Porcine Peripheral Blood Mononuclear Cells (PBMCs): Methods of Isolation, Cryopreservation, and Translational Applications in Human Studies
Abstract
1. Introduction
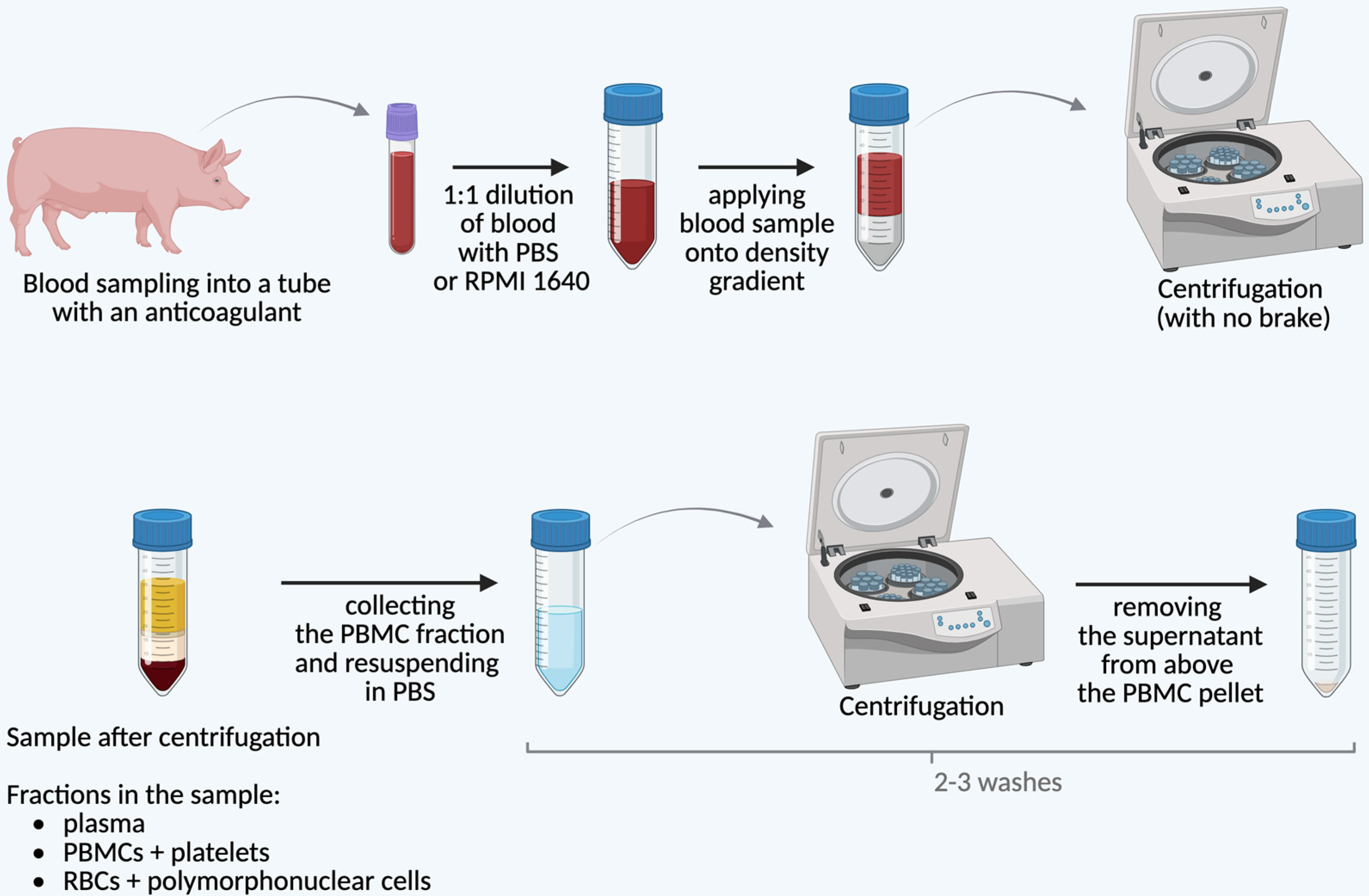
2. Isolation of pPBMCs
3. Cryopreservation of pPBMCs
3.1. Preparation for Cryopreservation
3.2. Control of the Freezing Rate
3.3. Thawing Process
4. Key Differences Between Human and Porcine PBMCs: Immunological Characteristics and Methodological Considerations
4.1. Immunological and Phenotypic Differences
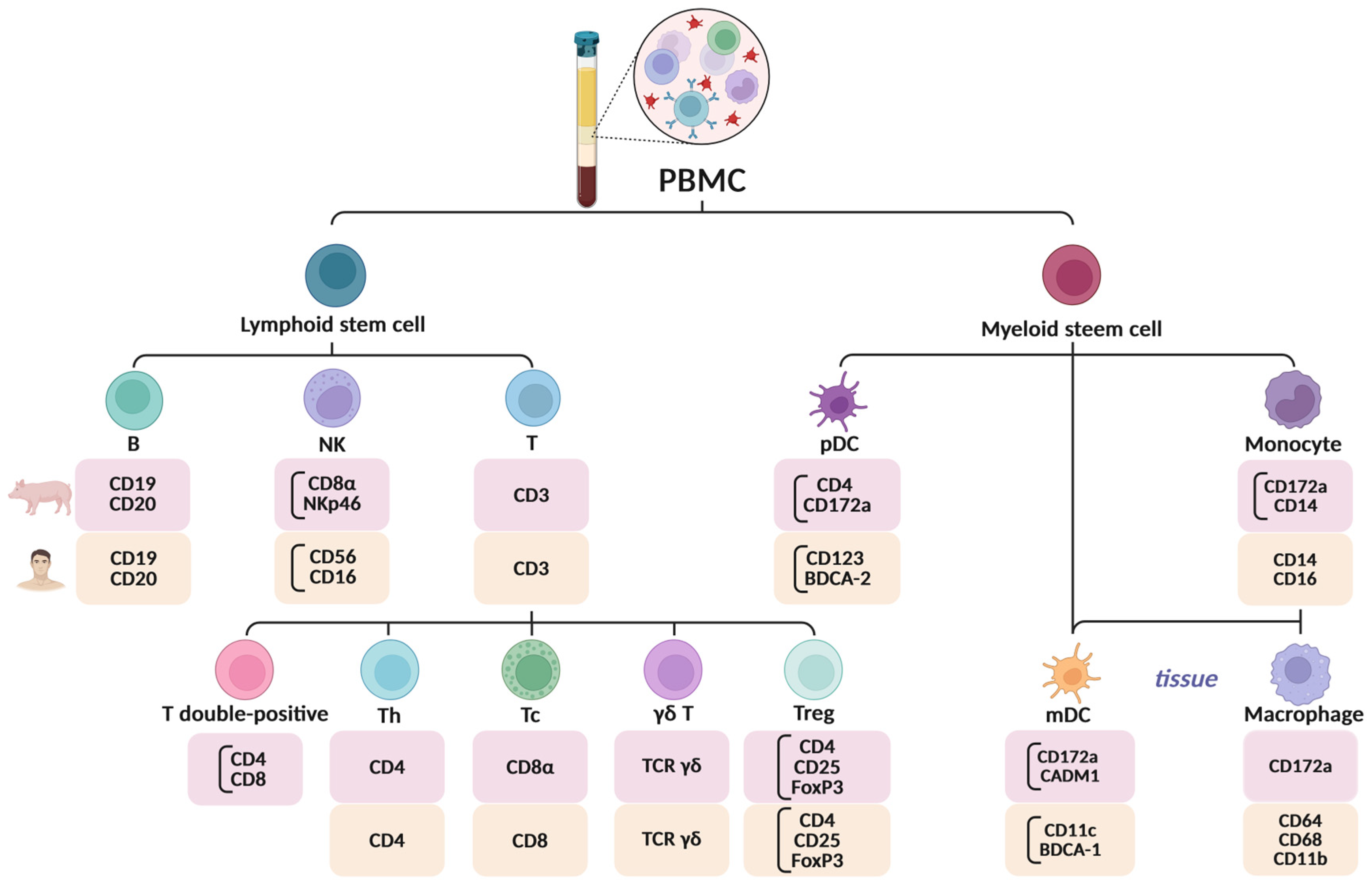
4.2. Methodological Considerations in pPBMC Isolation and Handling
| Species | Ref. | Anti- Coagulant | Ratio and Diluent | Density Gradient | Cell Separation Parameters | Number of Washes and Washing Solution | Washing Parameters |
|---|---|---|---|---|---|---|---|
| swine | [61] | EDTA | 1:2 PBS | Histopaque®-1077 | 400× g 30 min; RT | 2 washes, PBS | 250× g 10 min; RT |
| [62] | heparin | 1:1 RPMI 1640 | Histopaque®-1077 | 1100× g 25 min; RT | 2 washes, n.p. | n.p. | |
| [63] | heparin | n.p. | BD Vacutainer® CPT™ | 500× g 30 min | 3 washes, PBS | n.p. | |
| [64] | heparin | 1:2 RPMI 1640 | Histopaque®-1077 | 1100× g 25 min | 2 washes, RPMI 1640 | n.p. | |
| [65] | n.p. | 1:1 PBS | Histopaque®-1077 | 2200 rpm RT | 3 washes, PBS | 2500 rpm 15 min; 4 °C | |
| [66] | heparin | n.p. | BD Vacutainer® CPT™ | 1500× g 30 min; RT | 2 washes, PBS | 700× g 10 min | |
| [67] | EDTA | 1:1 PBS + 20% CD + 2% FBS | SepMate™-50 tube with Lymphoprep™ | 1100× g 10 min | until PLT removal, PBS + 20% ACD + 2% FBS | n.p. | |
| [68] | EDTA | 1:1 PBS | Ficoll-Paque™ | 400× g 30 min; 25 °C | 3 washes, n.p. | 1. 300× g 10 min; 4 °C; 2. 250× g 10 min; 4 °C | |
| [69] | heparin | 1:1 HBSS | Ficoll-Hypaque | 472× g 30 min; RT | 1 wash, HBSS | n.p. | |
| [70] | heparin | 2:1 PBS | Ficoll-Paque™ PLUS | 1455× g 30 min; RT | 3 washes, PBS | 930× g 5 min; 4 °C | |
| human | [71] | n.p. | 1:1 HBSS | Ficoll-Hypaque | 2000 r/min 20 min | 2 washes, HBSS | n.p. |
| [72] | EDTA | 1:1 PBS | Ficoll-Paque™ PLUS | 400× g 30 min; 20 °C | 1 wash, PBS | 300× g 10 min; 20 °C | |
| [73] | EDTA | 1:1 PBS | LSM™ | 400× g 30 min | 2 washes, PBS | n.p. | |
| [74] | heparin | n.p. | Histopaque®-1077 | 2000 r/min 20 min; 4 °C | 2 washes, PBS | 1500 r/min 10 min; 4 °C | |
| [75] | n.p. | 1:1 PBS + 2% FBS | Lymphoprep™ | 800× g 20 min; RT | n.p. | n.p. | |
| [76] | n.p. | PBS | Lympholyte® | 1500 r/min 30 min; RT | n.p. PBS | n.p. | |
| [77] | EDTA | 1:1 PBS | Histopaque®-1077 | 800× g 30 min; 22 °C | 1 wash, PBS | 300× g 3 min; 22 °C | |
| [78] | EDTA | 1:1 PBS | Ficoll-Paque™ PLUS | 400× g 30 min; 25 °C | 2 washes, PBS | 300× g 10 min; 25 °C | |
| [79] | EDTA | 1:1 PBS + 2% FBS | Lymphoprep™ | 800× g 20 min; RT | until the supernatant is clear, PBS | n.p. | |
| [80] | sodium citrate | 1:1 PBS | Lymphoprep™ | 1. 160× g 20 min; RT; 2. 800× g 20 min | 3 washes, RPMI | n.p. |
5. Utilization of Porcine PBMCs
5.1. Immunological and Translational Applications of pPBMCs
5.2. Pathogen-Specific Infectious Disease Research Using pPBMCs
5.3. Xenotransplantation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-Gal | alpha-galactosidase |
| γδ T | gamma–delta T cell |
| ASFV | African Swine Fever Virus |
