Emerging Advances in the Management of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
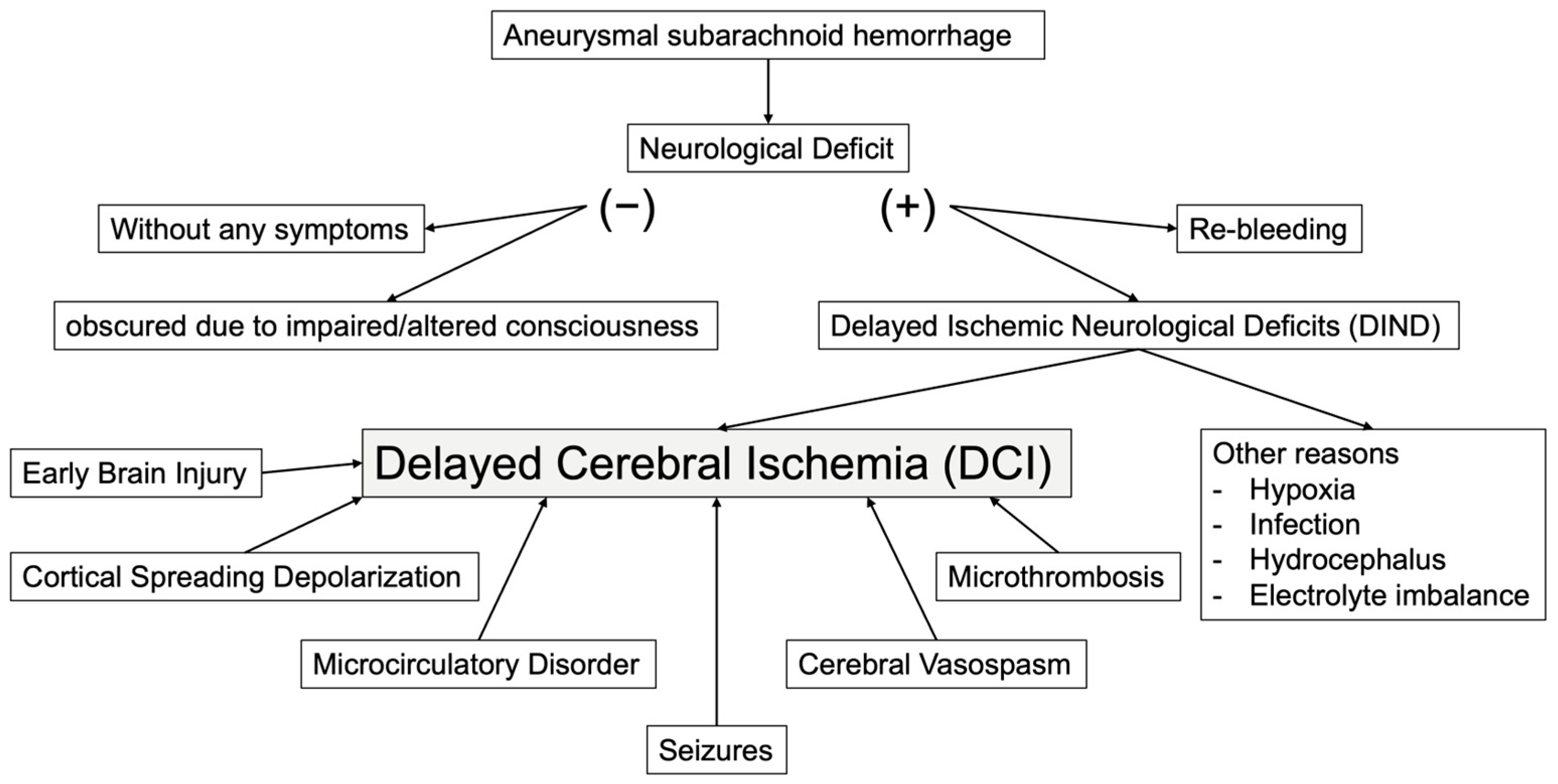
4. The Etiology of DCI
4.1. Cerebral Vasospasm and Subarachnoid Hematoma
4.2. Microcirculatory Disorder
4.3. Microthrombi
4.4. Cortical Spreading Depolarization (CSD)
5. The Management of DCI
6. The Prevention of DCI
6.1. Circulatory Management
6.2. Removal of Subarachnoid Hematoma
6.3. Calcium Channel Blockers
6.4. Clazosentan
6.5. Fasudil Hydrochloride
6.6. Antiplatelet and Anticoagulant Drugs
6.7. Statins
6.8. Magnesium
The Treatment of DCI
- Standardization of DCI Definition and Diagnostic CriteriaA universally accepted and clinically relevant definition of DCI is urgently needed. Current diagnostic paradigms vary widely across institutions and studies, incorporating clinical deterioration, radiographic evidence of infarction, or a combination thereof. This heterogeneity impedes cross-study comparisons and limits the generalizability of findings. The development and widespread adoption of standardized diagnostic criteria that integrate both clinical and imaging elements is essential. Furthermore, the incorporation of molecular biomarkers—including indicators of inflammation, thrombosis, and cerebral metabolic activity—may improve diagnostic accuracy. The application of machine learning techniques to synthesize multimodal data into predictive algorithms represents a promising strategy for the early detection of DCI.
