Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Descriptive Analysis
2.3. Disproportionality Analysis
3. Results
3.1. Descriptive Analysis
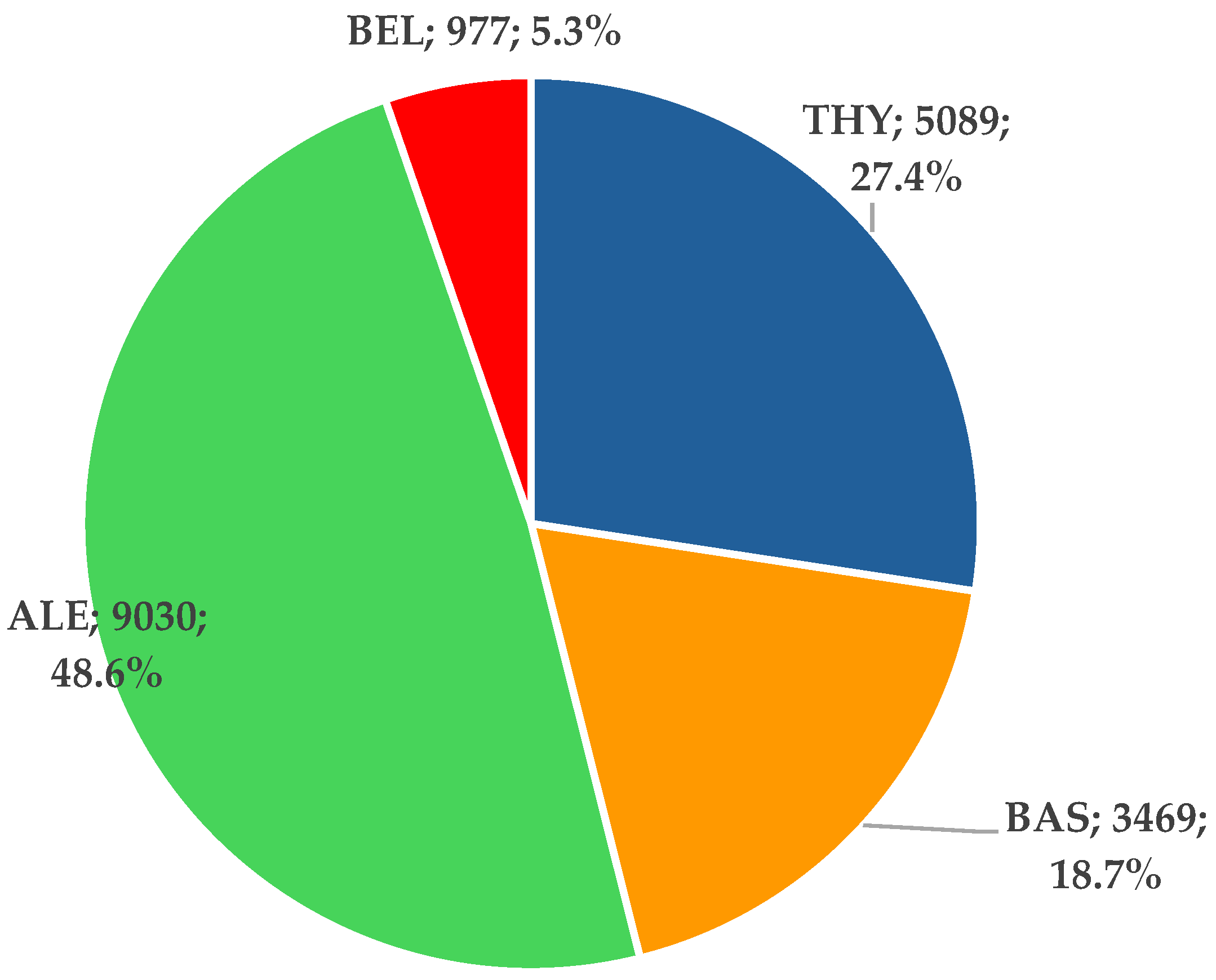
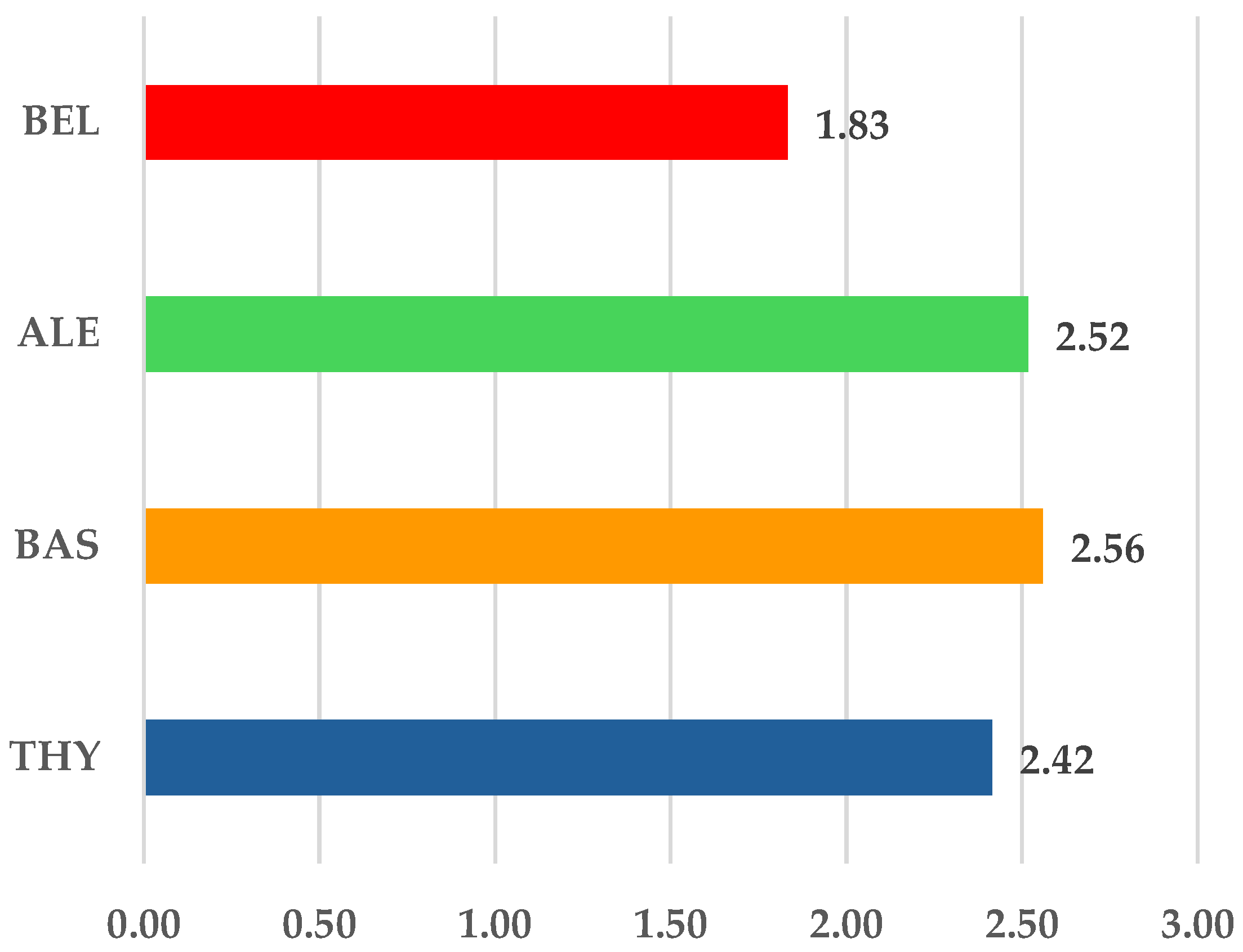
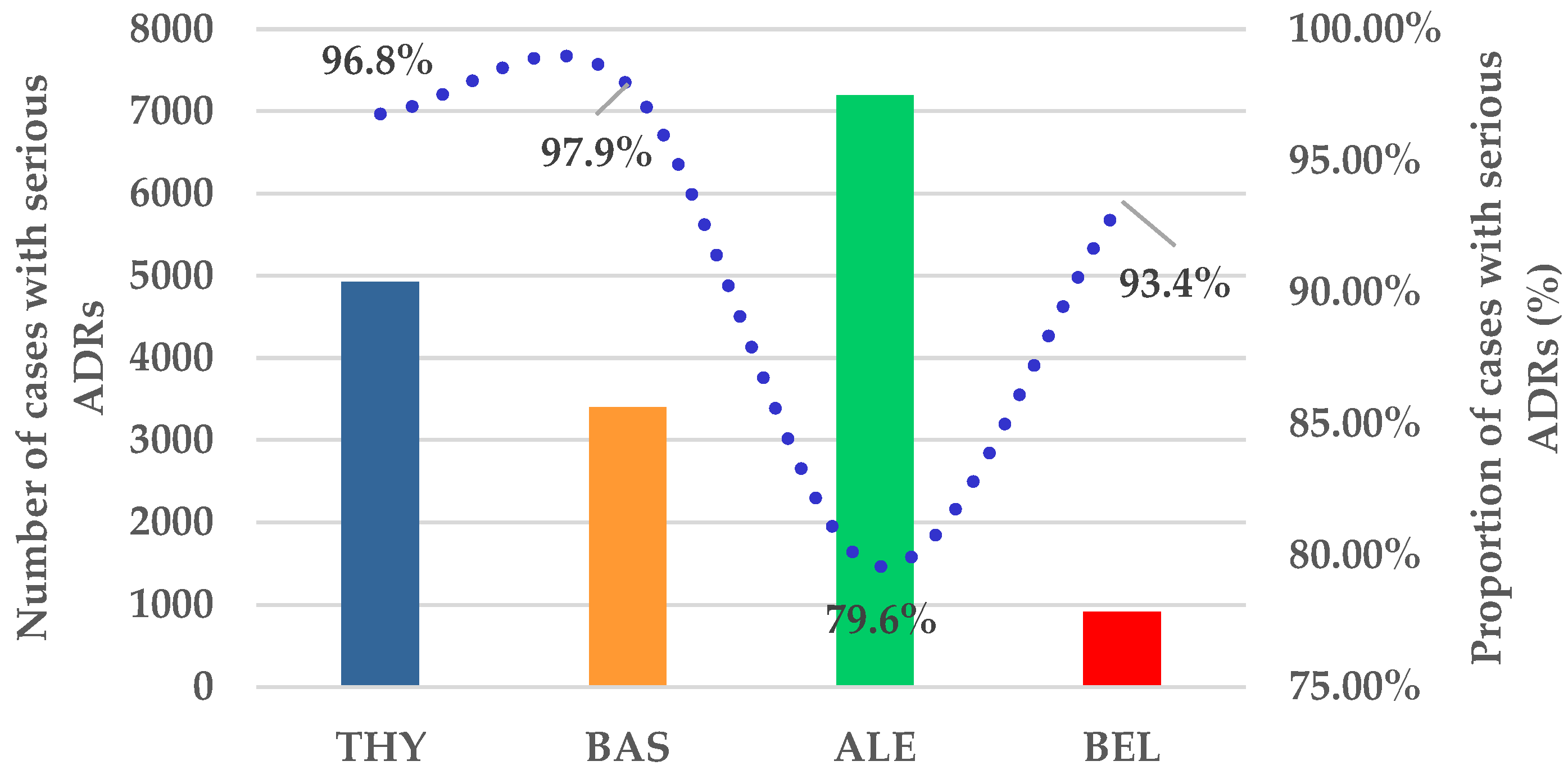
3.1.1. ICSR Analysis
3.1.2. ICSR Analysis
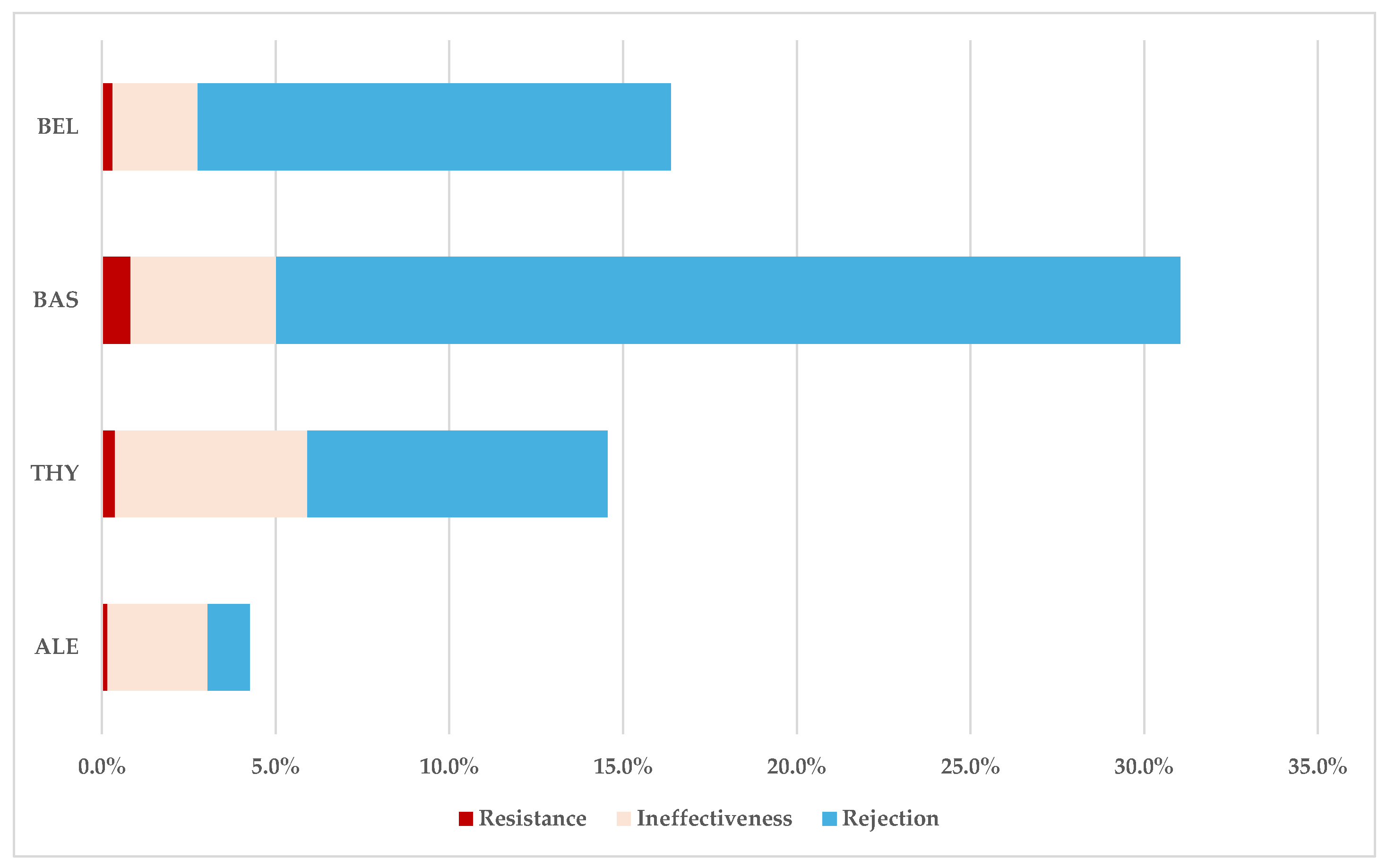
ADRs Related to Resistance, Ineffectiveness, and Transplant Rejection
3.2. Disproportionality Analysis
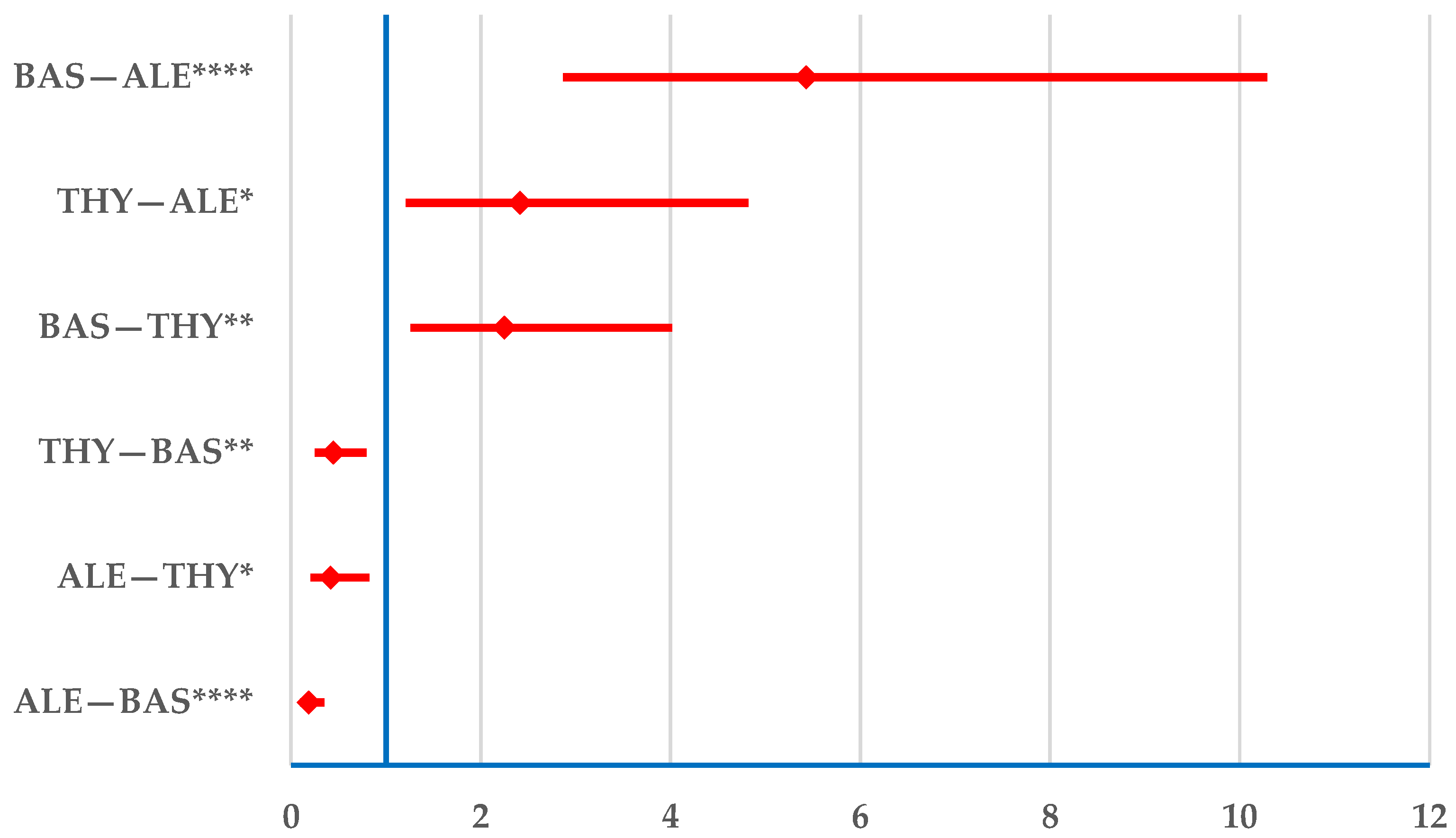
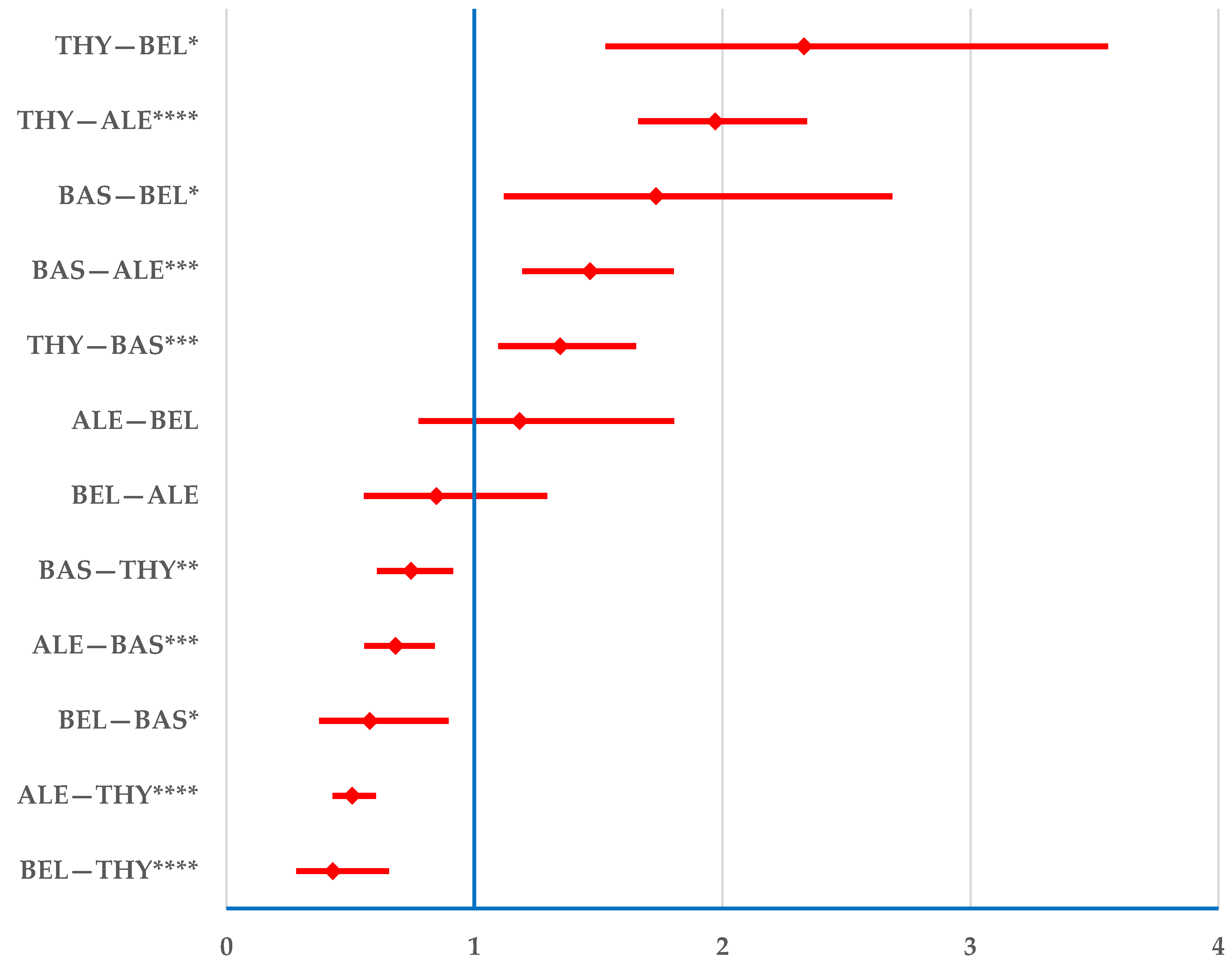
3.2.1. Drug Resistance
3.2.2. Drug Ineffectiveness