| B | B lymphocyte |
| CD | cluster of differentiation |
| DC | dendritic cell |
| DEX | dexamethasone |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediaminetetraacetic acid |
| ELISpot | enzyme-linked ImmunoSpot |
| FBS | fetal bovine serum |
| GGTA1 | gene encoding alpha1,3-galactosyltransferase |
| HANC-SOP | HIV/AIDS Network Coordination standard operating procedures |
| HBSS | Hank’s balanced salt solution |
| hPBMC | human peripheral blood mononuclear cell |
| IFN-γ | interferon gamma |
| IL | interleukin |
| LN2 | liquid nitrogen |
| LPS | lipopolysaccharide |
| mRNA | messenger RNA |
| MHC | major histocompatibility complex class |
| NK | natural killer cell |
| NKp | natural killer protein |
| n.p. | not provided |
| PBMC | peripheral blood mononuclear cell |
| PBS | phosphate-buffered saline |
| PCV2 | porcine circovirus type 2 |
| PolyI:C | polyinosinic:polycytidylic acid |
| pPBMC | porcine peripheral blood mononuclear cell |
| PRRSV | porcine reproductive and respiratory syndrome virus |
| RBC | red blood cell |
| RPMI 1640 | Roswell Park Memorial Institute 1640 (cell culture medium) |
| RT | room temperature |
| Tc | cytotoxic T cell |
| Th | T helper cell |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| Treg | regulatory T cells |
References
- Gerner, W.; Käser, T.; Saalmüller, A. Porcine T lymphocytes and NK cells—An update. Dev. Comp. Immunol. 2009, 33, 310–320. [Google Scholar] [CrossRef]
- Cunha, P.; Gilbert, F.B.; Bodin, J.; Godry, L.; Germon, P.; Holbert, S.; Martins, R.P. Simplified Approaches for the Production of Monocyte-Derived Dendritic Cells and Study of Antigen Presentation in Bovine. Front. Vet. Sci. 2022, 9, 891893. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska-Durczynska, K.; Wieczorek-Szukala, K.; Stefanski, B.; Zygmunt, A.; Stepniak, J.; Karbownik-Lewinska, M.; Lewinski, A. Percentage of Myeloid Dendritic Cells in Peripheral Venous Blood Is Negatively Related to Incidence of Graves’ Orbitopathy. Mediat. Inflamm. 2021, 2021, 8896055. [Google Scholar] [CrossRef]
- Larsson, A.M.; Nordström, O.; Johansson, A.; Rydén, L.; Leandersson, K.; Bergenfelz, C. Peripheral Blood Mononuclear Cell Populations Correlate with Outcome in Patients with Metastatic Breast Cancer. Cells 2022, 11, 1639. [Google Scholar] [CrossRef] [PubMed]
- Le Page, L.; Baldwin, C.L.; Telfer, J.C. γδ T cells in artiodactyls: Focus on swine. Dev. Comp. Immunol. 2022, 128, 104334. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Mair, K.H.; Sedlak, C.; Käser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The porcine innate immune system: An update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef]
- Sipos, W. Shifts in porcine PBMC populations from adolescence to adulthood. Vet. Immunol. Immunopathol. 2019, 211, 35–37. [Google Scholar] [CrossRef]
- Mattoo Sul, S.; Aganja, R.P.; Kim, S.C.; Jeong, C.G.; Nazki, S.; Khatun, A.; Kim, W.I.; Lee, S.M. A standardized method to study immune responses using porcine whole blood. J. Vet. Sci. 2023, 24, 1–14. [Google Scholar] [CrossRef]