- Toward Personalized Therapeutic StrategiesThere is a growing recognition of the need for individualized therapeutic approaches. Inter-patient variability in treatment response is well documented, rendering standardized treatment paradigms suboptimal. Advances in pharmacogenomics, such as cytochrome P450 polymorphism analysis, may enable personalized dosing regimens, particularly for agents like nimodipine and clazosentan. Furthermore, emerging analytic methodologies, including causal inference techniques and machine learning tools such as causal forests and SHAP (SHapley Additive exPlanations) analysis, offer powerful means to characterize treatment effect heterogeneity and support precision medicine frameworks. Stratifying patients according to predicted therapeutic responsiveness is an essential step toward optimizing individualized care.
- Identification and Evaluation of Novel Therapeutic TargetsThe discovery and rigorous assessment of novel pharmacological agents remain central to future research. While nimodipine is the only drug with consistent evidence for outcome improvement, its effect size remains modest. Investigational agents targeting inflammatory cascades, platelet aggregation, and endothelial dysfunction merit further exploration. Preliminary data suggest that cilostazol, tirofiban [84,85], nadroparin [86], and dapsone [87] may confer benefits in reducing DCI incidence or enhancing functional recovery. Additionally, novel targets such as soluble epoxide hydrolase inhibitors (e.g., GSK2256294) [88] hold promise, especially in trials employing biomarker-guided enrichment strategies.
- Advancement of Non-Invasive Neuromonitoring TechnologiesThe development and integration of real-time, non-invasive neuromonitoring modalities may transform DCI detection and management. Current monitoring techniques are often invasive, sporadic, or operator-dependent. Modalities including transcranial Doppler ultrasonography, near-infrared spectroscopy, electroencephalography, and cerebral microdialysis have demonstrated clinical utility but require further standardization and technological integration. The future lies in the creation of bedside, AI-assisted platforms that consolidate physiological and biochemical data to enable dynamic risk stratification and timely therapeutic interventions.
- Emphasis on Preventive Multimodal StrategiesGiven the multifactorial nature of DCI pathogenesis—including circulatory dysregulation, neuroinflammation, microthrombosis, and impaired autoregulation—prevention must involve multifaceted approaches. Optimization of systemic hemodynamics, early initiation of neuroprotective agents, and prophylactic endovascular techniques should be actively pursued. Intervening during the critical period of vulnerability, typically between days 4 and 10 post-hemorrhage, is of strategic importance.
- Focus on Long-Term, Patient-Centered OutcomesFuture research should place greater emphasis on long-term functional and quality-of-life outcomes. Traditional endpoints such as the 90-day modified Rankin Scale may not fully capture the breadth of patient recovery. Incorporating measures such as return to work, health-related quality of life (e.g., EQ-5D), and patient-reported outcomes (PROMs) will yield a more comprehensive assessment of long-term recovery. Longitudinal studies linking DCI with societal reintegration and sustained functional independence are warranted.
