When Real-World Outcomes Do Not Meet the Results of Clinical Trials: Transfemoral Transcatheter vs. Surgical Aortic Valve Replacement in an Intermediate-Age Population (The Outstanding Italy Study)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Study Outcomes
2.4. Propensity Score Matching
2.5. Statistical Analysis
3. Results
3.1. Propensity-Score-Matched Cohorts
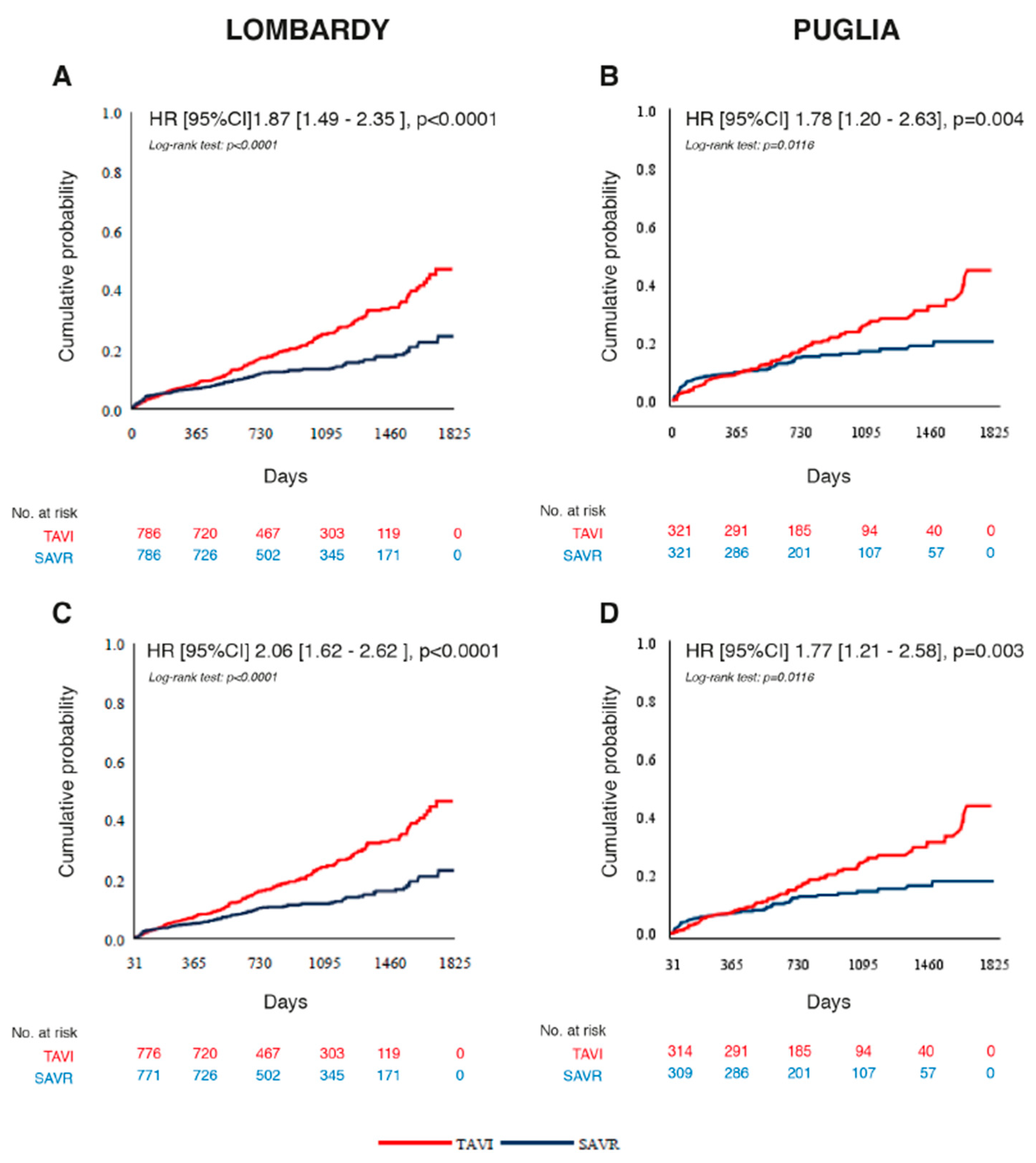
3.2. Primary Outcome in Propensity-Score-Matched Cohorts
3.3. Secondary Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| # | Comorbidities | Patients’ Record From | Classification | ICD-9CM Code |
| 1 | Cerebrovascular disease | Admission | ICD9 | from 430 * to 437 * |
| Admission | Procedures | 3811, 3812 | ||
| ER | ICD9 | from 430 * to 437 * | ||
| 2 | Myocardial infarction | Admission | ICD9 | 410 *, 412 * |
| ER | ICD9 | 410 *, 412 * | ||
| 3 | Other coronary disease | Admission | ICD9 | 413 *, 414 * |
| ER | ICD9 | 413 *, 414 * | ||
| 4 | Heart failure | Admission | ICD9 | 428 *, 39891, 40201, 40211, 40291, 40401, 40403, 40411, 40413, 40491, 40493 |
| ER Admission | ICD9 | 428 *, 39891, 40201, 40211, 40291, 40401, 40403, 40411, 40413, 40491, 40493 | ||
| 5 | Atrial fibrillation | ER Admission | ICD9 | 42731, 42732 |
| ICD9 | 42731, 42732 | |||
| 6 | Percutaneous transluminal coronary angioplasty | Admission | Procedures | from 0040 to 0048, 0066, 3606, 3607, 3609 |
| 7 | Coronary artery by-pass surgery | Admission | Procedures | 361, 362, 363, da 3610 a 3619, da 3631 a 3634, 3639 |
| 8 | Previous aortic valve replacement | Admission | Procedures | 3521, 3522 |
| 9 | Device therapy | Admission | Procedures | 0050, 0051, 0053, 0054, 3762, 3765, 3766, 3767, 3768, 3771, 3774, 3782, 3783, 3785, 3787, 3789, 3794, 3796, 3797, 3798 |
| 10 | Peripheral artery disease | Admission | ICD9 | 2507 *, 4402 *, 4403, 44030, 44032, 44381 |
| 10 11 | Peripheral artery disease Pulmonary embolia | Admission | Procedures ICD9 | 3808, 3818, 3925, 3926, 3929, 3950, 39902507 *, 4402 *, 4403, 44030, 44032, 44381 |
| 10 11 11 12 | Peripheral artery disease Pulmonary embolia Renal disease Pulmonary embolia | ER Admission | ICD9 Procedures | 2507 *, 4402 *, 4403, 44030, 44032, 44381 3808, 3818, 3925, 3926, 3929, 3950, 3990 |
| ER Admission | ICD9 | 4151, 41511, 415192507 *, 4402 *, 4403, 44030, 44032, 44381 | ||
| Admission | ICD9 | 585, V451, V561, V562, V563 *, 581814151, 41511, 41519 | ||
