Assessing the Impact of Inflammation on Erythropoietin Resistance in Hemodialysis: The Role of the NLR
Abstract
1. Introduction
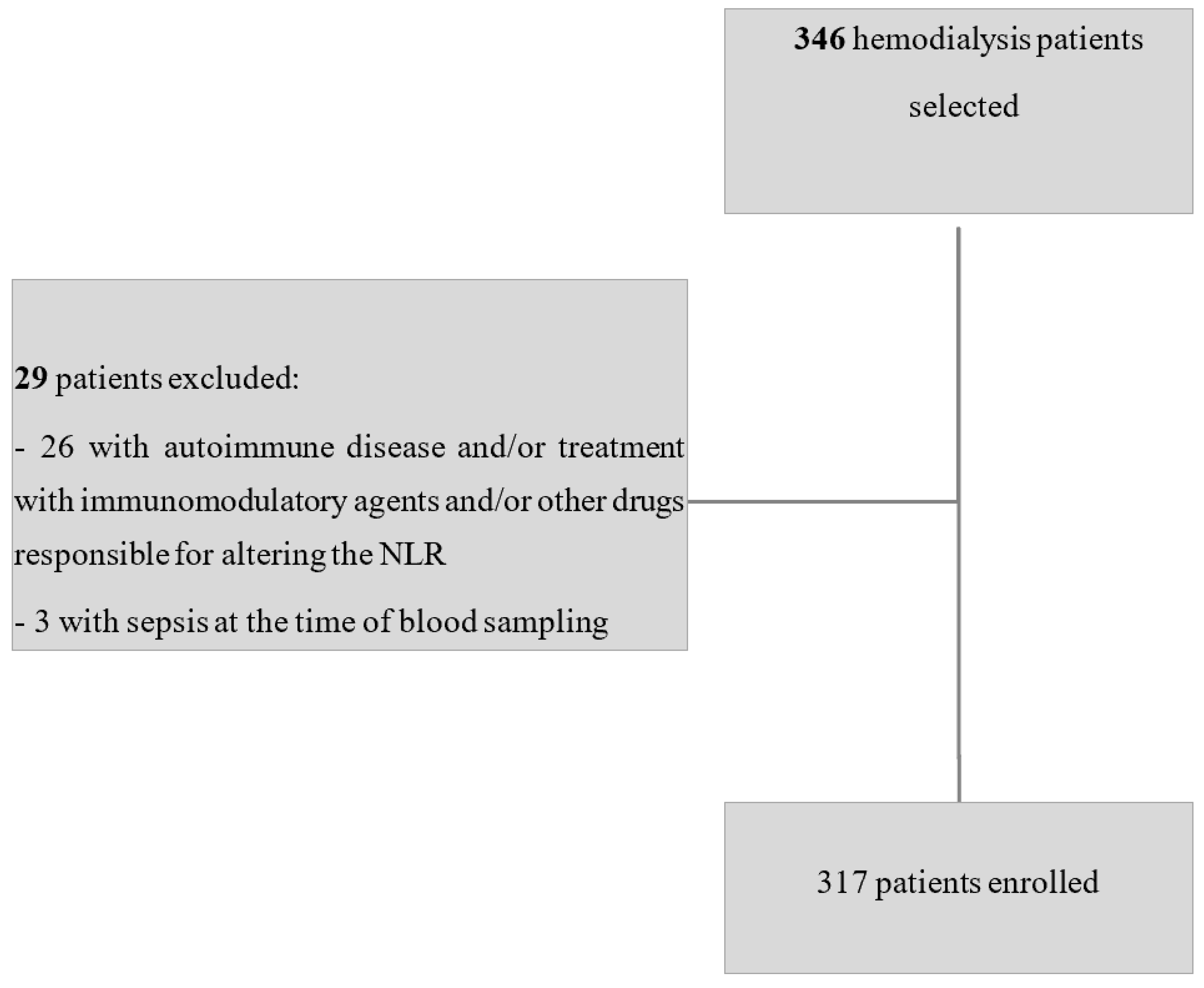
2. Materials and Methods
Statistical Analysis
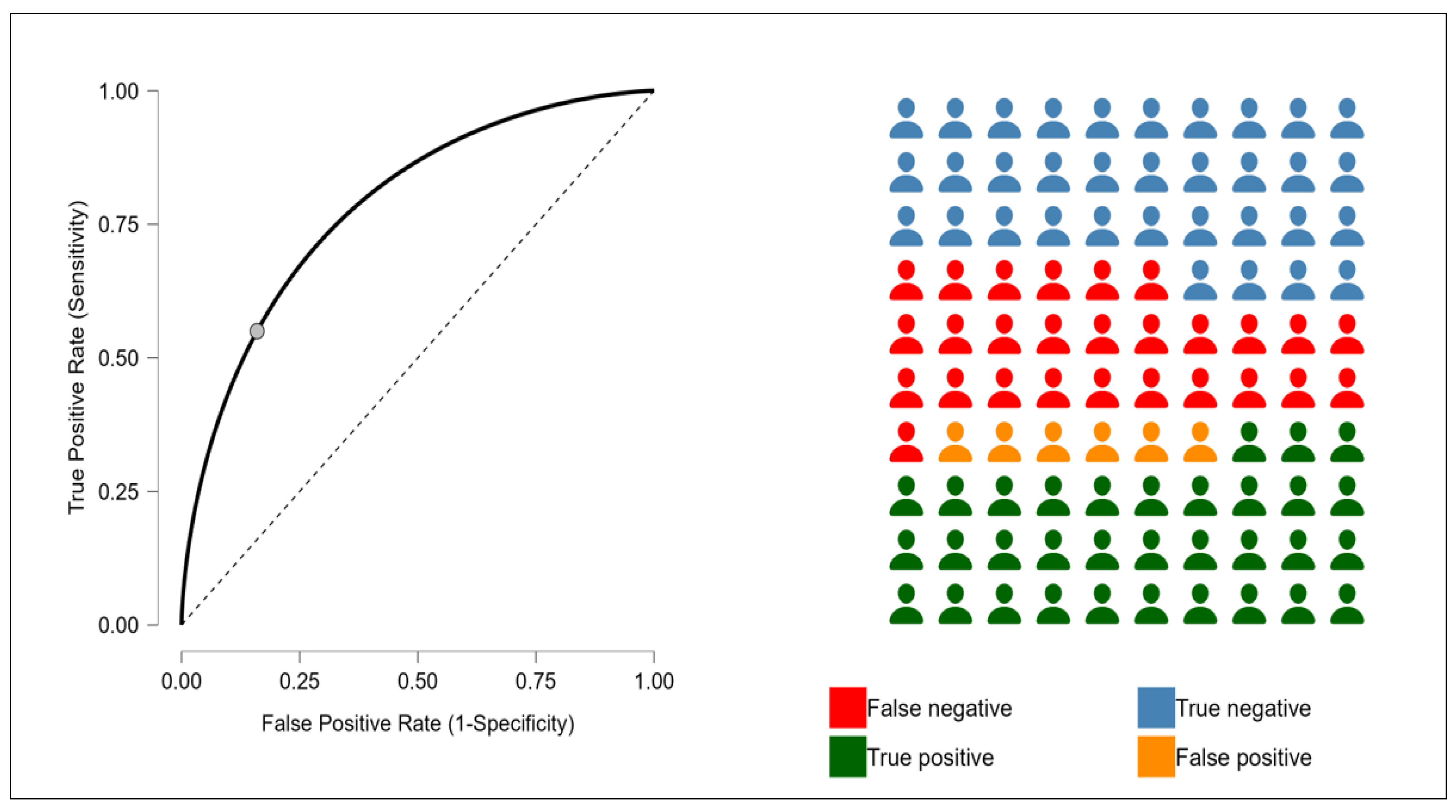
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanna, R.M.; Streja, E.; Kalantar-Zadeh, K. Burden of Anemia in Chronic Kidney Disease: Beyond Erythropoietin. Adv. Ther. 2021, 38, 52–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in chronic kidney disease: From pathophysiology and current treatments, to future agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef] [PubMed]
- Marques, O.; Weiss, G.; Muckenthaler, M.U. The role of iron in chronic inflammatory diseases: From mechanisms to treatment options in anemia of inflammation. Blood J. Am. Soc. Hematol. 2022, 140, 2011–2023. [Google Scholar] [CrossRef]
- Chung, E.Y.; Palmer, S.C.; Saglimbene, V.M.; Craig, J.C.; Tonelli, M.; Strippoli, G.F. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 2, CD010590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukuma, S.; Yamaguchi, T.; Hashimoto, S.; Nakai, S.; Iseki, K.; Tsubakihara, Y.; Fukuhara, S. Erythropoiesis-stimulating agent responsiveness and mortality in hemodialysis patients: Results from a cohort study from the dialysis registry in Japan. Am. J. Kidney Dis. 2012, 59, 108–116. [Google Scholar] [CrossRef]
- Ishigami, J.; Onishi, T.; Shikuma, S.; Akita, W.; Mori, Y.; Asai, T.; Kuwahara, M.; Sasaki, S.; Tsukamoto, Y. The impact of hyporesponsiveness to erythropoietin-stimulating agents on time-dependent mortality risk among CKD stage 5D patients: A single-center cohort study. Clin. Exp. Nephrol. 2013, 17, 106–114. [Google Scholar] [CrossRef]
- Weir, M.R. Managing anemia across the stages of kidney disease in those hyporesponsive to erythropoiesis-stimulating agents. Am. J. Nephrol. 2021, 52, 450–466. [Google Scholar] [CrossRef]
- Schneider, A.; Schneider, M.; Scharnagl, H.; Jardine, A.; Wanner, C.; Drechsler, C. Predicting erythropoietin resistance in hemodialysis patients with type 2 diabetes. BMC Nephrol. 2013, 14, 67–73. [Google Scholar] [CrossRef]
- Petrulienė, K.; Žiginskienė, E.; Kuzminskis, V.; Nedzelskienė, I.; Bumblytė, I. Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Medicina 2017, 53, 90–100. [Google Scholar] [CrossRef]
