Neurometabolite Changes After Transcranial Photobiomodulation in Major Depressive Disorder: A Randomized Controlled Trial Investigating Dose-Dependent Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization
2.3. Blinding
2.4. Eligibility Criteria
2.5. Intervention
2.6. Protocol Adherence and Site Standardization
2.7. Outcomes
2.8. Study Data Analysis Scheme
2.9. Statistical Analysis
3. Results
3.1. Three-Visit Analysis
3.1.1. Demographics
3.1.2. Changes in Neurometabolite Levels
3.1.3. Changes in Depression Severity (MADRS Scores)
3.1.4. Changes in Depression Severity (SDQ Scores)
3.2. Two-Visit Analysis
3.2.1. Demographics
3.2.2. Changes in Neurometabolite Levels
3.2.3. Changes in Depression Severity (MADRS Scores)
3.2.4. Changes in Depression Severity (SDQ Scores)
3.3. One-Visit Analysis
3.3.1. Demographics
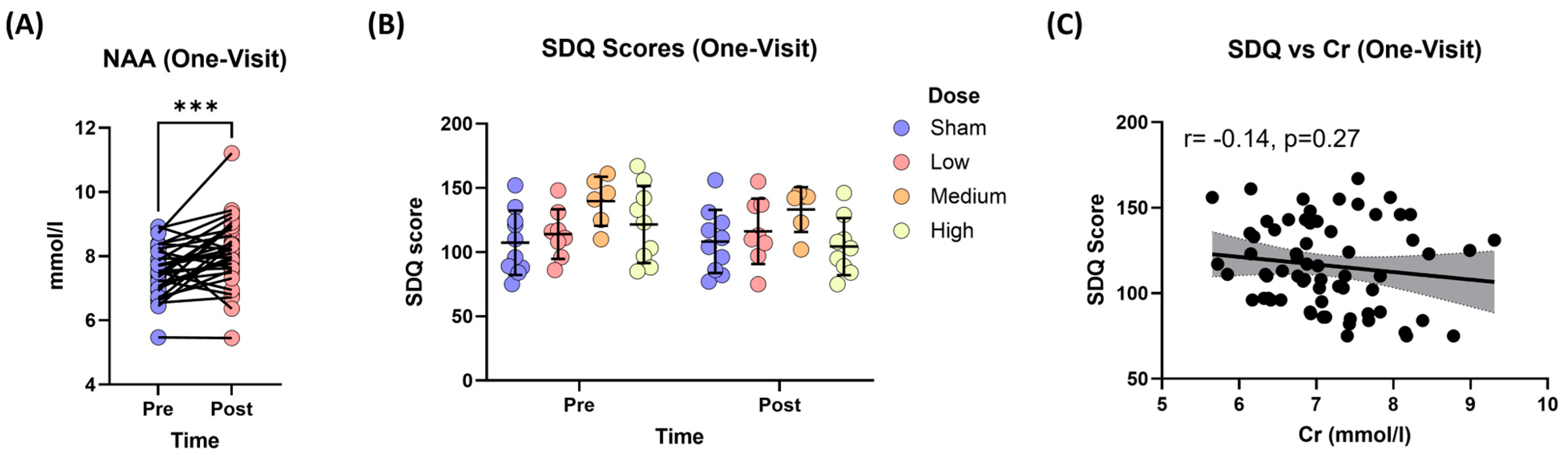
3.3.2. Changes in Neurometabolites
3.3.3. Changes in Depression Severity (MADRS Scores)
3.3.4. Changes in Depression Severity (SDQ Scores)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Araujo Coelho, D.R.; Vieira, W.F.; Moehlecke Iser, B.; Lampert, R.M.F.; Traebert, E.; da Silva, B.B.; de Oliveira, B.H.; Leão, G.M.; de Souza, G.; et al. Factors Associated with the Development of Depression and the Influence of Obesity on Depressive Disorders: A Narrative Review. Biomedicines 2024, 12, 1994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, X.; Xiao, C. Standardized Treatment Strategy for Depressive Disorder. Adv. Exp. Med. Biol. 2019, 1180, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining Light on the Head: Photobiomodulation