| 12 13 | Renal disease Chronic obstructive pulmonary disease Respiratory insufficiency | Admission | Procedures ICD9 | 3895, 3927, 3942, 3943, 3995, 5498585, V451, V561, V562, V563 *, 58181 |
| 12 13 13 14 | Renal disease Chronic obstructive pulmonary disease Respiratory insufficiency Chronic obstructive pulmonary disease Cancer | ER Admission | ICD9 Procedures | 585, V451, V561, V562, V563 *, 581813895, 3927, 3942, 3943, 3995, 5498 |
| ER Admission | ICD9 | 491 *, 492 *, 493 *, 494 *, 496 *, 51881, 51883, 51884585, V451, V561, V562, V563 *, 58181 | ||
| ER Admission | ICD9 | 491 *, 492 *, 493 *, 494 *, 496 *, 51881, 51883, 51884491 *, 492 *, 493 *, 494 *, 496 *, 51881, 51883, 51884 | ||
| 13 14 14 15 | Chronic obstructive pulmonary disease/Respiratory insufficiency Cancer Cancer Diabetes | Admission ER | ICD9 | from 140 * to 165 *, from 170 * to 208 *, from 210 * to 239 *, 2592491 *, 492 *, 493 *, 494 *, 496 *, 51881, 51883, 51884 |
| ER Admission | ICD9 | from 140 * to 165 *, from 170 * to 208 *, from 210 * to 239 *, 2592, from 140 * to 165 *, from 170 * to 208 *, from 210 * to 239 *, 2592 | ||
| 14 15 15 | Cancer Diabetes Diabetes | Drugs ER | ATC ICD9 | At least 2 A10 * prescriptions on two different dates in the 365 days preceding the index intervention from 140 * to 165 *, from 170 * to 208 *, from 210 * to 239 *, 2592 |
| ExemptionsDrugs | ATC | At least one record with exemption code 013, exemption start date before the index event and exemption end date missing or after the observation startAt least 2 A10 * prescriptions on twodifferent dates in the 365 days preceding the index intervention | ||
| 15 | Diabetes | Exemptions | At least one record with exemption code 013, exemption start date before the index event and exemption end date missing or after the observation start | |
| * Only the main category codes are shown in the table, but all subcategories are included in the selection. | ||||
Appendix B
| Diagnosis | ICD-9CM Code | ||
| Heart failure | 428,x; 398,91; 402,01; 402,11; 402,91; 404,01; 404,03; 404,11; 404,13; 404,91; 404,93 | ||
| Atrial fibrillation | 427,31; 427,32 | ||
| Unstable angina | 411; 411,0; 411,1; 411,8; 411,81; 411,89 | ||
| Myocardial infarction | 410,x; 412 | ||
| Percutaneous transluminal coronary angioplasty Coronary artery by-pass surgery | 36,1,x; 36,15; 36,2; 36,3 36,0,x 00,40 al 00,48; 00,66 | ||
| Stroke [ischemic, haemorragic] | 433,01; 433,11; 433,21; 433,31; 433,81; 433,91; 434,01; 434,91; 430; 431; 432; 432,0; 432,1; 432,9 | ||