- El Sewefy, D.A.; Farweez, B.A.; Behairy, M.A.; Yassin, N.R. Impact of serum hepcidin and inflammatory markers on resistance to erythropoiesis-stimulating therapy in haemodialysis patients. Int. Urol. Nephrol. 2019, 51, 325–334. [Google Scholar] [CrossRef] [PubMed]
- De Francisco, A.L.; Stenvinkel, P.; Vaulont, S. Inflammation and its impact on anaemia in chronic kidney disease: From haemoglobin variability to hyporesponsiveness. NDT Plus 2009, 2 (Suppl. S1), i18–i26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S11), 39–43. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzózka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The Influence of Inflammation on Anemia in CKD Patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-Ortiz, A.; Correa-Rotter, R.; Vázquez-Rangel, A.; Vega-Vega, O.; Espinosa-Cuevas, Á. Relationship between protein-energy wasting in adults with chronic hemodialysis and the response to treatment with erythropoietin. BMC Nephrol. 2019, 20, 316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stenvinkel, P. The role of inflammation in the anaemia of end-stage renal disease. Nephrol. Dial. Transplant. 2001, 16 (Suppl. S7), 36–40. [Google Scholar] [CrossRef]
- Rattanasompattikul, M.; Molnar, M.Z.; Zaritsky, J.J.; Hatamizadeh, P.; Jing, J.; Norris, K.C.; Kovesdy, C.P.; Kalantar-Zadeh, K. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol. Dial. Transplant. 2013, 28, 1936–1945. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carollo, C.; Mancia, E.; Sorce, A.; Altieri, C.; Altieri, D.; Brunori, G.; Mulè, G. Prognostic impact of neutrophil-to-lymphocyte ratio and vascular access in patients on chronic hemodialysis. J. Nephrol. 2025, 38, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Angkananard, T.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Thakkinstian, A. Neutrophil Lymphocyte Ratio and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 2703518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucius, K. Novel and Emerging Markers of Chronic or Low-Grade Inflammation. Integr. Complement. Therapies 2023, 29, 130–142. [Google Scholar] [CrossRef]
