-V450 | AB_10896140 | BD Bioscience |
| CD271-PE-Cy7 | AB_10894762 | BD Bioscience |
| Cat # | Source | |
| PE-hMSC negative cocktail | 51-9007661 | BD Bioscience |
| PE-hMSC isotype control negative cocktail | 51-9007662 | BD Bioscience |
| Stain Buffer (FBS) | 554656 | BD Bioscience |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsperger-Gleixner, M.; Li, C.; Wallon, N.; Kuckhahn, A.; Weisbach, V.; Weyand, M.; Heim, C. Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine. J. Clin. Med. 2025, 14, 3474. https://doi.org/10.3390/jcm14103474
Ramsperger-Gleixner M, Li C, Wallon N, Kuckhahn A, Weisbach V, Weyand M, Heim C. Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine. Journal of Clinical Medicine. 2025; 14(10):3474. https://doi.org/10.3390/jcm14103474
Chicago/Turabian StyleRamsperger-Gleixner, Martina, Chang Li, Nina Wallon, Annika Kuckhahn, Volker Weisbach, Michael Weyand, and Christian Heim. 2025. "Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine" Journal of Clinical Medicine 14, no. 10: 3474. https://doi.org/10.3390/jcm14103474
APA StyleRamsperger-Gleixner, M., Li, C., Wallon, N., Kuckhahn, A., Weisbach, V., Weyand, M., & Heim, C. (2025). Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine. Journal of Clinical Medicine, 14(10), 3474. https://doi.org/10.3390/jcm14103474