- Promotion of International Collaboration and Data HarmonizationMany existing studies are constrained by limited sample sizes, single-center designs, and methodological heterogeneity. Establishing multicenter prospective registries and conducting individual patient-level meta-analyses are critical for generating high-quality evidence and informing global clinical practice guidelines. International data-sharing initiatives are indispensable to accelerate discovery and facilitate the development of universally applicable, evidence-based management strategies.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; de Rooij, N.K.; Rinkel, G.J. Changes in case fatality of aneurysmal subarachnoid hemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid hemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Korja, M.; Silventoinen, K.; Laatikainen, T.; Jousilahti, P.; Salomaa, V.; Kaprio, J. Cause-specific mortality of 1-year survivors of subarachnoid hemorrhage. Neurology 2013, 80, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kassell, N.F.; Sasaki, T.; Colohan, A.R.; Nazar, G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1985, 16, 562–572. [Google Scholar] [CrossRef]
- Weir, B.; Grace, M.; Hansen, J.; Rothberg, C. Time course of vasospasm in man. J. Neurosurg. 1978, 48, 173–178. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: A review. J. Am. Heart Assoc. 2021, 10, e021845. [Google Scholar] [CrossRef]
- Charpentier, C.; Audibert, G.; Guillemin, F.; Civit, T.; Ducrocq, X.; Bracard, S.; Hepner, H.; Picard, L.; Laxenaire, M.C. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke 1999, 30, 1402–1408. [Google Scholar] [CrossRef]
- Brilstra, E.H.; Rinkel, G.J.; Algra, A.; van Gijn, J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000, 55, 1656–1660. [Google Scholar] [CrossRef]
- Rabinstein, A.A.; Friedman, J.A.; Weigand, S.D.; McClelland, R.L.; Fulgham, J.R.; Manno, E.M.; Atkinson, J.L.; Wijdicks, E.F. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2004, 35, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Locksley, H.B. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. J. Neurosurg. 1966, 25, 321–368. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.J.; Hadjipavlou, G.; Kelly, M.; Westbrook, J.; Pattinson, K.T. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vaso-spasm. Br. J. Anaesth. 2012, 109, 315–329. [Google Scholar] [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Toda, N.; Shimizu, K.; Ohta, T. Mechanism of cerebral arterial contraction induced by blood constituents. J. Neurosurg. 1980, 53, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Toda, N. Mechanisms of contracting action of oxyhemoglobin in isolated monkey and dog cerebral arteries. Am. J. Physiol. 1990, 258 Pt 2, H57–H63. [Google Scholar] [CrossRef] [PubMed]
- Macdonaid, L.; Weir, B.K.; Grace, M.G.; Martin, T.P.; Doi, M.; Cook, D.A. Morphometric analysis of monkey cerebral arteries exposed in vivo to whole blood, oxyhemoglobin, methemoglobin, and bilirubin. Blood Vessel. 1991, 28, 498–510. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Weir, B.K. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 1991, 22, 971–982. [Google Scholar] [CrossRef]
- Yundt, K.D.; Grubb, R.L.; Diringer, M.N.; Powers, W.J. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J. Cereb. Blood Flow. Metab. 1998, 18, 419–424. [Google Scholar] [CrossRef]
- Hart, M.N. Morphometry of brain parenchymal vessels following subarachnoid hemorrhage. Stroke 1980, 11, 653–655. [Google Scholar] [CrossRef]
- Udoetuk, J.D.; Stiefel, M.F.; Hurst, R.W.; Weigele, J.B.; LeRoux, P.D. Admission angiographic cerebral circulation time may predict subsequent angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 2007, 61, 1152–1159; discussion 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Browne, K.D.; Chen, X.-H.; Smith, D.H.; Graham, D.I. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: An autopsy study. Neurosurgery 2006, 59, 781–787; discussion 787–788. [Google Scholar] [CrossRef] [PubMed]