- Shah, H.H.; Uppal, N.N.; Fishbane, S. Inflammation and Erythropoiesis-Stimulating Agent Hyporesponsiveness: A Critical Connection. Kidney Med. 2020, 2, 245–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yılmaz, I.; Ozkok, A.; Kostek, O.; Kolukısa, A.; Duran, I.; Odabaş, A.R.; Işman, F.K.; Başok, B.I. C-reactive protein but not hepcidin, NGAL and transferrin determines the ESA resistance in hemodialysis patients. Ren. Fail. 2016, 38, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Kim, H.G.; Yun, Y.S.; Jeon, E.K.; Ko, Y.H.; Kim, Y.S.; Kim, Y.O.; Yoon, S.A. IL-6 is an independent risk factor for resistance to erythropoiesis-stimulating agents in hemodialysis patients without iron deficiency. Hemodial. Int. 2012, 16, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, J.; Bajo, M.A.; Caravaca, F.; Coronel, F.; García-Pérez, H.; González-Parra, E.; Granado, A.; Martín-Govantes, J.; Miguel, A.; Molina, A.; et al. Guías Sociedad Española de Nefrología. Guías de práctica clínica en diálisis peritoneal [Guidelines of the Spanish Society of Nephrology. Clinical practice guidelines for peritoneal dialysis]. Nefrologia 2006, 26 (Suppl. S4), 1–184. (In Spanish) [Google Scholar] [PubMed]
- Avilés, B.; Coronel, F.; Pérez-García, R.; Marcelli, D.; Orlandini, G.; Ayala, J.A.; Rentero, R. Control de la anemia en hemodiálisis. Base de datos EuCliD (European Clinical Database) en España [Anemia management in haemodialysis. EuCliD database in Spain]. Nefrologia 2002, 22, 555–563. (In Spanish) [Google Scholar] [PubMed]
- Yajima, T.; Yajima, K.; Takahashi, H. Association of the erythropoiesis-stimulating agent resistance index and the geriatric nutritional risk index with cardiovascular mortality in maintenance hemodialysis patients. PLoS ONE 2021, 16, e0245625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, S.; Zhao, D.L.; Li, P.; Sun, X.F.; Zhou, J.H.; Song, K.K.; Wang, Y.; Miao, L.N.; Ni, Z.H.; Lin, H.L.; et al. Relationships among the Dosage of Erythropoiesis-Stimulating Agents, Erythropoietin Resistance Index, and Mortality in Maintenance Hemodialysis Patients. Blood Purif. 2022, 51, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Pollock, C.A.; Macdougall, I.C. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology 2007, 12, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, M.; Wang, G. The neutrophil–lymphocyte ratio is associated with all-cause and cardiovascular mortality in cardiovascular patients. Sci. Rep. 2024, 14, 26692. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.H.; Oh, T.R.; Suh, S.H.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Kim, S.W.; Bae, E.H. Prognostic role of the neutrophil-to-lymphocyte ratio in patients with chronic kidney disease. Korean J. Intern. Med. 2023, 38, 725–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Lu, X.; Wang, S.; Li, H. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are associated with poor survival in patients with hemodialysis. Biomed. Res. Int. 2021, 2021, 9958081. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meier, P.; Dayer, E.; Blanc, E.; Wauters, J.-P. Early T cell activation correlates with expression of apoptosis-inducing molecules in chronic hemodialysis patients. Kidney Int. 2002, 62, 107–115. [Google Scholar]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.Y.; Babitt, J.L. Hepcidin regulation in the anemia of inflammation. Curr. Opin. Hematol. 2016, 23, 189–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sitter, T.; Bergner, A.; Schiffl, H. Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients. Nephrol. Dial. Transplant. 2000, 15, 1207–1211. [Google Scholar] [CrossRef][Green Version]
- Esa, T.; Budu, B.; Mulyono, B.; Soraya, G.V.; Usman, A.N.; Intansari, U.S. Correlation of serum interleukin-6 levels and neutrophil-lymphocyte ratio in the severity of COVID-19. F1000Research 2023, 12, 1189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heshmat-Ghahdarijani, K.; Sarmadi, V.; Heidari, A.; Falahati Marvasti, A.; Neshat, S.; Raeisi, S. The neutrophil-to-lymphocyte ratio as a new prognostic factor in cancers: A narrative review. Front Oncol. 2023, 13, 1228076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guarneri, M.; Scola, L.; Giarratana, R.M.; Bova, M.; Carollo, C.; Vaccarino, L.; Calandra, L.; Lio, D.; Balistreri, C.R.; Cottone, S. MIF rs755622 and IL6 rs1800795 Are Implied in Genetic Susceptibility to End-Stage Renal Disease (ESRD). Genes 2022, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Wang, S.; Li, H. Neutrophil-to-lymphocyte ratio and erythropoietin resistance among maintenance hemodialysis patients. Blood Purif. 2022, 51, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Valga, F.; Monzón, T.; Henriquez, F.; Santana-Del-Pino, A.; Antón-Pérez, G. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios as markers of erythropoietin resistance in chronic haemodialysis patients: A multicentre cross-sectional study. Nefrología Engl. Ed. 2020, 40, 320–327. [Google Scholar] [CrossRef]