3.2.3. Transplant Rejection
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Observatory on Donation and Transplantation. Available online: https://www.transplant-observatory.org/ (accessed on 19 January 2025).
- Scarpa, J. Improving Liver Transplant Outcomes with Transplant-Omics and Network Biology. Curr. Opin. Organ. Transplant. 2023, 28, 412–418. [Google Scholar] [CrossRef]
- Niederhaus, S.V. Pancreas Transplant Alone. Curr. Opin. Organ. Transplant. 2015, 20, 115–120. [Google Scholar] [CrossRef]
- Garcia, J.; Selvaggi, G.; Tekin, A.; Vianna, R. Intestinal Transplantation. Curr. Opin. Organ. Transplant. 2021, 26, 229–233. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Trahanas, J.; Xu, M.; Wells, Q.; Farber-Eger, E.; Pasrija, C.; Amancherla, K.; Debose-Scarlett, A.; Brinkley, D.M.; Lindenfeld, J.A.; et al. Outcomes of Heart Transplant Donation After Circulatory Death. J. Am. Coll. Cardiol. 2023, 82, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Gruttadauria, M.; Dunn, C.; Lin, J.; Kaminetsky, J.R.; Applebaum, K.; Portal, D.; Mohammed, O.; Rocca, J.; Greenstein, S. Patients’ Expectations for Longevity of Kidney Transplant. Prog. Transplant. 2018, 29, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wood-Trageser, M.A.; Xu, Q.; Zeevi, A.; Randhawa, P.; Lesniak, D.; Demetris, A.J. Precision Transplant Pathology. Curr. Opin. Organ. Transplant. 2020, 25, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Alelign, T.; Ahmed, M.M.; Bobosha, K.; Tadesse, Y.; Howe, R.; Petros, B. Kidney Transplantation: The Challenge of Human Leukocyte Antigen and Its Therapeutic Strategies. J. Immunol. Res. 2018, 2018, 5986740. [Google Scholar] [CrossRef]
- EAU Guidelines on Renal Transplantation; European Association of Urology: Arnhem, The Netherlands, 2024.