for Brain Disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Vieira, W.F.; Iosifescu, D.V.; McEachern, K.M.; Gersten, M.; Cassano, P. Photobiomodulation: An Emerging Treatment Modality for Depression. Psychiatr. Clin. N. Am. 2023, 46, 331–348. [Google Scholar] [CrossRef]
- Vieira, W.F.; Gersten, M.; Caldieraro, M.A.K.; Cassano, P. Photobiomodulation for Major Depressive Disorder: Linking Transcranial Infrared Light, Biophotons and Oxidative Stress. Harv. Rev. Psychiatry 2023, 31, 124–141. [Google Scholar] [CrossRef]
- Alberti, A.; Vieira, W.F.; Coelho, D.R.A.; Fernandes Martins, D. A Grant Report: Examining the Efficacy of Remote Photobiomodulation Therapy in Adolescents with Major Depressive Disorder. Photonics 2024, 11, 839. [Google Scholar] [CrossRef]
- Coelho, D.R.A.; Puerto, A.M.H.; Vieira, W.F.; Lohmann, C.A.; Shahab, M.H.; Gersten, M.B.; Vahedifard, F.; McEachern, K.M.; Clancy, J.A.; Cassano, P. Transcranial Photobiomodulation for Executive Function in Bipolar Disorder (TPEB): Study Protocol. Photonics 2024, 11, 761. [Google Scholar] [CrossRef]
- Gaggi, N.L.; Collins, K.A.; Gonzalez-Castillo, J.; Hurtado, A.M.; Castellanos, F.X.; Osorio, R.; Cassano, P.; Iosifescu, D.V. Transcranial Photobiomodulation Increases Intrinsic Brain Activity within Irradiated Areas in Early Alzheimer’s Disease: Potential Link with Cerebral Metabolism. Brain Stimul. 2024, 17, 208–210. [Google Scholar] [CrossRef]