- Overgaard, N.H.; Frøsig, T.M.; Welner, S.; Rasmussen, M.; Ilsøe, M.; Sørensen, M.R.; Andersen, M.H.; Buus, S.; Jungersen, G. Establishing the pig as a large animal model for vaccine development against human cancer. Front. Genet. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I. Rules of thumb to obtain, isolate, and preserve porcine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2022, 251, 110461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mateu, E.; Díaz, I. Impact of Cryopreservation on Viability, Phenotype, and Functionality of Porcine PBMC. Front. Immunol. 2021, 12, 765667. [Google Scholar] [CrossRef]
- Cao, Q.M.; Ni, Y.Y.; Cao, D.; Tian, D.; Yugo, D.M.; Heffron, C.L.; Overend, C. Recombinant Porcine Reproductive and Respiratory Syndrome Virus Expressing Membrane-Bound Interleukin-15 as an Immunomodulatory Adjuvant Enhances NK and γδ T Cell Responses and Confers Heterologous Protection. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Ma, F.; Ding, N.; Mi, S.; Zhao, Q.; Xing, Y.; Yang, T.; Xing, K.; Yu, Y.; et al. Pathogen stimulations and immune cells synergistically affect the gene expression profile characteristics of porcine peripheral blood mononuclear cells. BMC Genom. 2024, 25, 719. [Google Scholar] [CrossRef]
- Mu, S.; Chen, L.; Dong, H.; Li, S.; Zhang, Y.; Yin, S.; Tian, Y.; Ding, Y.; Sun, S.; Shang, S.; et al. Enhanced antigen-specific CD8 T cells contribute to early protection against FMDV through swine DC vaccination. J. Virol. 2024, 98, e02002-23. [Google Scholar] [CrossRef]
- Hernandez-Franco, J.F.; Xie, S.; Thimmapuram, J.; Ragland, D.; HogenEsch, H. Mechanism of activation of porcine dendritic cells by an α-D-glucan nanoparticle adjuvant and a nanoparticle/poly(I:C) combination adjuvant. Front. Immunol. 2022, 13, 990900. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; O’Brien, T.D.; Chattha, K.S.; Saif, L.J. Porcine lung mesenchymal stromal cells possess differentiation and immunoregulatory properties. Stem Cell Res. Ther. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Kapetanovic, R.; Fairbairn, L.; Beraldi, D.; Sester, D.P.; Archibald, A.L.; Tuggle, C.K.; Hume, D.A. Pig Bone Marrow-Derived Macrophages Resemble Human Macrophages in Their Response to Bacterial Lipopolysaccharide. J. Immunol. 2012, 188, 3382–3394. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Sipos, W.; Hartl, R.T.; Bayer, M.; Moldzio, R.; Stevenson, L.; Adair, B.; Gemeiner, M. Heparin and EDTA as anticoagulant differentially affect cytokine mRNA level of cultured porcine blood cells. J. Immunol. Methods 2007, 324, 38–47. [Google Scholar] [CrossRef]
- Talker, S.C.; Käser, T.; Reutner, K.; Sedlak, C.; Mair, K.H.; Koinig, H.; Graage, R.; Viehmann, M.; Klingler, E.; Ladinig, A.; et al. Phenotypic maturation of porcine NK- and T-cell subsets. Dev. Comp. Immunol. 2013, 40, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.; Wilker, S.; Roider, J.; Klettner, A. Isolation of porcine monocyte population: A simple and efficient method. Vet. Res. Commun. 2013, 37, 239–241. [Google Scholar] [CrossRef]
- Stabel, J.R.; Wherry, T.L.T. Comparison of methods to isolate peripheral blood mononuclear cells from cattle blood. J. Immunol. Methods 2023, 512, 113407. [Google Scholar] [CrossRef]