| Definitive pacemaker implantation | 00,50; 37,71; 37,72; 37,82; 37,83 | ||
| Renal insufficiency | 585,x; V451; 39,95; 54,98; V561; V562; V563; V563,1; V563,2; 38,95; 39,27; 39,42; 39,43; 581,81 | ||
| Bacterial endocarditis | 421,x | ||
| Aortic dissection | 4410 * | ||
| Senile dementia | 290,2; 290,3 | ||
| Vascular dementia | 290,4; 290,41; 290, 42; 290,43 | ||
| Mediastinitis | 519,2 | ||
| Respiratory failure | 518,81; 518,83; 518,84 | ||
| Gram- endotoxic septic shock | 785,52 | ||
| New valve replacement | 35,21; 35,22 | ||
| Events related to the aortic prosthetic valve | 996,02 | Mechanical complications from cardiac valve prosthesis | |
| 996,61 | Infection and inflammatory reaction from cardiac prostheses, implants and grafts | ||
| 996,71 | Other complications from aortic prosthetic valve | ||
| * Only the main category codes are shown in the table, but all subcategories are included in the selection. | |||
References
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Lee, G.; Chikwe, J.; Milojevic, M.; Wijeysundera, H.C.; Biondi-Zoccai, G.; Flather, M.; Gaudino, M.F.L.; Fremes, S.E.; Tam, D.Y. ESC/EACTS vs. ACC/AHA guidelines for the management of severe aortic stenosis. Eur. Heart J. 2023, 44, 796–812. [Google Scholar] [CrossRef]
- Coisne, A.; Lancellotti, P.; Habib, G.; Garbi, M.; Dahl, J.S.; Barbanti, M.; Vannan, M.A.; Vassiliou, V.S.; Dudek, D.; Chioncel, O.; et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2023, 82, 721–734. [Google Scholar] [CrossRef]
- Inanc, I.H.; Cilingiroglu, M.; Iliescu, C.; Ninios, V.; Matar, F.; Ates, I.; Toutouzas, K.; Hermiller, J.; Marmagkiolis, K. Comparison of American and European Guidelines for the Management of Patients With Valvular Heart Disease. Cardiovasc. Revasc. Med. 2023, 47, 76–85. [Google Scholar] [CrossRef]
- Virtanen, M.P.O.; Eskola, M.; Jalava, M.P.; Husso, A.; Laakso, T.; Niemelä, M.; Ahvenvaara, T.; Tauriainen, T.; Maaranen, P.; Kinnunen, E.-M.; et al. Comparison of Outcomes After Transcatheter Aortic Valve Replacement vs Surgical Aortic Valve Replacement Among Patients With Aortic Stenosis at Low Operative Risk. JAMA Netw. Open 2019, 2, e195742. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Khan, M.R.; Kayani, W.T.; Manan, M.; Munir, A.; Hamzeh, I.; Virani, S.S.; Birnbaum, Y.; Jneid, H.; Alam, M. Comparison of surgical versus transcatheter aortic valve replacement for patients with aortic stenosis at low-intermediate risk. Cardiovasc. Diagn. Ther. 2020, 10, 135–144. [Google Scholar] [CrossRef]