- Coelho, D.R.A.; Gersten, M.; Jimenez, A.S.; Fregni, F.; Cassano, P.; Vieira, W.F. Treating Neuropathic Pain and Comorbid Affective Disorders: Preclinical and Clinical Evidence. Pain. Pract. 2024, 24, 937–955. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Bicknell, B.; Laakso, E.-L.; Heller, G.; Jalilitabaei, P.; Tilley, S.; Mitrofanis, J.; Kiat, H. Improvements in Clinical Signs of Parkinson’s Disease Using Photobiomodulation: A Prospective Proof-of-Concept Study. BMC Neurol. 2021, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.R.A.; Renet, C.; López-Rodríguez, S.; Cassano, P.; Vieira, W.F. Transcranial Photobiomodulation for Neurodevelopmental Disorders: A Narrative Review. Photochem. Photobiol. Sci. 2024, 23, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.F.; Coelho, D.R.A.; Gersten, M.; Puerto, A.M.H.; Kalli, S.; Gonzalez-Garibay, G.; McEachern, K.; Clancy, J.A.; Skotko, B.G.; Abbeduto, L.; et al. TransPhoM-DS Study Grant Report: Rationale and Protocol for Investigating the Efficacy of Low-Power Transcranial Photobiomodulation on Language, Executive Function, Attention, and Memory in Down Syndrome. Photonics 2024, 11, 670. [Google Scholar] [CrossRef]
- Coelho, D.R.A.; Salvi, J.D.; Vieira, W.F.; Cassano, P. Inflammation in Obsessive-Compulsive Disorder: A Literature Review and Hypothesis-Based Potential of Transcranial Photobiomodulation. J. Neurosci. Res. 2024, 102, e25317. [Google Scholar] [CrossRef]
- Gaggi, N.L.; Roy, N.L.; Song, X.; Peterson, A.L.; Iosifescu, D.V.; Diaz-Arrastia, R.; Cassano, P.; Kim, J.J. Transcranial Photobiomodulation and Chronic Traumatic Brain Injury. Photonics 2024, 11, 260. [Google Scholar] [CrossRef]