- Grievink, H.W.; Luisman, T.; Kluft, C.; Moerland, M.; Malone, K.E. Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv. Biobank. 2016, 14, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Ruitenberg, J.J.; Mulder, C.B.; Maino, V.C.; Landay, A.L.; Ghanekar, S.A. VACUTAINER® CPTTM and Ficoll density gradient separation perform equivalently in maintaining the quality and function of PBMC from HIV seropositive blood samples. BMC Immunol. 2006, 7, 11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaiser, D.; Otto, N.M.; McCallion, O.; Hoffmann, H.; Zarrinrad, G.; Stein, M.; Beier, C.; Matz, I.; Herschel, M.; Hester, J.; et al. Freezing Medium Containing 5% DMSO Enhances the Cell Viability and Recovery Rate After Cryopreservation of Regulatory T Cell Products ex vivo and in vivo. Front. Cell Dev. Biol. 2021, 9, 750286. [Google Scholar] [CrossRef]
- Betsou, F.; Gaignaux, A.; Ammerlaan, W.; Norris, P.J.; Stone, M. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Curr. Pathobiol. Rep. 2019, 7, 17–27. [Google Scholar] [CrossRef]
- Browne, D.J.; Miller, C.M.; Doolan, D.L. Technical pitfalls when collecting, cryopreserving, thawing, and stimulating human T-cells. Front. Immunol. 2024, 15, 1382192. [Google Scholar] [CrossRef]
- Hope, C.M.; Huynh, D.; Wong, Y.Y.; Oakey, H.; Perkins, G.B.; Nguyen, T.; Binkowski, S.; Bui, M.; Choo, A.Y.L.; Gibson, E.; et al. Optimization of blood handling and peripheral blood mononuclear cell cryopreservation of low cell number samples. Int. J. Mol. Sci. 2021, 22, 9129. [Google Scholar] [CrossRef]
- Weinberg, A.; Song, L.Y.; Wilkening, C.L.; Fenton, T.; Hural, J.; Louzao, R.; Ferrari, G.; Etter, P.E.; Berrong, M.; Canniff, J.D.; et al. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J. Immunol. Methods 2010, 363, 42–50. [Google Scholar] [CrossRef]
- Cross-Network PBMC Processing SOP. Available online: https://www.hanc.info/resources/sops-guidelines-resources/laboratory/cross-network-pbmc-processing-sop.html (accessed on 10 May 2025).
- Hønge, B.L.; Petersen, M.S.; Olesen, R.; Møller, B.K.; Erikstrup, C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS ONE 2017, 12, e0187440. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Song, L.Y.; Wilkening, C.; Sevin, A.; Blais, B.; Louzao, R.; Stein, D. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. CVI 2009, 16, 1176–1186. [Google Scholar] [CrossRef]
- Yang, J.; Diaz, N.; Adelsberger, J.; Zhou, X.; Stevens, R.; Rupert, A.; Metcalf, J.A.; Baseler, M.; Barbon, C.; Imamichi, T.; et al. The effects of storage temperature on PBMC gene expression. BMC Immunol. 2016, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Larak, M.; Ellendorff, F. Comparative studies on in vitro reactivity of fresh and cryopreserved pig lymphocytes. Cryobiology 1991, 28, 405–412. [Google Scholar] [CrossRef]
- Cryopreserved PBMCs Retain Phenotype and Function. Available online: https://www.sigmaaldrich.com/PL/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/primary-cell-culture/cryopreserved-pbmcs-retain-phenotype-and-function (accessed on 10 May 2025).
- Thawing Cells—Cell Culture Protocols. Available online: https://www.thermofisher.com/pl/en/home/references/gibco-cell-culture-basics/cell-culture-protocols/thawing-cells.html (accessed on 10 May 2025).
- New CD Molecules. Available online: https://www.hcdm.org/index.php/hlda10-workshop/new-cd-molecules (accessed on 10 May 2025).