- Frijns, C.J.M.; Fijnheer, R.; Algra, A.; Van Mourik, J.A.; Van Gijn, J.; Rinkel, G.J.E. Early circulating levels of endothelial cell activation markers in aneurysmal subarachnoid haemorrhage: Associations with cerebral ischaemic events and outcome. J. Neurol. Neurosurg. Psychiatry 2006, 77, 77–83. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakamura, S.; Endo, S.; Kuwayama, N.; Naruse, Y.; Takaku, A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem. Res. 1997, 22, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Asakura, H.; Futami, K.; Yamashita, J. Coagulative and fibrinolytic activation in cerebrospinal fluid and plasma after subarachnoid hemorrhage. Neurosurgery 1997, 41, 344–349; discussion 349–350. [Google Scholar] [CrossRef]
- Ohkuma, H.; Suzuki, S.; Kimura, M.; Sobata, E. Role of platelet function in symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1991, 22, 854–859. [Google Scholar] [CrossRef]
- Peltonen, S.; Juvela, S.; Kaste, M.; Lassila, R. Hemostasis and fibrinolysis activation after subarachnoid hemorrhage. J. Neurosurg. 1997, 87, 207–214. [Google Scholar] [CrossRef]
- Dreier, J.P.; Major, S.; Manning, A.; Woitzik, J.; Drenckhahn, C.; Steinbrink, J.; Tolias, C.; Oliveira-Ferreira, A.I.; Fabricius, M.; Hartings, J.A.; et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009, 132 Pt 7, 1866–1881. [Google Scholar] [CrossRef]
- Dreier, J.P.; Woitzik, J.; Fabricius, M.; Bhatia, R.; Major, S.; Drenckhahn, C.; Lehmann, T.-N.; Sarrafzadeh, A.; Willumsen, L.; Hartings, J.A.; et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006, 129 Pt 12, 3224–3237. [Google Scholar] [CrossRef]
- Woitzik, J.; Dreier, J.P.; Hecht, N.; Fiss, I.; Sandow, N.; Major, S.; Winkler, M.; Dahlem, Y.A.; Manville, J.; Diepers, M.; et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow. Metab. 2012, 32, 203–212. [Google Scholar] [CrossRef]
- Brathwaite, S.; Macdonald, R.L. Current management of delayed cerebral ischemia: Update from results of recent clinical trials. Transl. Stroke Res. 2014, 5, 207–226. [Google Scholar] [CrossRef]
- Dayyani, M.; Sadeghirad, B.; Grotta, J.C.; Zabihyan, S.; Ahmadvand, S.; Wang, Y.; Guyatt, G.H.; Amin-Hanjani, S. Prophylactic Therapies for Morbidity and Mortality After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Network Meta-Analysis of Randomized Trials. Stroke 2022, 53, 1993–2005. [Google Scholar] [CrossRef]
- Hasan, D.; Wijdicks, E.F.; Vermeulen, M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Ann. Neurol. 1990, 27, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M.; Vermeulen, M.; Hijdra, A.; van Gijn, J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: Is fluid restriction harmful? Ann. Neurol. 1985, 17, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, M.M.; Walder, B.; Suter, P.M.; Romand, J.-A. Systematic review of the prevention of delayed ischemic neurological deficits with hypertension, hypervolemia, and hemodilution therapy following subarachnoid hemorrhage. J. Neurosurg. 2003, 98, 978–984. [Google Scholar] [CrossRef]
- Sakr, Y.; Dünisch, P.; Santos, C.; Matthes, L.; Zeidan, M.; Reinhart, K.; Kalff, R.; Ewald, C. Poor outcome is associated with less negative fluid balance in patients with aneurysmal subarachnoid hemorrhage treated with prophylactic vasopressor-induced hypertension. Ann. Intensive Care 2016, 6, 25. [Google Scholar] [CrossRef]
- Martini, R.P.; Deem, S.; Brown, M.; Souter, M.J.; Yanez, N.D.; Daniel, S.; Treggiari, M.M. The association between fluid balance and outcomes after subarachnoid hemorrhage. Neurocrit. Care 2012, 17, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370, Erratum in Stroke 2023, 54, e516. [Google Scholar] [CrossRef]
- Al-Tamimi, Y.Z.; Bhargava, D.; Feltbower, R.G.; Hall, G.; Goddard, A.J.; Quinn, A.C.; Ross, S.A. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: A prospective, randomized, controlled trial (LUMAS). Stroke 2012, 43, 677–682. [Google Scholar] [CrossRef]
- Kramer, A.H.; Fletcher, J.J. Locally-administered intrathecal thrombolytics following aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Neurocrit. Care 2011, 14, 489–499. [Google Scholar] [CrossRef]