- Bauer, A.C.; Franco, R.F.; Manfro, R.C. Immunosuppression in Kidney Transplantation: State of the Art and Current Protocols. Curr. Pharm. Des. 2020, 26, 3440–3450. [Google Scholar] [CrossRef]
- Brook, M.O.; Hennessy, C.; Hester, J.; Hammad, S.; Alzhrani, A.; Rombach, I.; Dutton, S.; Lombardi, G.; Wood, K.J.; Friend, P.; et al. Late Treatment with Autologous Expanded Regulatory T-Cell Therapy After Alemtuzumab Induction Is Safe and Facilitates Immunosuppression Minimization in Living Donor Renal Transplantation. Transplantation 2024, 108, 2278–2286. [Google Scholar] [CrossRef]
- Barnett, A.N.R.; Asgari, E.; Chowdhury, P.; Sacks, S.H.; Dorling, A.; Mamode, N. The Use of Eculizumab in Renal Transplantation. Clin. Transplant. 2013, 27, E216–E229. [Google Scholar] [CrossRef]
- Masset, C.; Kerleau, C.; Blancho, G.; Hourmant, M.; Walencik, A.; Ville, S.; Kervella, D.; Cantarovich, D.; Houzet, A.; Giral, M.; et al. Very Low Dose Anti-Thymocyte Globulins Versus Basiliximab in Non-Immunized Kidney Transplant Recipients. Transplant. Int. 2023, 36, 10816. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mier, G.; Moreno-Ley, P.I.; Budar-Fernández, L.F.; Méndez-López, M.T.; Allende-Castellanos, C.A.; Jiménez-López, L.A.; Barrera-Amoros, D.A.; Reyes-Ruiz, J.M. Low-Dose Thymoglobulin versus Basiliximab Induction Therapy in Low-Risk Living Related Kidney Transplant Recipients: Three-Year Follow-Up Study. Arch. Med. Res. 2024, 55, 103047. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, Z.; Ayvaci, M.U.S.; Hahsler, M.; Giacoma, T.; Gaston, R.S.; Tanriover, B. Cost-Effectiveness of Antibody-Based Induction Therapy in Deceased Donor Kidney Transplantation in the United States. Transplantation 2017, 101, 1234–1241. [Google Scholar] [CrossRef]
- Kamal, J.; Doyle, A. Immunosuppression and Kidney Transplantation. Handb. Exp. Pharmacol. 2021, 272, 165–179. [Google Scholar] [CrossRef]
- Ponticelli, C. Basiliximab: Efficacy and Safety Evaluation in Kidney Transplantation. Expert. Opin. Drug Saf. 2014, 13, 373–381. [Google Scholar] [CrossRef]
- Chauhan, K.; Mehta, A.A. Rituximab in Kidney Disease and Transplant. Animal Model. Exp. Med. 2019, 2, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Groves, R.W. Rituximab. Handbook of Systemic Drug Treatment in Dermatology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2024; pp. 233–239. [Google Scholar] [CrossRef]
- Guilcher, G.M.T.; Shah, R.; Shenoy, S. Principles of Alemtuzumab Immunoablation in Hematopoietic Cell Transplantation for Non-Malignant Diseases in Children: A Review. Pediatr. Transplant. 2018, 22, e13142. [Google Scholar] [CrossRef]
- Thomas, B.; Weir, M.R. Immunosuppression. In Nephrology Secrets, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–409. [Google Scholar] [CrossRef]
- Mashima, K.; Oh, I.; Fujiwara, K.; Izawa, J.; Takayama, N.; Nakano, H.; Kawasaki, Y.; Minakata, D.; Yamasaki, R.; Morita, K.; et al. Comparison of Alemtuzumab, Anti-Thymocyte Globulin, and Post-Transplant Cyclophosphamide for Graft-versus-Host Disease and Graft-versus-Leukemia in Murine Models. PLoS ONE 2021, 16, e0245232. [Google Scholar] [CrossRef]
- Acharya, S.; Lama, S.; Kanigicherla, D.A. Anti-Thymocyte Globulin for Treatment of T-Cell-Mediated Allograft Rejection. World J. Transplant. 2023, 13, 299–308. [Google Scholar] [CrossRef]
- Mueller, T.F. Mechanisms of Action of Thymoglobulin. Transplantation 2007, 84, S5–S10. [Google Scholar] [CrossRef]
- Dubrall, D.; Leitzen, S.; Toni, I.; Stingl, J.; Schulz, M.; Schmid, M.; Neubert, A.; Sachs, B. Descriptive Analysis of Adverse Drug Reaction Reports in Children and Adolescents from Germany: Frequently Reported Reactions and Suspected Drugs. BMC Pharmacol. Toxicol. 2021, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, K.; Szűcs, G.; Kőnig-Péter, A.; Balázs, O.; Vajda, P.; Botz, L.; Vida, R.G. Analysis of Pharmacovigilance Databases for Spontaneous Reports of Adverse Drug Reactions Related to Substandard and Falsified Medical Products: A Descriptive Study. Front. Pharmacol. 2022, 13, 964399. [Google Scholar] [CrossRef] [PubMed]
- European Database of Suspected Adverse Drug Reaction Reports—Search. Available online: https://www.adrreports.eu/en/search_subst.html# (accessed on 25 March 2025).
- Crisafulli, S.; Khan, Z.; Karatas, Y.; Tuccori, M.; Trifirò, G. An Overview of Methodological Flaws of Real-World Studies Investigating Drug Safety in the Post-Marketing Setting. Expert. Opin. Drug Saf. 2023, 22, 373–380. [Google Scholar] [CrossRef]
- Vintila, B.I.; Arseniu, A.M.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Axente, D.D.; Morgovan, C.; Gligor, F.G. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics 2023, 12, 918. [Google Scholar] [CrossRef]
- Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals 2023, 16, 455. [Google Scholar] [CrossRef]
- Grundmark, B.; Holmberg, L.; Garmo, H.; Zethelius, B. Reducing the Noise in Signal Detection of Adverse Drug Reactions by Standardizing the Background: A Pilot Study on Analyses of Proportional Reporting Ratios-by-Therapeutic Area. Eur. J. Clin. Pharmacol. 2014, 70, 627–635. [Google Scholar] [CrossRef]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef]
- Screening for Adverse Reactions in EudraVigilance. European Medicine Agency. Available online: https://www.ema.europa.eu/en/documents/other/screening-adverse-reactions-eudravigilance_en.pdf (accessed on 27 August 2024).
- Morgovan, C.; Dobrea, C.M.; Butuca, A.; Arseniu, A.M.; Frum, A.; Rus, L.L.; Chis, A.A.; Juncan, A.M.; Gligor, F.G.; Georgescu, C.; et al. Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance. Biomedicines 2024, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- MedCalc Software Ltd. Odds Ratio Calculator. Version 23.1.3. Available online: https://www.medcalc.org/manual/odds-ratio.php (accessed on 22 January 2025).