- Dawson, H.D.; Lunney, J.K. Porcine cluster of differentiation (CD) markers 2018 update. Res. Vet. Sci. 2018, 118, 199–246. [Google Scholar] [CrossRef]
- Corbett, R.J.; Luttman, A.M.; Herrera-Uribe, J.; Liu, H.; Raney, N.E.; Grabowski, J.M.; Loving, C.L.; Tuggle, C.K.; Ernst, C.W. Assessment of DNA methylation in porcine immune cells reveals novel regulatory elements associated with cell-specific gene expression and immune capacity traits. BMC Genom. 2022, 23, 575. [Google Scholar] [CrossRef]
- Loving, C.L.; Wiarda, J.E.; Sivasankaran, S.K.; Daharsh, L.; Liu, H.; Byrne, K.A.; Tuggle, C.K. Characterization of circulating porcine immune cells using single-cell RNA-sequencing and comparison to human datasets. J. Immunol. 2021, 206, 19.17. [Google Scholar] [CrossRef]
- Pernold, C.P.S.; Lagumdzic, E.; Stadler, M.; Dolezal, M.; Jäckel, S.; Schmitt, M.W.; Mair, K.H.; Saalmüller, A. Species comparison: Human and minipig PBMC reactivity under the influence of immunomodulating compounds in vitro. Front. Immunol. 2024, 14, 1327776. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.H.; Crossman, A.J.; Wagner, B.; Babasyan, S.; Noronha, L.; Boyd, P.; Zarlenga, D.; Stadler, M.; van Dongen, K.A.; Gerner, W.; et al. The Natural Cytotoxicity Receptor NKp44 (NCR2, CD336) Is Expressed on the Majority of Porcine NK Cells Ex Vivo Without Stimulation. Front. Immunol. 2022, 13, 767530. [Google Scholar] [CrossRef]
- Mair, K.H.; Essler, S.E.; Patzl, M.; Storset, A.K.; Saalmüller, A.; Gerner, W. NKp46 expression discriminates porcine NK cells with different functional properties. Eur. J. Immunol. 2012, 42, 1261–1271. [Google Scholar] [CrossRef]
- Fairbairn, L.; Kapetanovic, R.; Beraldi, D.; Sester, D.P.; Tuggle, C.K.; Archibald, A.L.; Hume, D.A. Comparative Analysis of Monocyte Subsets in the Pig. J. Immunol. 2013, 190, 6389–6396. [Google Scholar] [CrossRef] [PubMed]
- Yancy, H.; Ayers, S.L.; Farrell, D.E.; Day, A.; Myers, M.J. Differential cytokine mRNA expression in swine whole blood and peripheral blood mononuclear cell cultures. Vet. Immunol. Immunopathol. 2001, 79, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Trakooljul, N.; Hadlich, F.; Ponsuksili, S.; Wimmers, K.; Murani, E. Transcriptome analysis of porcine PBMCs reveals lipopolysaccharide-induced immunomodulatory responses and crosstalk of immune and glucocorticoid receptor signaling. Virulence 2021, 12, 1808–1824. [Google Scholar] [CrossRef]
- Herrera-Uribe, J.; Wiarda, J.E.; Sivasankaran, S.K.; Daharsh, L.; Liu, H.; Byrne, K.A.; Smith, T.P.L.; Lunney, J.K.; Loving, C.L.; Tuggle, C.K. Reference Transcriptomes of Porcine Peripheral Immune Cells Created Through Bulk and Single-Cell RNA Sequencing. Front. Genet. 2021, 12, 689406. [Google Scholar] [CrossRef] [PubMed]
- Tedder, T.F. CD19: A promising B cell target for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 572–577. [Google Scholar] [CrossRef]
- Mycko, M.P. The mechanism of action of anti-CD20 monoclonal antibodies used in the treatment of multiple sclerosis. Curr. Neurol. 2023, 23, 72–78. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Ebner, F.; Schwiertz, P.; Steinfelder, S.; Pieper, R.; Zentek, J.; Schütze, N.; Baums, C.G.; Alber, G.; Geldhof, P.; Hartmann, S. Pathogen-Reactive T Helper Cell Analysis in the Pig. Front. Immunol. 2017, 8, 565. [Google Scholar] [CrossRef]
- Hoog, A.; Villanueva-Hernández, S.; Razavi, M.A.; van Dongen, K.; Eder, T.; Piney, L.; Chapat, L.; de Luca, K.; Grebien, F.; Mair, K.H.; et al. Identification of CD4+ T cells with T follicular helper cell characteristics in the pig. Dev. Comp. Immunol. 2022, 134, 104462. [Google Scholar] [CrossRef]
- Käser, T.; Gerner, W.; Hammer, S.E.; Patzl, M.; Saalmüller, A. Phenotypic and functional characterisation of porcine CD4+CD25high regulatory T cells. Vet. Immunol. Immunopathol. 2008, 122, 153–158. [Google Scholar] [CrossRef]
- Auray, G.; Keller, I.; Python, S.; Gerber, M.; Bruggmann, R.; Ruggli, N.; Summerfield, A. Characterization and Transcriptomic Analysis of Porcine Blood Conventional and Plasmacytoid Dendritic Cells Reveals Striking Species-Specific Differences. J. Immunol. 2016, 197, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Li, Z.; Xie, Q.; Song, W. Phenotypic and functional differences of dendritic cells in tumor. J. Cancer Res. Ther. 2023, 19, 1509–1516. [Google Scholar] [CrossRef]
- Summerfield, A.; McCullough, K.C. The porcine dendritic cell family. Dev. Comp. Immunol. 2009, 33, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Porcine Antibodies—Innate Immunity. Available online: https://www.bio-rad-antibodies.com/porcine-antibodies-innate-immunity.html (accessed on 10 May 2025).