- Wolf, S.; Mielke, D.; Barner, C. Effectiveness of Lumbar Cerebrospinal Fluid Drain Among Patients With Aneurysmal Subarachnoid Hemorrhage: A Randomized Clinical Trial. JAMA Neurol. 2023, 80, 833–842, Erratum in JAMA Neurol. 2023, 80, 873. https://doi.org/10.1001/jamaneurol.2023.3002. [Google Scholar] [CrossRef]
- Mees, S.D.; Rinkel, G.J.; Feigin, V.L.; Algra, A.; Bergh, W.M.v.D.; Vermeulen, M.; van Gijn, J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007, 2007, Cd000277. [Google Scholar]
- Petruk, K.C.; West, M.; Mohr, G.; Weir, B.K.; Benoit, B.G.; Gentili, F.; Disney, L.B.; Khan, M.I.; Grace, M.; Holness, R.O.; et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J. Neurosurg. 1988, 68, 505–517. [Google Scholar] [CrossRef]
- Haley, E.C., Jr.; Kassell, N.F.; Torner, J.C. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J. Neurosurg. 1993, 78, 537–547. [Google Scholar] [CrossRef]
- Haley, E.C.; Kassell, N.F.; Torner, J.C.; Truskowski, L.L.; Germanson, T.P. A randomized trial of two doses of nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J. Neurosurg. 1994, 80, 788–796. [Google Scholar] [CrossRef]
- Zimmermann, M.; Seifert, V. Endothelin receptor antagonists and cerebral vasospasm. Clin. Auton. Res. 2004, 14, 143–145. [Google Scholar] [CrossRef]
- Kramer, A.; Fletcher, J. Do endothelin-receptor antagonists prevent delayed neurological deficits and poor outcomes after aneurysmal subarachnoid hemorrhage?: A meta-analysis. Stroke 2009, 40, 3403–3406. [Google Scholar] [CrossRef]
- Barth, M.; Capelle, H.-H.; Münch, E.; Thomé, C.; Fiedler, F.; Schmiedek, P.; Vajkoczy, P. Effects of the selective endothelin A (ET(A)) receptor antagonist clazosentan on cerebral perfusion and cerebral oxygenation following severe subarachnoid hemorrhage—Preliminary results from a randomized clinical series. Acta Neurochir. 2007, 149, 911–918. [Google Scholar] [CrossRef]
- Fujimura, M.; Joo, J.Y.; Kim, J.S.; Hatta, M.; Yokoyama, Y.; Tominaga, T. Preventive effect of clazosentan against cerebral vasospasm after clipping surgery for aneurysmal subarachnoid hemorrhage in Japanese and Korean patients. Cerebrovasc. Dis. 2017, 44, 59–67. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Kassell, N.F.; Mayer, S.; Ruefenacht, D.; Schmiedek, P.; Weidauer, S.; Frey, A.; Roux, S.; Pasqualin, A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008, 39, 3015–3021. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping: A randomized, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012, 43, 1463–1469. [Google Scholar] [CrossRef]
- Vajkoczy, P.; Meyer, B.; Weidauer, S.; Raabe, A.; Thome, C.; Ringel, F.; Breu, V.; Schmiedek, P.; The other Study Participants. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: Results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J. Neurosurg. 2005, 103, 9–17. [Google Scholar] [CrossRef]
- Endo, H.; Hagihara, Y.; Kimura, N.; Takizawa, K.; Niizuma, K.; Togo, O.; Tominaga, T. Effects of clazosentan on cerebral vasospasm-related morbidity and all-cause mortality after aneurysmal subarachnoid hemorrhage: Two randomized phase 3 trials in Japanese patients. J. Neurosurg. 2022, 137, 1707–1717. [Google Scholar] [CrossRef]
- Muraoka, S.; Asai, T.; Fukui, T.; Ota, S.; Shimato, S.; Koketsu, N.; Nishizawa, T.; Araki, Y.; Saito, R. Real-world data of clazosentan in combination therapy for aneurysmal subarachnoid hemorrhage: A multicenter retrospective cohort study. Neurosurg. Rev. 2023, 46, 195. [Google Scholar] [CrossRef]
- Muraoka, S.; Izumi, T.; Nishida, K.; Chretien, B.; Ishii, K.; Takeuchi, I.; Nishihori, M.; Goto, S.; Maesawa, S.; Shimato, S.; et al. RECOVER (REtrospective study of Clazosentan and fasudil in subarachnOid hemorrhage: Vasospasm prEvention and patient pRognosis) Study—Comparison of efficacy and safety of clazosentan and fasudil in patients with aneurysmal subarachnoid hemorrhage: A multicenter retrospective cohort study. J. Neurosurg. 2025, in press.