- Cassano, P.; Cusin, C.; Mischoulon, D.; Hamblin, M.R.; De Taboada, L.; Pisoni, A.; Chang, T.; Yeung, A.; Ionescu, D.F.; Petrie, S.R.; et al. Near-Infrared Transcranial Radiation for Major Depressive Disorder: Proof of Concept Study. Psychiatry J. 2015, 2015, 352979. [Google Scholar] [CrossRef]
- Cassano, P.; Petrie, S.R.; Mischoulon, D.; Cusin, C.; Katnani, H.; Yeung, A.; De Taboada, L.; Archibald, A.; Bui, E.; Baer, L.; et al. Transcranial Photobiomodulation for the Treatment of Major Depressive Disorder. The ELATED-2 Pilot Trial. Photomed. Laser Surg. 2018, 36, 634–646. [Google Scholar] [CrossRef]
- Weerasekera, A.; Coelho, D.R.A.; Ratai, E.-M.; Collins, K.A.; Puerto, A.M.H.; De Taboada, L.; Gersten, M.B.; Clancy, J.A.; Hoptman, M.J.; Irvin, M.K.; et al. Dose-Dependent Effects of Transcranial Photobiomodulation on Brain Temperature in Patients with Major Depressive Disorder: A Spectroscopy Study. Lasers Med. Sci. 2024, 39, 249. [Google Scholar] [CrossRef]
- Bustillo, J.R. Use of Proton Magnetic Resonance Spectroscopy in the Treatment of Psychiatric Disorders: A Critical Update. Dialogues Clin. Neurosci. 2013, 15, 329–337. [Google Scholar] [CrossRef]
- Schuff, N.; Meyerhoff, D.J.; Mueller, S.; Chao, L.; Sacrey, D.T.; Laxer, K.; Weiner, M.W. N-Acetylaspartate as a Marker of Neuronal Injury in Neurodegenerative Disease. Adv. Exp. Med. Biol. 2006, 576, 241–363. [Google Scholar]
- Pajares, S.; Arias, A.; García-Villoria, J.; Briones, P.; Ribes, A. Role of Creatine as Biomarker of Mitochondrial Diseases. Mol. Genet. Metab. 