- Pineault, J.; Lamarche, C.; Bell, R.; Lafrance, J.-P.; Ouellet, G.; Leblanc, M.; Pichette, V.; Bezzaoucha, S.; Vallée, M. Association of neutrophil-to-lymphocyte ratio with inflammation and erythropoietin resistance in chronic dialysis patients. Can. J. Kidney Health Dis. 2017, 4, 2054358117735563. [Google Scholar] [CrossRef]
- Jadeja, Y.P.; Kher, V. Protein energy wasting in chronic kidney disease: An update with focus on nutritional interventions to improve outcomes. Indian J. Endocrinol. Metab. 2012, 16, 246–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feret, W.; Safranow, K.; Kwiatkowska, E.; Daniel, A.; Ciechanowski, K. Malnutrition and Erythropoietin Resistance among Patients with End-Stage Kidney Disease: Where Is the Perpetrator of Disaster? Nutrients 2022, 14, 5318. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Bint Harun, K.U.H.; Kawser, M.; Nabi, M.H.; Mitra, D.K. Factors associated with the malnutrition inflammation score (MIS) among hemodialysis patients in Dhaka city: A cross-sectional study in tertiary care hospitals. Porto. Biomed. J. 2024, 9, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| 1° TERTILE n = 105 | 2° TERTILE n = 106 | 3° TERTILE n = 106 | p | |
|---|---|---|---|---|
| Age, years | 68 (56–76) | 71 (61–80) | 73 (61–81) | 0.0504 |
| Male sex, n (%) | 75 (71) | 78 (74) | 76 (72) | 0.1010 |
| Weight, kg | 68 (59–79) | 67 (58–77) | 66 (57–78) | 0.7641 |
| Dialysis vintage, months | 33 (16–46) | 37 (17–68) | 42 (23–71) | 0.0030 |
| ERI | 13 (6–13) | 15 (13–18) | 20 (17–23) | 0.0022 |
| PLT, 109/L | 200 (148–258) | 209 (160–253) | 202 (154–257) | 0.672 |
| CRP, mg/dL | 2.7 (1.9–7.6) | 3.9 (2.2–10.2) | 9.3 (3.3–25) | 0.0002 |
| PLR | 108 (87–140) | 155 (125–206) | 239 (190–324) | <0.000001 |
| Neutrophils, 109/L | 4300 (3900–4600) | 4300 (4100–4700) | 4500 (4200–4800) | 0.4233 |
| Lymphocyte, 109/L | 1840 (1380–2200) | 1330 (1000–1645) | 845 (660–1100) | <0.000001 |
| d-NLR | 1.29 (0.99–1.47) | 2.1 (1.8–2.4) | 3.4 (3–4.1) | <0.000001 |
| Total proteins, g/dL | 6.5 (6.2–6.8) | 6.5 (6.2–6.9) | 6.5 (6.1–6.8) | 0.6050 |
| Albumin, g/L | 32 (29–36) | 33 (31–35) | 32 (28–35) | 0.0944 |