- Shibuya, M.; Suzuki, Y.; Sugita, K.; Saito, I.; Sasaki, T.; Takakura, K.; Nagata, I.; Kikuchi, H.; Takemae, T.; Hidaka, H.; et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J. Neurosurg. 1992, 76, 571–577. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, D.; Guo, J.; Ren, Z.; Zhou, L.; Wang, S.; Zhang, Y.; Xu, B.; Zhao, K.; Wang, R.; et al. Efficacy and Safety of Fasudil in Patients With Subarachnoid Hemorrhage: Final Results of a Randomized Trial of Fasudil Versus Nimodipine. Neurol. Med.-Chir. 2011, 51, 679–683. [Google Scholar] [CrossRef]
- Liu, G.J.; Wang, Z.J.; Wang, Y.F.; Xu, L.L.; Wang, X.L.; Liu, Y.; Luo, G.J.; He, G.H.; Zeng, Y.J. Systematic assessment and meta-analysis of the efficacy and safety of fasudil in the treatment of cerebral vasospasm in patients with subarachnoid hemorrhage. Eur. J. Clin. Pharmacol. 2012, 68, 131–139. [Google Scholar] [CrossRef]
- Mees, S.D.; Bergh, W.M.v.D.; Algra, A.; Rinkel, G.J. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007, 2007, Cd006184. [Google Scholar]
- Sun, G.-U.; Park, E.; Kim, D.-W.; Kang, S.-D. Dual antiplatelet treatment associated with reduced risk of symptomatic vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J. Cerebrovasc. Endovasc. Neurosurg. 2020, 22, 134–140. [Google Scholar] [CrossRef]
- Oppong, M.D.; Gembruch, O.; Pierscianek, D.; Köhrmann, M.; Kleinschnitz, C.; Deuschl, C.; Mönninghoff, C.; Kaier, K.; Forsting, M.; Sure, U.; et al. Post-treatment Antiplatelet Therapy Reduces Risk for Delayed Cerebral Ischemia due to Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2019, 85, 827–833. [Google Scholar] [CrossRef]
- Matsuda, N.; Naraoka, M.; Ohkuma, H.; Shimamura, N.; Ito, K.; Asano, K.; Hasegawa, S.; Takemura, A. Effect of Cilostazol on Cerebral Vasospasm and Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Randomized, Double-Blind, Placebo-Controlled Trial. Cerebrovasc. Dis. 2016, 42, 97–105. [Google Scholar] [CrossRef]
- Senbokuya, N.; Kinouchi, H.; Kanemaru, K.; Ohashi, Y.; Fukamachi, A.; Yagi, S.; Shimizu, T.; Furuya, K.; Uchida, M.; Takeuchi, N.; et al. Effects of cilostazol on cerebral vasospasm after aneurysmal subarachnoid hemorrhage: A multicenter prospective, randomized, open-label blinded end point trial. J. Neurosurg. 2013, 118, 121–130. [Google Scholar] [CrossRef]
- Suzuki, S.; Sayama, T.; Nakamura, T.; Nishimura, H.; Ohta, M.; Inoue, T.; Mannoji, H.; Takeshita, I. Cilostazol improves outcome after subarachnoid hemorrhage: A preliminary report. Cerebrovasc. Dis. 2011, 32, 89–93. [Google Scholar] [CrossRef]
- Wurm, G.; Tomancok, B.; Nussbaumer, K.; Adelwöhrer, C.; Holl, K. Reduction of ischemic sequelae following spontaneous subarachnoid hemorrhage: A double-blind, randomized comparison of enoxaparin versus placebo. Clin. Neurol. Neurosurg. 2004, 106, 97–103. [Google Scholar] [CrossRef]
- Siironen, J.; Juvela, S.; Varis, J.; Porras, M.; Poussa, K.; Ilveskero, S.; Hernesniemi, J.; Lassila, R. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled clinical trial. J. Neurosurg. 2003, 99, 953–959. [Google Scholar] [CrossRef]
- James, R.F.; Khattar, N.K.; Aljuboori, Z.S.; Page, P.S.; Shao, E.Y.; Carter, L.M.; Meyer, K.S.; Daniels, M.W.; Craycroft, J.; Gaughen, J.R.; et al. Continuous infusion of low-dose unfractionated heparin after aneurysmal subarachnoid hemorrhage: A preliminary study of cognitive outcomes. J. Neurosurg. 2019, 130, 1460–1467. [Google Scholar] [CrossRef]
- Kole, M.J.; Wessell, A.P.; Ugiliweneza, B.; Cannarsa, G.J.; Fortuny, E.; Stokum, J.A.; Shea, P.; Chryssikos, T.; Khattar, N.K.; Crabill, G.A.; et al. Low-Dose Intravenous Heparin Infusion After Aneurysmal Subarachnoid Hemorrhage is Associated With Decreased Risk of Delayed Neurological Deficit and Cerebral Infarction. Neurosurgery 2021, 88, 523–530. [Google Scholar] [CrossRef]
- Simard, J.M.; Aldrich, E.F.; Schreibman, D.; James, R.F.; Polifka, A.; Beaty, N. Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: A preliminary assessment. J. Neurosurg. 2013, 119, 1611–1619. [Google Scholar] [CrossRef]
- Shen, J.; Huang, K.Y.; Zhu, Y.; Pan, J.W.; Jiang, H.; Weng, Y.X.; Zhan, R.Y. Effect of statin treatment on vasospasm-related morbidity and functional outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 291–301. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Zhai, Q.; Zhang, X.; Jing, H.; Li, K.; Liu, S.; Han, S.; Li, L.; Shi, X.; et al. Optimal course of statins for patients with aneurysmal subarachnoid hemorrhage: Is longer treatment better? A meta-analysis of randomized controlled trials. Front. Neurosci. 2021, 15, 757505. [Google Scholar] [CrossRef]