- Mallone, R.; Mannering, S.I.; Brooks-Worrell, B.M.; Durinovic-Belló, I.; Cilio, C.M.; Wong, F.S.; Schloot, N.C. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: Position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin. Exp. Immunol. 2010, 163, 33–49. [Google Scholar] [CrossRef]
- Scheible, K.; Secor-Socha, S.; Wightman, T.; Wang, H.; Mariani, T.J.; Topham, D.J.; Pryhuber, G.; Quataert, S. Stability of T cell phenotype and functional assays following heparinized umbilical cord blood collection. Cytom. Part A 2012, 81, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Dozois, C.M.; Oswald, E.; Gautier, N.; Serthelon, J.P.; Fairbrother, J.M.; Oswald, I.P. A reverse transcription-polymerase chain reaction method to analyze porcine cytokine gene expression. Vet. Immunol. Immunopathol. 1997, 58, 287–300. [Google Scholar] [CrossRef]
- Begni, B.; Amadori, M.; Ritelli, M.; Podavini, D. Effects of IFN-α on the Inflammatory Response of Swine Leukocytes to Bacterial Endotoxin. JICR 2005, 25, 202–208. [Google Scholar] [CrossRef]
- Wikström, F.H.; Meehan, B.M.; Berg, M.; Timmusk, S.; Elving, J.; Fuxler, L.; Magnusson, M.; Allan, G.M.; McNeilly, F.; Fossum, C. Structure-Dependent Modulation of Alpha Interferon Production by Porcine Circovirus 2 Oligodeoxyribonucleotide and CpG DNAs in Porcine Peripheral Blood Mononuclear Cells. J. Virol. 2007, 81, 4919–4927. [Google Scholar] [CrossRef]
- Amadori, M.; Cristiano, A.; Ferrari, M. Constitutive expression of interferons in swine leukocytes. Res. Vet. Sci. 2010, 88, 64–71. [Google Scholar] [CrossRef]
- Sah, V.; Kumar, A.; Dhar, P.; Upmanyu, V.; Tiwari, A.K.; Wani, S.A.; Sahu, A.R.; Kumar, A.; Badasara, S.K.; Pandey, A.; et al. Signature of genome wide gene expression in classical swine fever virus infected macrophages and PBMCs of indigenous vis-a-vis crossbred pigs. Gene 2020, 731, 144356. [Google Scholar] [CrossRef]
- Platt, R.; Vincent, A.L.; Gauger, P.C.; Loving, C.L.; Zanella, E.L.; Lager, K.M.; Kehrli, M.E., Jr.; Kimura, K.; Roth, J.A. Comparison of humoral and cellular immune responses to inactivated swine influenza virus vaccine in weaned pigs. Vet. Immunol. Immunopathol. 2011, 142, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Cha, S.H.; Grimm, A.L.; Ajithdoss, D.; Rzepka, J.; Chung, G.; Yu, J.; Davis, W.C.; Ho, C.S. Pigs that recover from porcine reproduction and respiratory syndrome virus infection develop cytotoxic CD4+CD8+ and CD4+CD8- T-cells that kill virus infected cells. PLoS ONE 2018, 13, e0203482. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.C.; Schaffner, R.; Fraefel, W.; Kihm, U. The relative density of CD44-positive porcine monocytic cell populations varies between isolations and upon culture and influences susceptibility to infection by African swine fever virus. Immunol. Lett. 1993, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Huang, X.; Zhu, H.; Chen, H.; Wang, W.; Liu, Y. Analysis of microRNA expression profiles in porcine PBMCs after LPS stimulation. Innate Immunol. 2020, 26, 435–446. [Google Scholar] [CrossRef]
- Franzoni, G.; Kurkure, N.V.; Essler, S.E.; Pedrera, M.; Everett, H.E.; Bodman-Smith, K.B.; Crooke, H.R.; Graham, S.P. Proteome-Wide Screening Reveals Immunodominance in the CD8 T Cell Response against Classical Swine Fever Virus with Antigen-Specificity Dependent on MHC Class I Haplotype Expression. PLoS ONE 2013, 8, 84246. [Google Scholar] [CrossRef]
- Shui, X.; Chen, S.; Lin, J.; Kong, J.; Zhou, C.; Wu, J. Knockdown of lncRNA NEAT1 inhibits Th17/CD4 + T cell differentiation through reducing the STAT3 protein level. J. Cell Physiol. 2019, 234, 22477–22484. [Google Scholar] [CrossRef]
- Costa, A.; Reynés, B.; Konieczna, J.; Martín, M.; Fiol, M.; Palou, A.; Romaguera, D.; Oliver, P. Use of human PBMC to analyse the impact of obesity on lipid metabolism and metabolic status: A proof-of-concept pilot study. Sci. Rep. 2021, 11, 18329. [Google Scholar] [CrossRef]
- Madden, L.A.; Vince, R.V.; Laden, G. The effect of acute hyperoxia in vivo on NF kappa B expression in human PBMC. Cell Biochem. Funct. 2011, 29, 71–73. [Google Scholar] [CrossRef]
- Chen, R.; Curran, J.; Pu, F.; Zhuola, Z.; Bayon, Y.; Hunt, J. In Vitro Response of Human Peripheral Blood Mononuclear Cells (PBMC) to Collagen Films Treated with Cold Plasma. Polymers 2017, 9, 254. [Google Scholar] [CrossRef]
- Robert, J.; Button, E.B.; Stukas, S.; Boyce, G.K.; Gibbs, E.; Cowan, C.M.; Gilmour, M.; Cheng, W.H.; Soo, S.K.; Yuen, B.; et al. High-density lipoproteins suppress Aβ-induced PBMC adhesion to human endothelial cells in bioengineered vessels and in monoculture. Mol. Neurodegener. 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Steller, D.; Scheibert, A.; Sturmheit, T.; Hakim, S.G. Establishment and validation of an in vitro co-culture model for oral cell lines using human PBMC-derived osteoclasts, osteoblasts, fibroblasts and keratinocytes. Sci. Rep. 2020, 10, 16861. [Google Scholar] [CrossRef] [PubMed]
- Salustri, A.; De Maio, F.; Palmieri, V.; Santarelli, G.; Palucci, I.; Mercedes Bianco, D.; Marchionni, F.; Bellesi, S.; Ciasca, G.; Perini, G.; et al. Evaluation of the Toxic Activity of the Graphene Oxide in the Ex Vivo Model of Human PBMC Infection with Mycobacterium tuberculosis. Microorganisms 2023, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Cifre, M.; Díaz-Rúa, R.; Varela-Calviño, R.; Reynés, B.; Pericás-Beltrán, J.; Palou, A.; Oliver, P. Human peripheral blood mononuclear cell in vitro system to test the efficacy of food bioactive compounds: Effects of polyunsaturated fatty acids and their relation with BMI. Mol. Nutr. Food. Res. 2017, 61, 1600353. [Google Scholar] [CrossRef]