| Serum iron, µg/dL | 51 (37–70) | 48 (38–60) | 39 (29–52) | 0.0005 |
| Transferrin, mg/dL | 184 (160–200) | 184 (160–210) | 170 (130–190) | 0.0067 |
| Total cholesterol, mg/dL | 147 (127–168) | 151 (124–178) | 149 (123–175) | 0.8962 |
| Plasma urea, mg/dL | 156 ± 40 | 153 ± 38 | 150 ± 45 | 0.7341 |
| Potassium, mmol/L | 5 ± 0.7 | 5.1 ± 0.8 | 5.1 ± 0.9 | 0.1843 |
| Phosporus, mg/dL | 5.3 (4.4–6.3) | 5.1 (4.1–6.5) | 5.1 (4.4–6.3) | 0.6466 |
| Blood glucose, mg/dL | 116 (97–139) | 121 (97–150) | 125 (104–157) | 0.1500 |
| Model | Unstandardized | Standard Error | Standardized | t | p | |
|---|---|---|---|---|---|---|
| M₁ | (Intercept) | 21.133 | 5.493 | 3.847 | <0.001 | |
| Hemoglobin | −0.067 | 0.069 | −0.061 | −0.965 | 0.02 | |
| NLR | 0.848 | 0.423 | 0.124 | 2.004 | 0.046 | |
| Serum Iron | −0.003 | 0.004 | −0.057 | −0.887 | 0.001 |
| Unstandardized | SE | p | Exp(b) | 95% CI of Exp(b) | |
|---|---|---|---|---|---|
| Serum Iron | −0.004489 | 0.003810 | 0.0035 | 0.7710 | 0.9882 to 1.4229 |
| NLR | 0.01964 | 0.03415 | 0.0021 | 1.2198 | 0.9541 to 1.2412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, C.; Sorce, A.; Mancia, E.; Cirafici, E.; Ciuppa, M.E.; De Biasio, B.; Mulè, G.; Brunori, G. Assessing the Impact of Inflammation on Erythropoietin Resistance in Hemodialysis: The Role of the NLR. J. Clin. Med. 2025, 14, 3411. https://doi.org/10.3390/jcm14103411
Carollo C, Sorce A, Mancia E, Cirafici E, Ciuppa ME, De Biasio B, Mulè G, Brunori G. Assessing the Impact of Inflammation on Erythropoietin Resistance in Hemodialysis: The Role of the NLR. Journal of Clinical Medicine. 2025; 14(10):3411. https://doi.org/10.3390/jcm14103411
Chicago/Turabian StyleCarollo, Caterina, Alessandra Sorce, Ettore Mancia, Emanuele Cirafici, Maria Elena Ciuppa, Benedetto De Biasio, Giuseppe Mulè, and Giuliano Brunori. 2025. "Assessing the Impact of Inflammation on Erythropoietin Resistance in Hemodialysis: The Role of the NLR" Journal of Clinical Medicine 14, no. 10: 3411. https://doi.org/10.3390/jcm14103411
APA StyleCarollo, C., Sorce, A., Mancia, E., Cirafici, E., Ciuppa, M. E., De Biasio, B., Mulè, G., & Brunori, G. (2025). Assessing the Impact of Inflammation on Erythropoietin Resistance in Hemodialysis: The Role of the NLR. Journal of Clinical Medicine, 14(10), 3411. https://doi.org/10.3390/jcm14103411