2013, 108, 119–124. [Google Scholar] [CrossRef]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline Transport for Phospholipid Synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Cai, K.; Singh, A.; Hariharan, H.; Reddy, R. In Vivo Mapping of Brain Myo-Inositol. Neuroimage 2011, 54, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Şendur, İ.; Kalkan Oğuzhanoğlu, N.; Sözeri Varma, G. Study on Dorsolateral Prefrontal Cortex Neurochemical Metabolite Levels of Patients with Major Depression Using H-MRS Technique. Turk. Psikiyatr. Derg. 2020, 31, 75–83. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Y.; Ma, L.; Yang, W.; Pu, J.; Shen, Y.; Liu, Y.; Zhang, H.; Lv, F.; Hu, L. Altered Neurometabolite Levels in the Brains of Patients with Depression: A Systematic Analysis of Magnetic Resonance Spectroscopy Studies. J. Affect. Disord. 2023, 328, 95–102. [Google Scholar] [CrossRef]
- Iosifescu, D.V.; Collins, K.A.; Hurtado-Puerto, A.; Irvin, M.K.; Clancy, J.A.; Sparpana, A.M.; Sullivan, E.F.; Parincu, Z.; Ratai, E.-M.; Funes, C.J.; et al. Grant Report on the Transcranial near Infrared Radiation and Cerebral Blood Flow in Depression (TRIADE) Study. Photonics 2023, 10, 90. [Google Scholar] [CrossRef]
- Bottomley, P.A. Spatial localization in NMR spectroscopy in vivo. Ann. N. Y. Acad. Sci. 1987, 508, 333–348. [Google Scholar] [CrossRef]
- Harris, A.D.; Puts, N.A.J.; Edden, R.A.E. Tissue Correction for GABA-Edited MRS: Considerations of Voxel Composition, Tissue Segmentation, and Tissue Relaxations. J. Magn. Reson. Imaging 2015, 42, 1431–1440. [Google Scholar] [CrossRef]
- Penny, W.; Friston, K.; Ashburner, J.; Kiebel, S.; Nichols, T. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar] [CrossRef]