- Luukkainen, A.; Puan, K.J.; Yusof, N.; Lee, B.; Tan, K.S.; Liu, J.; Yan, Y.; Toppila-Salmi, S.; Renkonen, R.; Chow, V.T.; et al. A Co-culture Model of PBMC and Stem Cell Derived Human Nasal Epithelium Reveals Rapid Activation of NK and Innate T Cells Upon Influenza A Virus Infection of the Nasal Epithelium. Front. Immunol. 2018, 9, 2514. [Google Scholar] [CrossRef]
- Kaya, E.; Grassi, L.; Benedetti, A.; Maisetta, G.; Pileggi, C.; Di Luca, M.; Batoni, G.; Esin, S. In vitro Interaction of Pseudomonas aeruginosa Biofilms with Human Peripheral Blood Mononuclear Cells. Front. Cell Infect. Microbiol. 2020, 10, 187. [Google Scholar] [CrossRef]
- Bode, G.; Clausing, P.; Gervais, F.; Loegsted, J.; Luft, J.; Nogues, V.; Sims, J. The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Methods 2010, 62, 196–220. [Google Scholar] [CrossRef]
- Lunney, J.K. Advances in Swine Biomedical Model Genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef]
- Vodička, P.; Smetana, K.; Dvořánková, B.; Emerick, T.; Xu, Y.; Ourednik, J.; Ourednik, V.; Motlík, J. The Miniature Pig as an Animal Model in Biomedical Research. Ann. N. Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Helke, K.L.; Swindle, M.M. Animal models of toxicology testing: The role of pigs. Expert Opin. Drug. Metab. Toxicol. 2013, 9, 127–139. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Aigner, B.; Renner, S.; Kessler, B.; Klymiuk, N.; Kurome, M.; Wünsch, A.; Wolf, E. Transgenic pigs as models for translational biomedical research. J. Mol. Med. 2010, 88, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Prather, R.S.; Lorson, M.; Ross, J.W.; Whyte, J.J.; Walters, E. Genetically Engineered Pig Models for Human Diseases. Annu. Rev. Anim. Biosci. 2013, 1, 203–219. [Google Scholar] [CrossRef]
- Wolf, E.; Braun-Reichhart, C.; Streckel, E.; Renner, S. Genetically engineered pig models for diabetes research. Transgenic Res. 2014, 23, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Kyrova, K.; Stepanova, H.; Rychlik, I.; Polansky, O.; Leva, L.; Sekelova, Z.; Faldyna, M.; Volf, J. The response of porcine monocyte derived macrophages and dendritic cells to SalmonellaTyphimurium and lipopolysaccharide. BMC Vet. Res. 2014, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Czyżewska-Dors, E.; Kwit, K.; Pejsak, Z. Enrofloxacin in therapeutic doses alters cytokine production by porcine PBMCs induced by lipopolysaccharide. Drug Chem. Toxicol. 2017, 40, 295–299. [Google Scholar] [CrossRef]
- Adler, M.; Murani, E.; Brunner, R.; Ponsuksili, S.; Wimmers, K. Transcriptomic Response of Porcine PBMCs to Vaccination with Tetanus Toxoid as a Model Antigen. PLoS ONE 2013, 8, e58306. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus. Res. 2013, 173, 122–130. [Google Scholar] [CrossRef]
- Canter, J.A.; Aponte, T.; Ramirez-Medina, E.; Pruitt, S.; Gladue, D.P.; Borca, M.V.; Zhu, J.J. Serum Neutralizing and Enhancing Effects on African Swine Fever Virus Infectivity in Adherent Pig PBMC. Viruses 2022, 14, 1249. [Google Scholar] [CrossRef]
- Costers, S.; Lefebvre, D.J.; Goddeeris, B.; Delputte, P.L.; Nauwynck, H.J. Functional impairment of PRRSV-specific peripheral CD3 + CD8 high cells. Vet. Res. 2009, 40, 46. [Google Scholar] [CrossRef]
- Franzoni, G.; Pedrera, M.; Sánchez-Cordón, P.J. African Swine Fever Virus Infection and Cytokine Response In Vivo: An Update. Viruses 2023, 15, 233. [Google Scholar] [CrossRef]
- Islam, M.d.A.; Große-Brinkhaus, C.; Pröll, M.J.; Uddin, M.J.; Aqter Rony, S.; Tesfaye, D.; Tholen, E.; Hoelker, M.; Schellander, K.; Neuhoff, C. PBMC transcriptome profiles identifies potential candidate genes and functional networks controlling the innate and the adaptive immune response to PRRSV vaccine in Pietrain pig. PLoS ONE 2017, 12, e0171828. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, F.; Zhai, L.; He, W.; Tan, Z.; Sun, Y.; Wang, Y.; Liu, L.; Ning, C.; Zhou, W.; et al. Transcriptome of Porcine PBMCs over Two Generations Reveals Key Genes and Pathways Associated with Variable Antibody Responses post PRRSV Vaccination. Sci. Rep. 2018, 8, 2460. [Google Scholar] [CrossRef] [PubMed]
- Reiske, L.; Schmucker, S.; Steuber, J.; Stefanski, V. Glucocorticoids and Catecholamines Affect In Vitro Functionality of Porcine Blood Immune Cells. Animals 2019, 9, 545. [Google Scholar] [CrossRef]
- Li, Y.; Díaz, I.; Martín-Valls, G.; Beyersdorf, N.; Mateu, E. Systemic CD4 cytotoxic T cells improve protection against PRRSV-1 transplacental infection. Front. Immunol. 2023, 13, 1020227. [Google Scholar] [CrossRef] [PubMed]
- Olesen, A.S.; Kodama, M.; Skovgaard, K.; Møbjerg, A.; Lohse, L.; Limborg, M.T.; Bøtner, A.; Belsham, G.J. Influence of African Swine Fever Virus on Host Gene Transcription within Peripheral Blood Mononuclear Cells from Infected Pigs. Viruses 2022, 14, 2147. [Google Scholar] [CrossRef]
- Fort, M.; Fernandes, L.T.; Nofrarias, M.; Díaz, I.; Sibila, M.; Pujols, J.; Mateu, E.; Segalés, J. Development of cell-mediated immunity to porcine circovirus type 2 (PCV2) in caesarean-derived, colostrum-deprived piglets. Vet. Immunol. Immunopathol. 2009, 129, 101–107. [Google Scholar] [CrossRef]
- Knoetig, S.M.; Summerfield, A.; Spagnuolo-Weaver, M.; McCullough, K.C. Immunopathogenesis of classical swine fever: Role of monocytic cells. Immunology 1999, 97, 359–366. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.J.; Feng, L.; Cai, X.B.; Tang, H.B.; Sun, S.K.; Zhang, H.Y.; Liang, J.J.; Luo, T.R. Global transcriptional profiles in peripheral blood mononuclear cell during classical swine fever virus infection. Virus. Res. 2010, 148, 60–70. [Google Scholar] [CrossRef]