- Ciancio, G.; Burke, G.W.; Miller, J. Induction Therapy in Renal Transplantation: An Overview of Current Developments. Drugs 2007, 67, 2667–2680. [Google Scholar] [CrossRef]
- Antlanger, M.; Noordzij, M.; van de Luijtgaarden, M.; Carrero, J.J.; Palsson, R.; Finne, P.; Hemmelder, M.H.; Aresté-Fosalba, N.; Reisæter, A.V.; Cases, A.; et al. Sex Differences in Kidney Replacement Therapy Initiation and Maintenance. Clin. J. Am. Soc. Nephrol. 2019, 14, 1616–1625. [Google Scholar] [CrossRef]
- Holmøy, T.; Fevang, B.; Olsen, D.B.; Spigset, O.; Bø, L. Adverse Events with Fatal Outcome Associated with Alemtuzumab Treatment in Multiple Sclerosis. BMC Res. Notes 2019, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, X.; Zhang, J. Safety Assessment of Basiliximab Using Real-World Adverse Event Data from the FDA Adverse Event Reporting System Database: A Retrospective Observational Study. Medicine 2024, 103, e39537. [Google Scholar] [CrossRef]
- van der Zwan, M.; Clahsen-Van Groningen, M.C.; van den Hoogen, M.W.; Kho, M.M.; Roodnat, J.I.; Mauff, K.A.L.; Roelen, D.L.; van Agteren, M.; Baan, C.C.; Hesselink, D.A. Comparison of alemtuzumab and anti-thymocyte globulin treatment for acute kidney allograft rejection. Transplantation 2020, 104, S367. [Google Scholar] [CrossRef]
- Guthoff, M.; Berger, K.; Althaus, K.; Mühlbacher, T.; Bakchoul, T.; Steurer, W.; Nadalin, S.; Königsrainer, A.; Heyne, N. Low-Dose Alemtuzumab Induction in a Tailored Immunosuppression Protocol for Sensitized Kidney Transplant Recipients. BMC Nephrol. 2020, 21, 178. [Google Scholar] [CrossRef]
- Khanolkar, R.A.; Dookie, S.; Li, N.; Khan, F.; Storek, J. Proinflammatory Cytokine Release and Infusional Side-Effects after Anti-Thymocyte Globulin Serotherapy for Graft-Versus-Host Disease Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Blood 2023, 142, 6782. [Google Scholar] [CrossRef]
- Mendes, B.; Figueiredo, C.; Cabral, M.; Borba, A.; Mineiro, A.; Cardoso, J.; Calvinho, P.; Semedo, L.; Fragata, J. Basiliximab vs. Antithymocyte Globulin as Initial Induction Therapy for Lung Transplantation: A National Two Years Review. Transplantology 2022, 3, 267–274. [Google Scholar] [CrossRef]
- Zaza, G.; Tomei, P.; Granata, S.; Boschiero, L.; Lupo, A. Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects. Toxins 2014, 6, 869–891. [Google Scholar] [CrossRef] [PubMed]
- Hannan, S.J.; Iasella, C.J.; Sutton, R.M.; Popescu, I.D.; Koshy, R.; Burke, R.; Chen, X.; Zhang, Y.; Pilewski, J.M.; Hage, C.A.; et al. Lung Transplant Recipients with Telomere-Mediated Pulmonary Fibrosis Have Increased Risk for Hematologic Complications. Am. J. Transplant. 2023, 23, 1590–1602. [Google Scholar] [CrossRef]
- Sasaki, H.; Chikaraishi, T.; Furuhata, S.; Tsutsumi, H.; Miyano, S.; Nakano, T.; Sato, Y.; Kimura, K.; Takahashi, T. Anaphylactic Reaction After Initial Exposure of Basiliximab: Case Reports. Transplant. Proc. 2007, 39, 3457–3459. [Google Scholar] [CrossRef]
- Guillermo, F.; Patricia, L.; Vanina, B.; Rodolfo, M. Anaphylactic Reaction After Initial Exposure to Basiliximab. Res. Rev. J. Med. Health Sci. 2018, 7, 3457–3459. [Google Scholar]
- Wang, K.; Xu, X.; Fan, M. Induction Therapy of Basiliximab versus Antithymocyte Globulin in Renal Allograft: A Systematic Review and Meta-Analysis. Clin. Exp. Nephrol. 2018, 22, 684–693. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, L.K.; van der Zwan, M.; Clahsen-van Groningen, M.C.; van Agteren, M.; Hullegie-Peelen, D.M.; De Winter, B.C.M.; Reinders, M.E.J.; Miranda Afonso, P.; Hesselink, D.A. A Decade of Experience with Alemtuzumab Therapy for Severe or Glucocorticoid-Resistant Kidney Transplant Rejection. Transplant. Int. 2023, 36, 11834. [Google Scholar] [CrossRef]
- Rai, J.; Sinha, S. EP-506 Kidney Transplant Rejection Rate Comparing Alemtuzumab versus Basiliximab during Covid Pandemic against Pre-Covid Period. Br. J. Surg. 2022, 109, znac245.123. [Google Scholar] [CrossRef]
- Lacave, F.; de Terwangne, C.; Darius, T.; Buemi, A.; Mourad, M.; France, Y.; Cardoso Coelho, J.; Fernandes, G.; Goffin, E.; Devresse, A.; et al. Basiliximab vs. No Induction Therapy in Kidney Transplant Recipients with a Low Immunological Risk Profile Receiving Tacrolimus/Mycophenolate/Steroids Maintenance Immunosuppression. J. Clin. Med. 2024, 13, 6151. [Google Scholar] [CrossRef] [PubMed]
- La Hoz, R.M.; Baddley, J.W. Infectious Complications of Immune Modulatory Agents. Curr. Infect. Dis. Rep. 2013, 15, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Terasaki, P.I. Induction Immunosuppression Improves Long-Term Graft and Patient Outcome in Organ Transplantation: An Analysis of United Network for Organ Sharing Registry Data. Transplantation 2010, 90, 1511–1515. [Google Scholar] [CrossRef]
- Lentine, K.L.; Schnitzler, M.A.; Xiao, H.; Brennan, D.C. Long-Term Safety and Efficacy of Antithymocyte Globulin Induction: Use of Integrated National Registry Data to Achieve Ten-Year Follow-up of 10-10 Study Participants. Trials 2015, 16, 365. [Google Scholar] [CrossRef]