- Ahmad, Y.; Howard, J.P.; Arnold, A.D.; Madhavan, M.V.; Cook, C.M.; Alu, M.; Mack, M.J.; Reardon, M.J.; Thourani, V.H.; Kapadia, S.; et al. Transcatheter versus surgical aortic valve replacement in lower-risk and higher-risk patients: A meta-analysis of randomized trials. Eur. Heart J. 2023, 44, 836–852. [Google Scholar] [CrossRef]
- Tarantini, G.; Fovino, L.N.; D′Errigo, P.; Rosato, S.; Barbanti, M.; Tamburino, C.; Ranucci, M.; Santoro, G.; Badoni, G.; Seccareccia, F.; et al. Factors influencing the choice between transcatheter and surgical treatment of severe aortic stenosis in patients younger than 80 years: Results from the OBSERVANT study. Catheter. Cardiovasc. Interv. 2020, 95, E186–E195. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Andreotti, F.; Kołodziejczak, M.; Wanha, W.; Lauten, A.; Veulemans, V.; Frediani, L.; Kubica, J.; de Cillis, E.; Wojakowski, W.; et al. Age-Related 2-Year Mortality After Transcatheter Aortic Valve Replacement: The YOUNG TAVR Registry. Mayo Clin. Proc. 2019, 94, 1457–1466. [Google Scholar] [CrossRef]
- Robusto, F.; Lepore, V.; D’Ettorre, A.; Lucisano, G.; De Berardis, G.; Bisceglia, L.; Tognoni, G.; Nicolucci, A. The Drug Derived Complexity Index (DDCI) Predicts Mortality, Unplanned Hospitalization and Hospital Readmissions at the Population Level. PLoS ONE 2016, 11, e0149203. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes From the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]
- Thourani, V.H.; Habib, R.; Szeto, W.Y.; Sabik, J.F.; Romano, J.C.; MacGillivray, T.E.; Badhwar, V. Survival After Surgical Aortic Valve Replacement in Low-Risk Patients: A Contemporary Trial Benchmark. Ann. Thorac. Surg. 2024, 117, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Barili, F.; Freemantle, N.; Musumeci, F.; Martin, B.; Anselmi, A.; Rinaldi, M.; Kaul, S.; Rodriguez-Roda, J.; Di Mauro, M.; Folliguet, T.; et al. Five-year outcomes in trials comparing transcatheter aortic valve implantation versus surgical aortic valve replacement: A pooled meta-analysis of reconstructed time-to-event data. Eur. J. Cardiothorac. Surg. 2022, 61, 977–987. [Google Scholar] [CrossRef]
- Cardiac Interventions Today. STS Reports Study Findings of SAVR Versus TAVR in Patients Aged < 60 Years. Available online: https://citoday.com/news/sts-reports-study-findings-of-savr-versus-tavr-in-patients-aged-60-years (accessed on 30 April 2024).