- Gasparovic, C.; Song, T.; Devier, D.; Bockholt, H.J.; Caprihan, A.; Mullins, P.G.; Posse, S.; Jung, R.E.; Morrison, L.A. Use of Tissue Water as a Concentration Reference for Proton Spectroscopic Imaging. Magn. Reson. Med. 2006, 55, 1219–1226. [Google Scholar] [CrossRef]
- Träber, F.; Block, W.; Lamerichs, R.; Gieseke, J.; Schild, H.H. 1H Metabolite Relaxation Times at 3.0 Tesla: Measurements of T1 and T2 Values in Normal Brain and Determination of Regional Differences in Transverse Relaxation. J. Magn. Reson. Imaging 2004, 19, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wansapura, J.P.; Holland, S.K.; Dunn, R.S.; Ball, W.S. NMR Relaxation Times in the Human Brain at 3.0 Tesla. J. Magn. Reson. Imaging 1999, 9, 531–538. [Google Scholar] [CrossRef]
- Davidson, J.; Turnbull, C.D.; Strickland, R.; Miller, R.; Graves, K. The Montgomery-Asberg Depression Scale: Reliability and Validity. Acta Psychiatr. Scand. 1986, 73, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Pedrelli, P.; Blais, M.A.; Alpert, J.E.; Shelton, R.C.; Walker, R.S.W.; Fava, M. Reliability and Validity of the Symptoms of Depression Questionnaire (SDQ). CNS Spectr. 2014, 19, 535–546. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 5 August 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, M.A.A. N-Acetylaspartate in the CNS: From Neurodiagnostics to Neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Tassone, M.; Tozzi, F.; Rutigliano, G. Proton Magnetic Resonance Spectroscopy of N-Acetyl Aspartate in First Depressive Episode and Chronic Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2024, 355, 265–282. [Google Scholar] [CrossRef]