- Koyawala, N.; Silber, J.H.; Rosenbaum, P.R.; Wang, W.; Hill, A.S.; Reiter, J.G.; Niknam, B.A.; Even-Shoshan, O.; Bloom, R.D.; Sawinski, D.; et al. Comparing Outcomes between Antibody Induction Therapies in Kidney Transplantation. J. Am. Soc. Nephrol. 2017, 28, 2188–2200. [Google Scholar] [CrossRef]
- Hong, S.Y.; Kim, Y.S.; Jin, K.; Han, S.; Yang, C.W.; Chung, B.H.; Park, W.Y. The Comparative Efficacy and Safety of Basiliximab and Antithymocyte Globulin in Deceased Donor Kidney Transplantation: A Multicenter Cohort Study; The Korean Society of Nephrology: Seoul, Republic of Korea, 2023; Volume 42, p. 138. [Google Scholar]
- Furian, L.; Bestard, O.; Budde, K.; Cozzi, E.; Diekmann, F.; Mamode, N.; Naesens, M.; Pengel, L.H.M.; Schwartz Sorensen, S.; Vistoli, F.; et al. European Consensus on the Management of Sensitized Kidney Transplant Recipients: A Delphi Study. Transplant. Int. 2024, 37, 12475. [Google Scholar] [CrossRef]
- Asderakis, A.; Sabah, T.K.; Watkins, W.J.; Khalid, U.; Szabo, L.; Stephens, M.R.; Griffin, S.; Chavez, R. Thymoglobulin Versus Alemtuzumab Versus Basiliximab Kidney Transplantation from Donors After Circulatory Death. Kidney Int. Rep. 2022, 7, 732–740. [Google Scholar] [CrossRef]
- Furukawa, M.; Chan, E.G.; Ryan, J.P.; Hyzny, E.J.; Sacha, L.M.; Coster, J.N.; Pilewski, J.M.; Lendermon, E.A.; Kilaru, S.D.; McDyer, J.F.; et al. Induction Strategies in Lung Transplantation: Alemtuzumab vs. Basiliximab a Single-Center Experience. Front. Immunol. 2022, 13, 864545. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.C.; Daller, J.A.; Lake, K.D.; Cibrik, D.; Del Castillo, D. Rabbit Antithymocyte Globulin versus Basiliximab in Renal Transplantation. N. Engl. J. Med. 2006, 355, 1967–1977. [Google Scholar] [CrossRef]
- Kim, J.M.; Jang, H.R.; Kwon, C.H.D.; Huh, W.S.; Kim, G.S.; Kim, S.J.; Joh, J.W.; Oh, H.Y. Rabbit Antithymocyte Globulin Compared with Basiliximab in Kidney Transplantation: A Single-Center Study. Transplant. Proc. 2012, 44, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Hardinger, K.L.; Brennan, D.C.; Schnitzler, M.A. Rabbit Antithymocyte Globulin Is More Beneficial in Standard Kidney than in Extended Donor Recipients. Transplantation 2009, 87, 1372–1376. [Google Scholar] [CrossRef]
- Basiliximab: Off-Label Use Warnings for Cardiac Transplantation. React. Wkly. 2014, 1520, 1. [CrossRef]
- CHMP—Simulect: Annex I Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/simulect-epar-product-information_en.pdf (accessed on 23 March 2025).
- Spada, M.; Petz, W.; Bertani, A.; Riva, S.; Sonzogni, A.; Giovannelli, M.; Torri, E.; Torre, G.; Colledan, M.; Gridelli, B. Randomized Trial of Basiliximab Induction versus Steroid Therapy in Pediatric Liver Allograft Recipients under Tacrolimus Immunosuppression. Am. J. Transplant. 2006, 6, 1913–1921. [Google Scholar] [CrossRef]
- Swarup, R.; Allenspach, L.L.; Nemeh, H.W.; Stagner, L.D.; Betensley, A.D. Timing of Basiliximab Induction and Development of Acute Rejection in Lung Transplant Patients. J. Heart Lung Transplant. 2011, 30, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Archdeacon, P.; Dixon, C.; Belen, O.; Albrecht, R.; Meyer, J. Summary of the US FDA Approval of Belatacept. Am. J. Transplant. 2012, 12, 554–562. [Google Scholar] [CrossRef]
- Holdaas, H.; Mjøen, G.; Jardine, A.G. Belatacept: Where the BENEFITS Outweigh the Risk. Am. J. Kidney Dis. 2017, 69, 561–563. [Google Scholar] [CrossRef]
- Abramowicz, D.; Oberbauer, R.; Heemann, U.; Viklicky, O.; Peruzzi, L.; Mariat, C.; Crespo, M.; Budde, K.; Oniscu, G.C. Recent Advances in Kidney Transplantation: A Viewpoint from the Descartes Advisory Board. Nephrol. Dial. Transplant. 2018, 33, 1699–1707. [Google Scholar] [CrossRef]
- Vincenti, F.; Chandran, S.; Raja, R.; Mason, K.; Mall, S.; Maruthamuthu, S.; Martin, T.; Breeden, C.; Silva, J. Impact of Daratumumab and Belatacept on HlA Antibodies in Kidney Transplant Candidates with 100% CPRA: Early Results of ATTAIN (ITN090ST). Nephrol. Dial. Transplant. 2023, 38, gfad063a_6402. [Google Scholar] [CrossRef]
- Pinheiro, L.; Miguel, E.; Maciá, Á.; Stroe, R. Spontaneous Adverse Drug Reactions Subgroup Report César Hernandez García, ES (Subgroup Lead). Available online: https://www.ema.europa.eu/en/documents/report/spontaneous-adverse-drug-reactions-subgroup-report_en.pdf (accessed on 23 March 2025).
- Calapai, F.; Ammendolia, I.; Cardia, L.; Currò, M.; Calapai, G.; Esposito, E.; Mannucci, C. Pharmacovigilance of Risankizumab in the Treatment of Psoriasis and Arthritic Psoriasis: Real-World Data from EudraVigilance Database. Pharmaceutics 2023, 15, 1933. [Google Scholar] [CrossRef]
- European Database of Suspected Adverse Drug Reaction Reports—Disclaimer. Available online: https://www.adrreports.eu/en/disclaimer.html (accessed on 22 March 2025).