- Auer, J.; Krotka, P.; Reichardt, B.; Traxler, D.; Wendt, R.; Mildner, M.; Ankersmit, H.J.; Graf, A. Selection for transcatheter versus surgical aortic valve replacement and mid-term survival: Results of the AUTHEARTVISIT study. Eur. J. Cardiothorac. Surg. 2024, 66, ezae214. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.-H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef] [PubMed]

| Lombardy | Puglia | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | TAVI n = 1753 | SAVR n = 3537 | p Value | SMD | TAVI n = 540 | SAVR n = 1491 | p Value | SMD |
| Age, mean ± SD | 76.3 ± 3.5 | 73.4 ± 4.3 | <0.0001 | 0.77 | 76.5 ± 3.6 | 73.6 ± 4.2 | <0.0001 | 0.72 |
| Median [Q1–Q3] | 77 (74–79) | 74.0 (70–77) | <0.0001 | 78 (74–79) | 74 (70–78) | <0.0001 | ||
| Min–Max | (65–80) | (65–80) | (65–81) | (65–81) | ||||
| Sex | ||||||||
| Women. n (%) | 815 (46.5) | 1.339 (37.9) | <0.0001 | 0.18 | 295 (54.6) | 591 (39.6) | <0.0001 | 0.08 |
| Men. n (%) | 938 (53.5) | 2.198 (62.1) | 245 (45.4) | 900 (60.4) | ||||
| Two-year period | ||||||||
| 2018–2019. y | 783 (44.7) | 2.083 (58.9) | <0.0001 | −0.29 | 139 (43.3) | 133 (41.4) | <0.0001 | 0.04 |
| 2020–2021. y | 970 (55.3) | 1.454 (41.1) | 182 (56.7) | 188 (58.6) | ||||
| Comorbidities of interest (in previous 5 years), n (%) | ||||||||
| Cerebrovascular disease | 179 (10.2) | 218 (6.2) | <0.0001 | 0.15 | 79 (14.6) | 116 (7.8) | <0.0001 | 0.22 |
| Myocardial infarction | 210 (12.0) | 195 (5.5) | <0.0001 | 0.23 | 67 (12.4) | 96 (6.4) | <0.0001 | −0.21 |
| Other coronary disease | 572 (32.6) | 580 (16.4) | <0.0001 | 0.38 | 189 (35.0) | 257 (17.2) | <0.0001 | 0.41 |
| Heart failure | 570 (32.5) | 525 (14.8) | <0.0001 | 0.43 | 182 (33.7) | 240 (16.1) | <0.0001 | 0.42 |
| Atrial fibrillation | 355 (20.3) | 356 (10.1) | <0.0001 | 0.29 | 130 (24.1) | 195 (13.1) | <0.0001 | 0.29 |
| Percutaneous transluminal coronary angioplasty | 390 (22.2) | 270 (7.6) | <0.0001 | 0.42 | 102 (18.9) | 75 (5.0) | <0.0001 | 0.44 |
| Coronary artery bypass surgery | 29 (1.7) | 8 (0.2) | <0.0001 | 0.15 | 10 (1.8) | 0 (0.0) | <0.0001 | 0.19 |
| Previous aortic valve replacement | 6 (0.3) | 9 (0.3) | 0.59 | 0.02 | 8 (1.5) | 6 (0.4) | 0.009 | 0.11 |
| Device therapy | 144 (8.2) | 124 (3.5) | <0.0001 | 0.20 | 36 (6.7) | 34 (2.3) | <0.0001 | 0.21 |
| Peripheral artery disease | 113 (6.4) | 79 (2.2) | <0.0001 | 0.21 | 39 (7.2) | 40 (2.7) | <0.0001 | 0.21 |