- Su, S.H.; Xu, W.; Hai, J.; Wu, Y.F.; Yu, F. Effects of statins-use for patients with aneurysmal subarachnoid hemorrhage: A meta-analysis of randomized controlled trials. Sci. Rep. 2014, 4, 4573. [Google Scholar] [CrossRef]
- Rabinstein, A.A. Vasospasm and statin therapy: Yet another cautionary tale. Neurocrit. Care 2010, 12, 310–312. [Google Scholar] [CrossRef]
- Odom, M.J.; Zuckerman, S.L.; Mocco, J. The role of magnesium in the management of cerebral vasospasm. Neurol. Res. Int. 2013, 2013, 943914. [Google Scholar] [CrossRef]
- Jeon, J.S.; Sheen, S.H.; Hwang, G.; Kang, S.H.; Heo, D.H.; Cho, Y.J. Intravenous magnesium infusion for the prevention of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J. Korean Neurosurg. Soc. 2012, 52, 75–79. [Google Scholar] [CrossRef]
- Reddy, D.; Fallah, A.; Petropoulos, J.A.; Farrokhyar, F.; Macdonald, R.L.; Jichici, D. Prophylactic magnesium sulfate for aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Neurocrit. Care 2014, 21, 356–364. [Google Scholar] [CrossRef]
- Jost, S.C.; Diringer, M.N.; Zazulia, A.R.; Videen, T.O.; Aiyagari, V.; Grubb, R.L.; Powers, W.J. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. J. Neurosurg. 2005, 103, 25–30. [Google Scholar] [CrossRef]
- Francoeur, C.L.; Mayer, S.A. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit. Care 2016, 20, 277. [Google Scholar] [CrossRef]
- Zwienenberg-Lee, M.; Hartman, J.; Rudisill, N.; Madden, L.K.; Smith, K.; Eskridge, J.; Newell, D.; Verweij, B.; Bullock, M.R.; Baker, A.; et al. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: Results of a phase II multicenter, randomized, clinical trial. Stroke 2008, 39, 1759–1765. [Google Scholar] [CrossRef]
- Chalouhi, N.; Tjoumakaris, S.; Thakkar, V.; Theofanis, T.; Hammer, C.; Hasan, D.; Starke, R.M.; Wu, C.; Gonzalez, L.F.; Rosenwasser, R.; et al. Endovascular management of cerebral vasospasm following aneurysm rupture: Outcomes and predictors in 116 patients. Clin. Neurol. Neurosurg. 2014, 118, 26–31. [Google Scholar] [CrossRef]
- Rosenwasser, R.H.; Armonda, R.A.; Thomas, J.E.; Benitez, R.P.; Gannon, P.M.; Harrop, J. Therapeutic modalities for the management of cerebral vasospasm: Timing of endovascular options. Neurosurgery 1999, 44, 975–979; discussion 979–980. [Google Scholar] [CrossRef]
- Hollingworth, M.; Chen, P.R.; Goddard, A.J.; Coulthard, A.; Söderman, M.; Bulsara, K.R. Results of an International Survey on the Investigation and Endovascular Management of Cerebral Vasospasm and Delayed Cerebral Ischemia. World Neurosurg. 2015, 83, 1120–1126.e1. [Google Scholar] [CrossRef]
- Zanaty, M.; Allan, L.; Samaniego, E.A.; Piscopo, A.; Ryan, E.; Torner, J.C.; Hasan, D. Phase 1/2a Trial of ISPASM. Stroke 2021, 52, 3750–3758. [Google Scholar] [CrossRef]
- Zanaty, M.; Osorno-Cruz, C.; Byer, S.; Roa, J.A.; Limaye, K.; Ishii, D.; Nakagawa, D.; Torner, J.; Yongjun, L.; Ortega-Gutiérrez, S.; et al. Tirofiban Protocol Protects Against Delayed Cerebral Ischemia: A Case-Series Study. Neurosurgery 2020, 87, E552–E556. [Google Scholar] [CrossRef]
- Post, R.; Zijlstra, I.A.J.; Berg, R.V.D.; Coert, B.A.; Verbaan, D.; Vandertop, W.P. High-Dose Nadroparin Following Endovascular Aneurysm Treatment Benefits Outcome After Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2018, 83, 281–287. [Google Scholar] [CrossRef]
- García-Pastor, C.; de Llano, J.P.N.-G.; Balcázar-Padrón, J.C.; Tristán-López, L.; Rios, C.; Díaz-Ruíz, A.; Rodríguez-Hernandez, L.A.; Nathal, E. Neuroprotective effect of dapsone in patients with aneurysmal subarachnoid hemorrhage: A prospective, randomized, double-blind, placebo-controlled clinical trial. Neurosurg. Focus 2022, 52, E12. [Google Scholar] [CrossRef]
- Martini, R.P.; Siler, D.; Cetas, J.; Alkayed, N.J.; Allen, E.; Treggiari, M.M. A Double-Blind, Randomized, Placebo-Controlled Trial of Soluble Epoxide Hydrolase Inhibition in Patients with Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2022, 36, 905–915. [Google Scholar] [CrossRef]