- Gonsalves, M.A.; White, T.L.; Barredo, J.; Fukuda, A.M.; Joyce, H.E.; Harris, A.D.; Carpenter, L.L. Repetitive Transcranial Magnetic Stimulation-Associated Changes in Neocortical Metabolites in Major Depression: A Systematic Review. Neuroimage Clin. 2022, 35, 103049. [Google Scholar] [CrossRef]
- Naeser, M.A.; Martin, P.I.; Ho, M.D.; Krengel, M.H.; Bogdanova, Y.; Knight, J.A.; Hamblin, M.R.; Fedoruk, A.E.; Poole, L.G.; Cheng, C.; et al. Transcranial Photobiomodulation Treatment: Significant Improvements in Four Ex-Football Players with Possible Chronic Traumatic Encephalopathy. J. Alzheimers Dis. Rep. 2023, 7, 77–105. [Google Scholar] [CrossRef]
- Roy, P.; Tomassoni, D.; Nittari, G.; Traini, E.; Amenta, F. Effects of Choline Containing Phospholipids on the Neurovascular Unit: A Review. Front. Cell Neurosci. 2022, 16, 988759. [Google Scholar] [CrossRef]
- Riley, C.A.; Renshaw, P.F. Brain Choline in Major Depression: A Review of the Literature. Psychiatry Res. Neuroimaging 2018, 271, 142–153. [Google Scholar] [CrossRef]
- Yang, X.-R.; Langevin, L.M.; Jaworska, N.; Kirton, A.; Lebel, R.M.; Harris, A.D.; Jasaui, Y.; Wilkes, T.C.; Sembo, M.; Swansburg, R.; et al. Proton Spectroscopy Study of the Dorsolateral Prefrontal Cortex in Youth with Familial Depression. Psychiatry Clin. Neurosci. 2016, 70, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Meftahi, G.H.; Hatef, B.; Pirzad Jahromi, G. Creatine Activity as a Neuromodulator in the Central Nervous System. Arch. Razi Inst. 2023, 78, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, P.; Paioni, S.L.; Kozhuharova, P.; Orlov, N.; Lythgoe, D.J.; Daniju, Y.; Morgenroth, E.; Barker, H.; Allen, P. Relationship between Depression, Prefrontal Creatine and Grey Matter Volume. J. Psychopharmacol. 2021, 35, 1464–1472. [Google Scholar] [CrossRef]