| Pulmonary embolia | 15 (0.9) | 17 (0.5) | 0.0977 | 0.05 | 3 (0.6) | 2 (0.1) | 0.009 | 0.09 |
| Renal disease | 210 (12.0) | 103 (2.9) | <0.0001 | 0.35 | 78 (14.4) | 61 (4.1) | <0.0001 | 0.36 |
| Chronic obstructive pulmonary disease | 262 (14.9) | 203 (5.7) | <0.0001 | 0.31 | 110 (20.4) | 175 (11.7) | <0.0001 | 0.24 |
| Cancer | 250 (14.3) | 309 (8.7) | <0.0001 | 0.17 | 60 (11.1) | 120 (8.0) | 0.03 | 0.11 |
| Diabetes | 665 (37.9) | 841 (23.8) | <0.0001 | 0.31 | 219 (40.6) | 419 (28.1) | <0.0001 | 0.27 |
| Lombardy | Puglia | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | TAVI n = 786 | SAVR n = 786 | p Value | SMD | TAVI n =321 | SAVR n = 321 | p Value | SMD |
| Age, mean±SD | 75.5 ± 3.9) | 75.8 ± 3.5 | 0.37 | 0.09 | 75.9 ± 3.8) | 75.8 ± 3.6) | 0.88 | 0.03 |
| Median [Q1–Q3] | 76 (73–79) | 77 (74–79) | 0.37 | 0.02 | 77 (74–79) | 76.8 (73–79) | 0.88 | |
| Min–Max | (65–80) | (65–80) | −0.07 | (65–81) | (65–81) | |||
| Sex | ||||||||
| Women. n (%) | 346 (44.0) | 368 (46.8) | 0.27 | −0.06 | 166 (51.7) | 153 (47.7) | 0.31 | 0.08 |
| Men. n (%) | 440 (56.0) | 418 (53.2) | 155 (48.3) | 168 (52.3) | ||||
| Two-year period | ||||||||
| 2018–2019. y | 411 (52.3) | 403 (51.3) | 0.69 | 0.02 | 139 (43.3) | 133 (41.4) | 0.63 | 0.04 |
| 2020–2021. y | 375 (47.7) | 383 (48.7) | 182 (56.7) | 188 (58.6) | ||||
| Comorbidities of interest (in previous 5 years), n (%) | ||||||||
| Cerebrovascular disease | 87 (11.1) | 70 (8.9) | 0.15 | 0.07 | 41 (12.8) | 37 (11.5) | 0.63 | 0.04 |
| Myocardial infarction | 91 (11.6) | 74 (9.4) | 0.16 | 0.07 | 30 (9.3) | 34 (10.6) | 0.60 | −0.04 |
| Other coronary disease | 223 (28.4) | 218 (27.7) | 0.78 | 0.01 | 91 (28.3) | 88 (27.4) | 0.79 | 0.00 |
| Heart failure | 219 (27.9) | 204 (26.0) | 0.39 | 0.04 | 86 (26.8) | 94 (29.3) | 0.48 | −0.06 |
| Atrial fibrillation | 135 (17.2) | 147 (18.7) | 0.43 | −0.04 | 71 (22.1) | 69 (21.5) | 0.85 | 0.01 |
| Percutaneous transluminal coronary angioplasty | 141 (17.9) | 129 (16.4) | 0.42 | 0.04 | 40 (12.5) | 38 (11.8) | 0.80 | 0.02 |
| Coronary artery bypass surgery | 10 (1.3) | 6 (0.8) | 0.31 | 0.05 | 0 (0.0) | 0 (0.0) | - | - |
| Previous aortic valve replacement | 4 (0.5) | 3 (0.4) | 1.00 | 0.02 | 3 (0.9) | 2 (0.6) | 0.65 | 0.04 |
| Device therapy | 49 (6.2) | 47 (6.0) | 0.83 | 0.01 | 15 (4.7) | 20 (6.2) | 0.38 | −0.07 |
| Peripheral artery disease | 44 (5.6) | 34 (4.3) | 0.25 | 0.06 | 17 (5.3) | 17 (5.3) | 1.00 | 0.00 |