| Drugs | Sample Size/Study Type | Primary Endpoints | Effect on DCI Prevention | Improvement in Functional Outcome | Comments |
|---|---|---|---|---|---|
| Nimodipine | Intra-arterial administration (n = 10) + pharmacogenomic review | Vasospasm, cerebral oxygenation, GOS | Yes (vasospasm prevention, improves pO₂) | Yes (all patients had favorable GOS) | Gold standard treatment with proven outcome benefit. |
| Nicardipine Implant | Meta-analysis (n = 284) + NMA (n = 866) | DCI, mRS, angiographic vasospasm | Yes (OR 0.21–0.30 in meta-analysis) | Trend toward improvement (not significant) | Effective adjunct in clipping cases; outcome benefit inconclusive. |
| Clazosentan | RCT (n = 409) + retrospective (n = 221) + meta-analysis (n = 2778) | DCI incidence, mRS, rescue therapy | Yes (strong; reduces vasospasm and DCI) | Mixed (no effect in RCTs, benefit in retrospective study) | Prevents vasospasm and DCI but increases adverse events; functional benefit limited. |
| Fasudil | Retrospective study (n = 221, PS-matched n = 27) | DCI, vasospasm, 6-month mRS | Limited (less effective than clazosentan) | Some improvement reported | Traditional Japanese treatment with limited efficacy. |
| Cilostazol | Meta-analysis including RCTs (n = 543) | Symptomatic vasospasm, infarction, poor outcome | Yes (reduces CV, infarction, DCI) | Yes (OR 0.40 for good outcome) | Low-risk drug with significant effect on both DCI and functional recovery. |
| Statins | Meta-analysis (n = 1885) | DCI, mRS, mortality | Yes (short-term use effective) | No clear improvement | Suppresses DCI, but no consistent improvement in outcomes. |
| Magnesium | RCT + retrospective (~n = 250) | DCI, mRS, vasospasm incidence | Uncertain (mixed results) | Some improvement in retrospective reports | Mixed findings; not recommended for routine use yet. |
| Tirofiban | RCT (n = 30) + retrospective (n = 102) | DCI, vasospasm, bleeding events | Yes (significant reduction in small RCT) | Improvement seen in small RCT | Reduces vasospasm and DCI; bleeding risk must be considered. |
| Nadroparin | Retrospective cohort (n = 158, high- vs. low-dose) | DCI, mortality, discharge destination | No for DCI, but reduced mortality and better discharge | Yes (lower mortality, better disposition) | High-dose group showed reduced mortality; no increase in adverse events. |
| Dapsone | Double-blind RCT (n = 48) | DCI, cerebral infarction, mRS (discharge, 3 mo) | Yes (DCI: 26.9% vs. 63.6%, p = 0.011) | Yes (favorable mRS and lower infarction rate) | Promising results in all endpoints; needs validation in larger trials. |
| GSK2256294 | Phase Ib RCT (n = 19) | Safety, DCI, biomarkers | Possibly (trends in small trial; needs further study) | Not assessable; further trials needed | sEH inhibitor with good safety profile; further investigation warranted. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraoka, S.; Izumi, T.; Nishihori, M.; Goto, S.; Takeuchi, I.; Saito, R. Emerging Advances in the Management of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Narrative Review. J. Clin. Med. 2025, 14, 3403. https://doi.org/10.3390/jcm14103403
Muraoka S, Izumi T, Nishihori M, Goto S, Takeuchi I, Saito R. Emerging Advances in the Management of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Narrative Review. Journal of Clinical Medicine. 2025; 14(10):3403. https://doi.org/10.3390/jcm14103403
Chicago/Turabian StyleMuraoka, Shinsuke, Takashi Izumi, Masahiro Nishihori, Shunsaku Goto, Issei Takeuchi, and Ryuta Saito. 2025. "Emerging Advances in the Management of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Narrative Review" Journal of Clinical Medicine 14, no. 10: 3403. https://doi.org/10.3390/jcm14103403
APA StyleMuraoka, S., Izumi, T., Nishihori, M., Goto, S., Takeuchi, I., & Saito, R. (2025). Emerging Advances in the Management of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Narrative Review. Journal of Clinical Medicine, 14(10), 3403. https://doi.org/10.3390/jcm14103403