| ADR | PT |
|---|---|
| Drug resistance | Drug resistance |
| Multiple-drug resistance | |
| Drug ineffectiveness | Drug ineffective |
| Drug effect less than expected | |
| Therapeutic product effect decreased | |
| Therapeutic product effect incomplete | |
| Therapeutic product ineffective | |
| Therapeutic response decreased | |
| Therapeutic response shortened | |
| Therapy non-responder | |
| Therapy partial responder | |
| Treatment failure | |
| Decreased activity | |
| Transplant rejection | Bone marrow transplant rejection |
| Heart transplant rejection | |
| Intestine transplant rejection | |
| Kidney transplant rejection | |
| Liver transplant rejection | |
| Lung transplant rejection | |
| Multiple organ transplant rejection | |
| Pancreas transplant rejection | |
| Renal and pancreas transplant rejection | |
| Skin graft rejection | |
| Transplant rejection |
| THY | BAS | ALE | BEL | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Age | ||||||||
| NS | 1085 | 21.3% | 760 | 21.9% | 2858 | 31.7% | 404 | 41.4% |
| 0–1 Month | 7 | 0.1% | 1 | 0.0% | 13 | 0.1% | 2 | 0.2% |
| 2 Months–2 Years | 225 | 4.4% | 81 | 2.3% | 156 | 1.7% | 0 | 0.0% |
| 3–11 Years | 494 | 9.7% | 170 | 4.9% | 209 | 2.3% | 1 | 0.1% |
| 12–17 Years | 335 | 6.6% | 142 | 4.1% | 156 | 1.7% | 3 | 0.3% |
| 18–64 Years | 2590 | 50.9% | 2027 | 58.4% | 5122 | 56.7% | 375 | 38.4% |
| 65–85 Years | 353 | 6.9% | 288 | 8.3% | 504 | 5.6% | 192 | 19.7% |
| >85 Years | 0 | 0.0% | 0 | 0.0% | 12 | 0.1% | 0 | 0.00% |
| Sex | ||||||||
| Female | 1900 | 37.3% | 1103 | 31.8% | 5120 | 56.7% | 360 | 36.9% |
| Male | 2397 | 47.1% | 1910 | 55.1% | 2854 | 31.6% | 500 | 51.2% |
| NS | 792 | 15.6% | 456 | 13.1% | 1056 | 11.7% | 117 | 12.0% |
| Origin | ||||||||
| EEA | 1648 | 32.4% | 932 | 26.9% | 5595 | 62.0% | 360 | 36.9% |
| NON-EEA | 3441 | 67.6% | 2537 | 73.1% | 3435 | 38.1% | 617 | 63.2% |
| NS | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Reporter | ||||||||
| HP | 4902 | 96.3% | 3342 | 96.3% | 7769 | 86.0% | 893 | 91.4% |
| Non-HP | 178 | 3.5% | 118 | 3.4% | 1254 | 13.9% | 84 | 8.6% |
| Not Specified | 9 | 0.2% | 9 | 0.3% | 7 | 0.1% | 0 | 0.0% |
| System Organ Classes | THY | BAS | ALE | BEL |
|---|---|---|---|---|
| Blood and lymphatic system disorders | 8.6% | 5.2% | 7.8% | 3.7% |
| Cardiac disorders | 3.1% | 1.7% | 3.4% | 2.9% |
| Congenital, familial, and genetic disorders | 0.3% | 0.1% | 0.2% | 0.1% |
| Ear and labyrinth disorders | 0.1% | 0.1% | 0.4% | 0.3% |
| Endocrine disorders | 0.2% | 0.1% | 4.5% | 0.1% |
| Eye disorders | 0.5% | 0.5% | 1.3% | 1.1% |
| Gastrointestinal disorders | 4.3% | 4.6% | 4.4% | 4.0% |
| General disorders and administration site conditions | 11.5% | 8.2% | 11.9% | 8.8% |
| Hepatobiliary disorders | 2.5% | 1.7% | 1.4% | 0.3% |
| Immune system disorders | 10.7% | 12.5% | 2.9% | 8.5% |
| Infections and infestations | 15.4% | 20.3% | 11.1% | 23.1% |
| Injury, poisoning, and procedural complications | 7.3% | 7.7% | 6.0% | 15.0% |
| Investigations | 6.5% | 7.6% | 8.3% | 3.4% |
| Metabolism and nutrition disorders | 1.8% | 2.6% | 1.4% | 1.1% |
| Musculoskeletal and connective tissue disorders | 1.3% | 1.1% | 3.0% | 1.3% |
| Neoplasms benign, malignant, and unspecified (incl. cysts and polyps) | 3.6% | 3.7% | 2.7% | 4.0% |
| Nervous system disorders | 3.3% | 2.6% | 8.5% | 4.4% |
| Pregnancy, puerperium, and perinatal conditions | 0.1% | 0.2% | 0.3% | 0.3% |
| Product issues | 0.1% | 0.1% | 0.0% | 0.5% |
| Psychiatric disorders | 0.6% | 0.7% | 2.0% | 0.8% |
| Renal and urinary disorders | 4.3% | 7.47% | 2.6% | 5.4% |
| Reproductive system and breast disorders | 0.3% | 0.3% | 0.6% | 0.3% |
| Respiratory, thoracic, and mediastinal disorders | 5.6% | 4.2% | 4.8% | 3.7% |
| Skin and subcutaneous tissue disorders | 2.9% | 1.9% | 7.0% | 2.4% |
| Social circumstances | 0.1% | 0.0% | 0.3% | 0.1% |
| Surgical and medical procedures | 0.6% | 1.4% | 0.6% | 2.2% |
| Vascular disorders | 4.7% | 3.7% | 2.5% | 2.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butuca, A.; Stoicescu, L.; Popa, M.L.; Dobrea, C.M.; Muntean, A.; Morgovan, C.; Pienar, C.; Gligor, F.G.; Ghibu, S.; Popa Ilie, I.R.; et al. Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study. J. Clin. Med. 2025, 14, 3409. https://doi.org/10.3390/jcm14103409
Butuca A, Stoicescu L, Popa ML, Dobrea CM, Muntean A, Morgovan C, Pienar C, Gligor FG, Ghibu S, Popa Ilie IR, et al. Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study. Journal of Clinical Medicine. 2025; 14(10):3409. https://doi.org/10.3390/jcm14103409
Chicago/Turabian StyleButuca, Anca, Laurentiu Stoicescu, Mirela Livia Popa, Carmen Maximiliana Dobrea, Adriana Muntean, Claudiu Morgovan, Corina Pienar, Felicia Gabriela Gligor, Steliana Ghibu, Ioana Rada Popa Ilie, and et al. 2025. "Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study" Journal of Clinical Medicine 14, no. 10: 3409. https://doi.org/10.3390/jcm14103409
APA StyleButuca, A., Stoicescu, L., Popa, M. L., Dobrea, C. M., Muntean, A., Morgovan, C., Pienar, C., Gligor, F. G., Ghibu, S., Popa Ilie, I. R., & Frum, A. (2025). Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study. Journal of Clinical Medicine, 14(10), 3409. https://doi.org/10.3390/jcm14103409