- Pensaert, M. Viremia and effect of fetal infection with porcine viruses with special reference to porcine circovirus 2 infection. Vet. Microbiol. 2004, 98, 175–183. [Google Scholar] [CrossRef][Green Version]
- Hohnstein, F.S.; Meurer, M.; de Buhr, N.; von Köckritz-Blickwede, M.; Baums, C.G.; Alber, G.; Schütze, N. Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro. Pathogens 2020, 9, 40. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Rebel, J.M.J.; Smits, M.A.; van Putten, J.P.M.; Smith, H.E. Lgt Processing Is an Essential Step in Streptococcus suis Lipoprotein Mediated Innate Immune Activation. PLoS ONE 2011, 6, e22299. [Google Scholar] [CrossRef][Green Version]
- Hara, H.; Long, C.; Lin, Y.J.; Tai, H.C.; Ezzelarab, M.; Ayares, D.; Cooper, D.K.C. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl. Int. 2008, 21, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.; Ramackers, W.; Tiede, A.; Lucas-Hahn, A.; Herrmann, D.; Barg-Kues, B.; Schuettler, W.; Friedrich, L.; Schwinzer, R.; Winkler, M.; et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation 2009, 16, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of α1,3-Galactosyltransferase-Deficient Pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef]
- Butler, J.R.; Martens, G.R.; Estrada, J.L.; Reyes, L.M.; Ladowski, J.M.; Galli, C.; Perota, A.; Cunningham, C.M.; Tector, M.; Tector, A.J. Silencing porcine genes significantly reduces human-anti-pig cytotoxicity profiles: An alternative to direct complement regulation. Transgenic Res. 2016, 25, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Dorling, A.; Pierson, R.N.; Rees, M.; Seebach, J.; Yazer, M.; Ohdan, H.; Awwad, M.; Ayares, D. α1,3-Galactosyltransferase Gene-Knockout Pigs for Xenotransplantation: Where Do We Go From Here? Transplantation 2007, 84, 1–7. [Google Scholar] [CrossRef]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef]
- Gock, H.; Nottle, M.; Lew, A.M.; d’Apice, A.J.F.; Cowan, P. Genetic modification of pigs for solid organ xenotransplantation. Transplant. Rev. 2011, 25, 9–20. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.W.; Cheong, H.T.; Greenstein, J.L.; Im, G.S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of α-1,3-Galactosyltransferase Knockout Pigs by Nuclear Transfer Cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef]
- Sachs, D.H.; Galli, C. Genetic manipulation in pigs. Curr. Opin. Organ Transplant. 2009, 14, 148–153. [Google Scholar] [CrossRef]
- Yamada, K.; Yazawa, K.; Shimizu, A.; Iwanaga, T.; Hisashi, Y.; Nuhn, M.; O’Malley, P.; Nobori, S.; Vagefi, P.A.; Patience, C.; et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 2005, 11, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Laval, F.; Audonnet, J.-C.; Andreoni, C.; Juillard, V. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology 2001, 102, 396–404. [Google Scholar] [CrossRef]
- Buermann, A.; Petkov, S.; Petersen, B.; Hein, R.; Lucas-Hahn, A.; Baars, W.; Brinkmann, A.; Niemann, H.; Schwinzer, R. Pigs expressing the human inhibitory ligand PD-L1 (CD274) provide a new source of xenogeneic cells and tissues with low immunogenic properties. Xenotransplantation 2018, 25, e12387. [Google Scholar] [CrossRef] [PubMed]
- Golbus, A.L.; Ochoa, B.V.; Hardy, W.A.; Helke, K.L.; Kavarana, M.N.; Kwon, J.H.; Rajab, T.K. Immunosuppressive regimens in porcine transplantation models. Transplant. Rev. 2022, 36, 100725. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.N.; Malde, P.; Rogers, N.J.; Jackson, I.M.; Lechler, R.I.; Dorling, A. Porcine CTLA4-Ig Lacks a MYPPPY Motif, Binds Inefficiently to Human B7 and Specifically Suppresses Human CD4+ T Cell Responses Costimulated by Pig But Not Human B7. J. Immunol. 2000, 165, 3175–3181. [Google Scholar] [CrossRef]
- Hara, H.; Witt, W.; Crossley, T.; Long, C.; Isse, K.; Fan, L.; Phelps, C.J.; Ayares, D.; Cooper, D.K.C.; Dai, Y.; et al. Human dominant-negative class II transactivator transgenic pigs—Effect on the human anti-pig T-cell immune response and immune status. Immunology 2013, 140, 39–46. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, M.; Chaszczewska-Markowska, M.; Zemelka-Wiacek, M. Porcine Peripheral Blood Mononuclear Cells (PBMCs): Methods of Isolation, Cryopreservation, and Translational Applications in Human Studies. J. Clin. Med. 2025, 14, 3432. https://doi.org/10.3390/jcm14103432
Pietrzak M, Chaszczewska-Markowska M, Zemelka-Wiacek M. Porcine Peripheral Blood Mononuclear Cells (PBMCs): Methods of Isolation, Cryopreservation, and Translational Applications in Human Studies. Journal of Clinical Medicine. 2025; 14(10):3432. https://doi.org/10.3390/jcm14103432
Chicago/Turabian StylePietrzak, Magdalena, Monika Chaszczewska-Markowska, and Magdalena Zemelka-Wiacek. 2025. "Porcine Peripheral Blood Mononuclear Cells (PBMCs): Methods of Isolation, Cryopreservation, and Translational Applications in Human Studies" Journal of Clinical Medicine 14, no. 10: 3432. https://doi.org/10.3390/jcm14103432
APA StylePietrzak, M., Chaszczewska-Markowska, M., & Zemelka-Wiacek, M. (2025). Porcine Peripheral Blood Mononuclear Cells (PBMCs): Methods of Isolation, Cryopreservation, and Translational Applications in Human Studies. Journal of Clinical Medicine, 14(10), 3432. https://doi.org/10.3390/jcm14103432