| Pulmonary embolia | 7 (0.9) | 2 (0.3) | 0.18 | 0.08 | 1 (0.3) | 0 (0.0) | 0.32 | 0.14 |
| Renal disease | 65 (8.3) | 55 (7.0) | 0.34 | 0.05 | 24 (7.5) | 29 (9.0) | 0.47 | −0.05 |
| Chronic obstructive pulmonary disease | 100 (12.7) | 84 (10.7) | 0.21 | 0.06 | 56 (17.4) | 55 (17.1) | 0.92 | 0.01 |
| Cancer | 103 (13.1) | 113 (14.4) | 0.47 | −0.04 | 31 (9.7) | 22 (6.8) | 0.20 | 0.11 |
| Diabetes | 269 (34.2) | 270 (34.4) | 0.96 | −0.003 | 112 (34.9) | 124 (38.6) | 0.33 | −0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranucci, M.; Staszewsky, L.; Cartabia, M.; Tettamanti, M.; Lepore, V.; Robusto, F.; Clavenna, A.; D’Ettorre, A.; Arbustini, E.; Baldassarre, D.; et al. When Real-World Outcomes Do Not Meet the Results of Clinical Trials: Transfemoral Transcatheter vs. Surgical Aortic Valve Replacement in an Intermediate-Age Population (The Outstanding Italy Study). J. Clin. Med. 2025, 14, 3471. https://doi.org/10.3390/jcm14103471
Ranucci M, Staszewsky L, Cartabia M, Tettamanti M, Lepore V, Robusto F, Clavenna A, D’Ettorre A, Arbustini E, Baldassarre D, et al. When Real-World Outcomes Do Not Meet the Results of Clinical Trials: Transfemoral Transcatheter vs. Surgical Aortic Valve Replacement in an Intermediate-Age Population (The Outstanding Italy Study). Journal of Clinical Medicine. 2025; 14(10):3471. https://doi.org/10.3390/jcm14103471
Chicago/Turabian StyleRanucci, Marco, Lidia Staszewsky, Massimo Cartabia, Mauro Tettamanti, Vito Lepore, Fabio Robusto, Antonio Clavenna, Antonio D’Ettorre, Eloisa Arbustini, Damiano Baldassarre, and et al. 2025. "When Real-World Outcomes Do Not Meet the Results of Clinical Trials: Transfemoral Transcatheter vs. Surgical Aortic Valve Replacement in an Intermediate-Age Population (The Outstanding Italy Study)" Journal of Clinical Medicine 14, no. 10: 3471. https://doi.org/10.3390/jcm14103471
APA StyleRanucci, M., Staszewsky, L., Cartabia, M., Tettamanti, M., Lepore, V., Robusto, F., Clavenna, A., D’Ettorre, A., Arbustini, E., Baldassarre, D., La Rovere, M. T., Montorfano, M., Parati, G., Pedretti, R. F. E., Raffa, G. M., Santini, F., Stefanini, G., Volterrani, M., Fortino, I., ... Latini, R. (2025). When Real-World Outcomes Do Not Meet the Results of Clinical Trials: Transfemoral Transcatheter vs. Surgical Aortic Valve Replacement in an Intermediate-Age Population (The Outstanding Italy Study). Journal of Clinical Medicine, 14(10), 3471. https://doi.org/10.3390/jcm14103471