- Guo, Z.-L.; Liu, T.C.-Y. Quantitative and Integrative Photobiomodulation. Photobiomodul. Photomed. Laser Surg. 2022, 40, 659–660. [Google Scholar] [CrossRef]
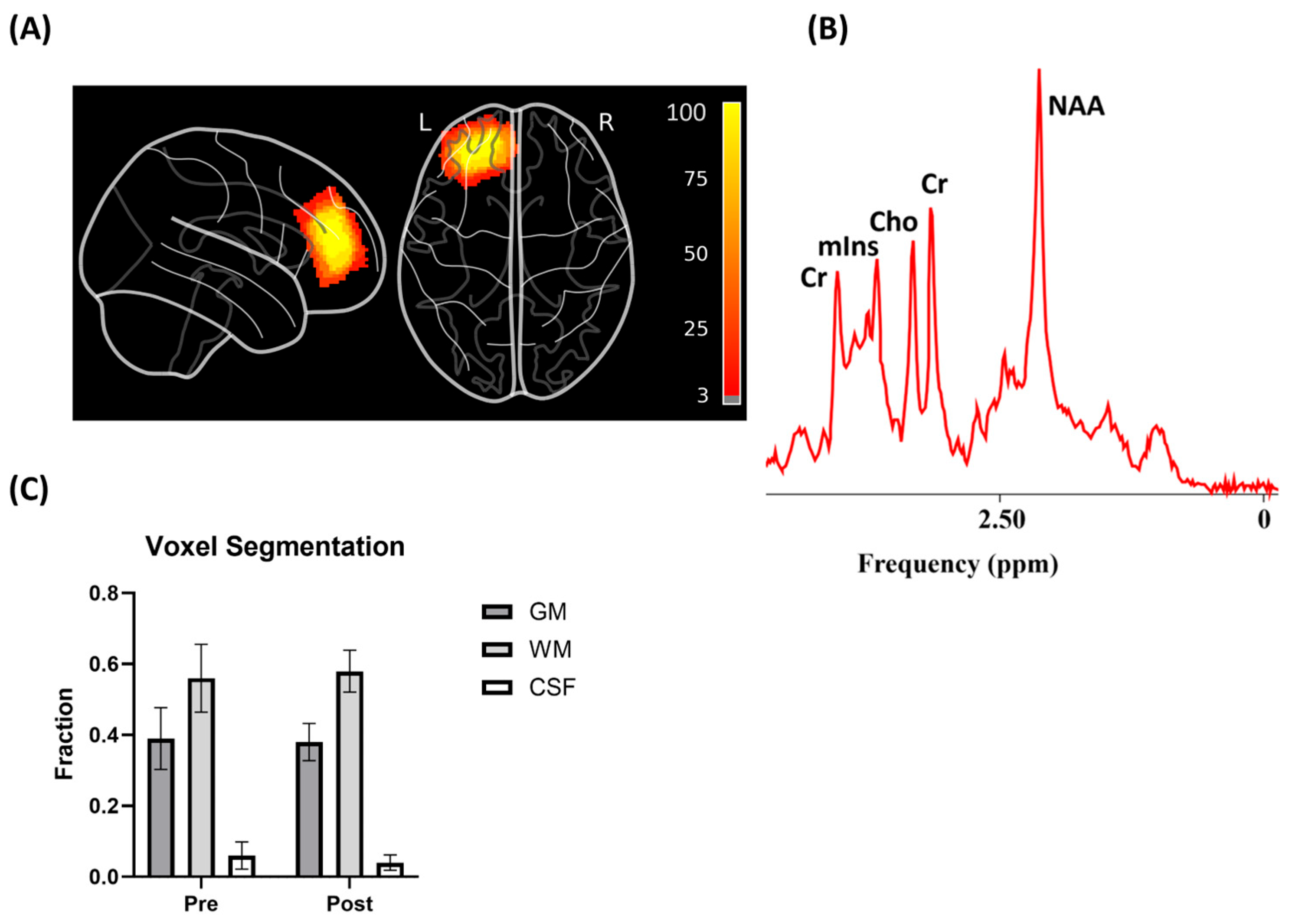

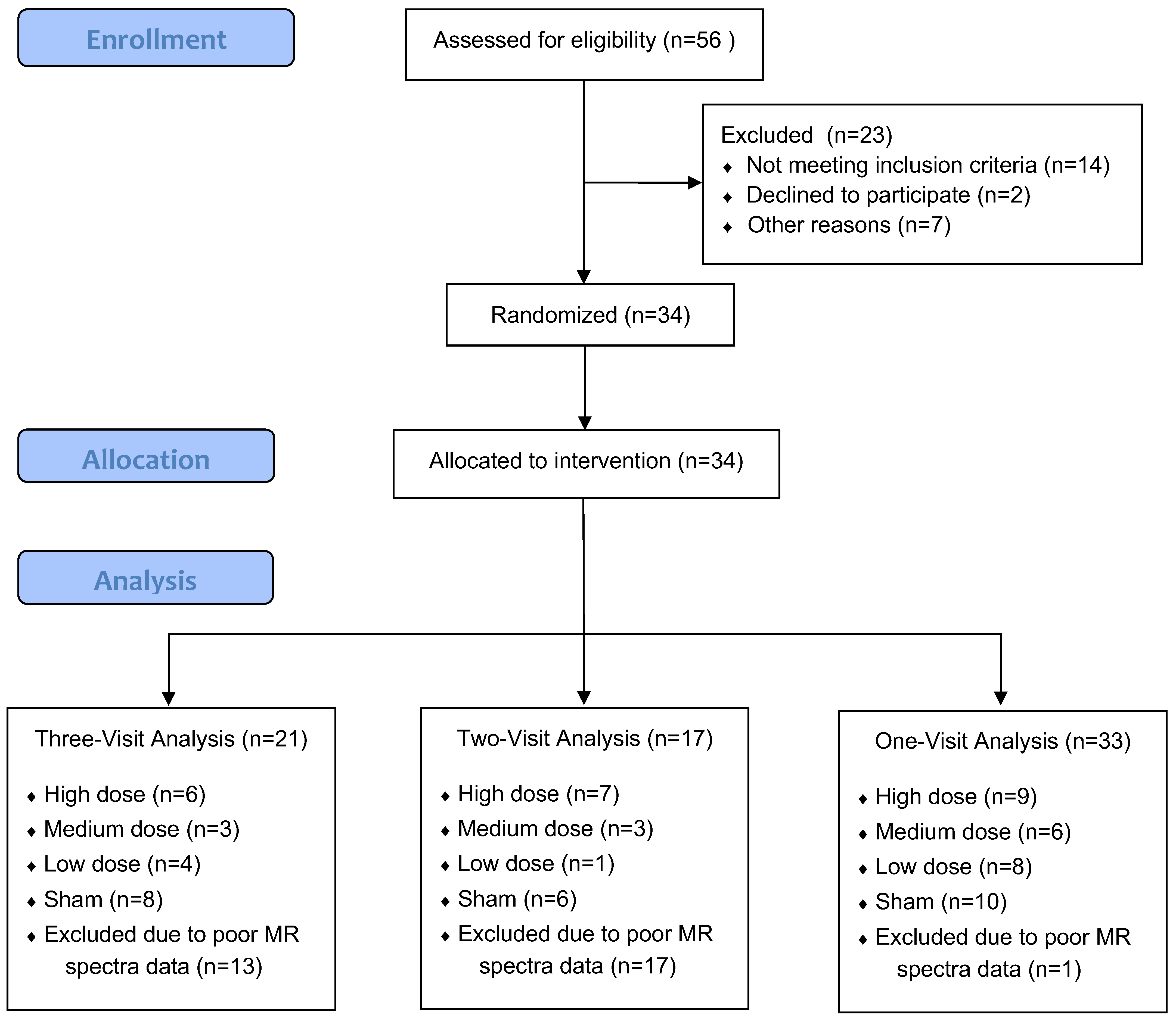
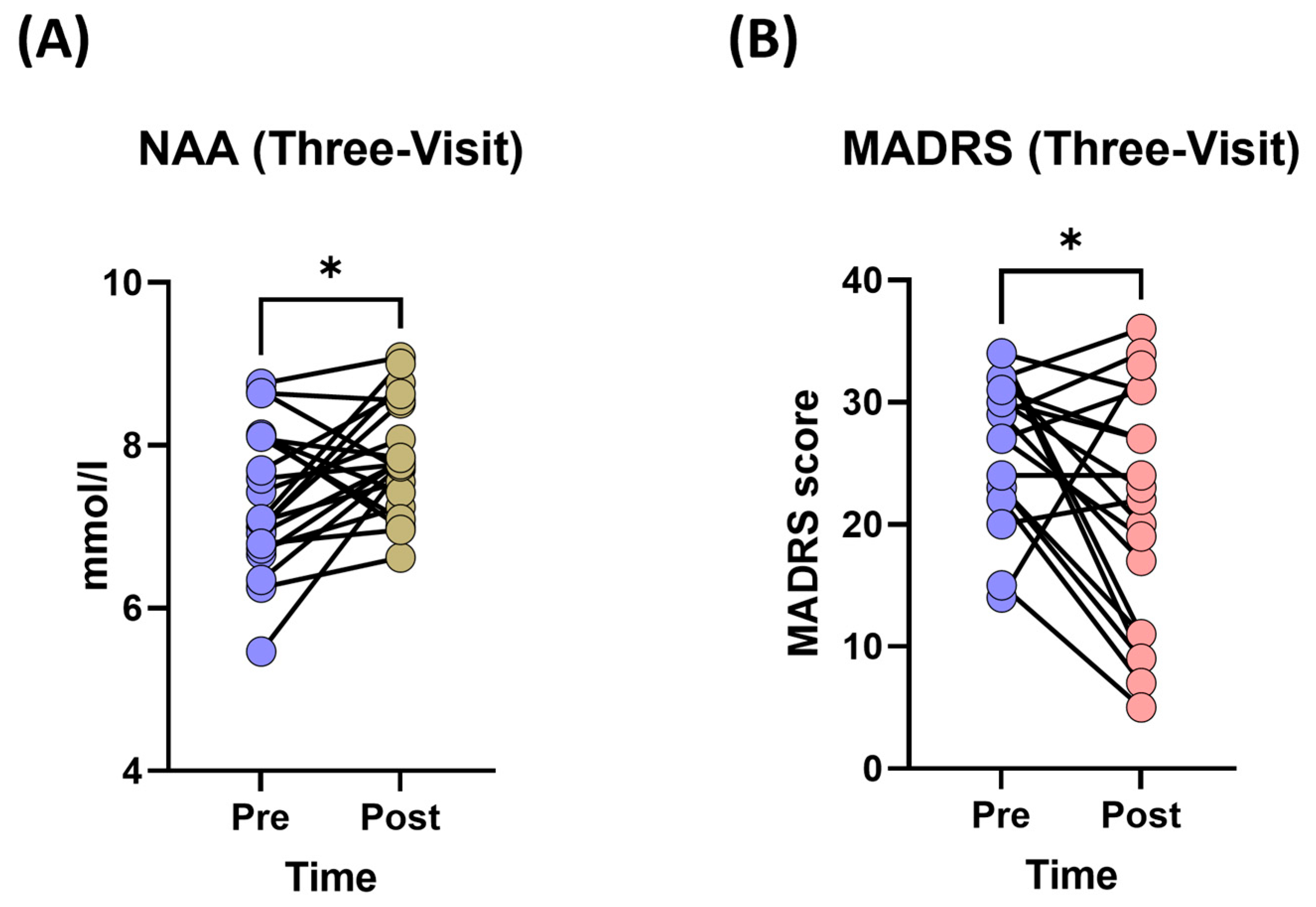
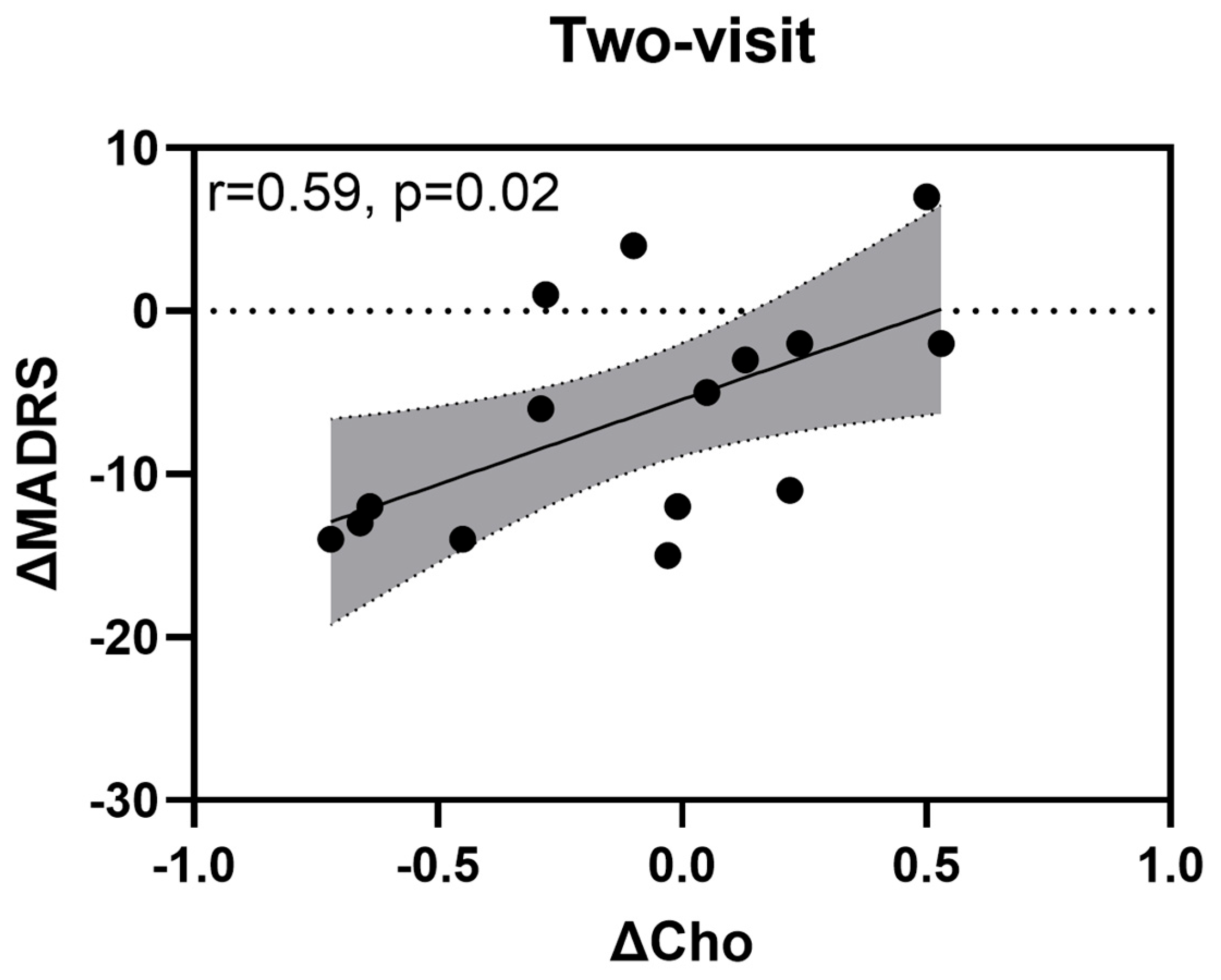

| Parameters | Low Dose | Medium Dose | High Dose | Sham |
|---|---|---|---|---|
| NIR Source | Laser | Laser | Laser | N/A |
| Wavelength (nm) | 808 | 808 | 808 | N/A |
| Area of Exposure (cm2) | 24 | 24 | 24 | 24 |
| Peak Irradiance (mW/cm2) | ~50 | ~300 | ~850 | 0 |
| Average Irradiance (mW/cm2) | ~50 | ~300 | ~300 | 0 |
| Exposure Time (s) | 1200 | 333 | 600 | 0 |
| Average Fluence (J/cm2) | ~60 | ~100 | ~180 | 0 |
| Total Energy (kJ) | ~1.4 | ~2.4 | ~4.3 | 0 |
| Wave Mode | Continuous | Continuous | Pulsed | N/A |
| Duty Cycle (%) | N/A | N/A | 33 | N/A |
| Pulse Rate (Hz) | N/A | N/A | ~40 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, D.R.A.; Tural, Ü.; Hurtado Puerto, A.M.; Collins, K.A.; Gersten, M.B.; Parincu, Z.; Siu, K.; Iosifescu, D.V.; Ratai, E.-M.; Cassano, P.; et al. Neurometabolite Changes After Transcranial Photobiomodulation in Major Depressive Disorder: A Randomized Controlled Trial Investigating Dose-Dependent Effects. J. Clin. Med. 2025, 14, 3402. https://doi.org/10.3390/jcm14103402
Coelho DRA, Tural Ü, Hurtado Puerto AM, Collins KA, Gersten MB, Parincu Z, Siu K, Iosifescu DV, Ratai E-M, Cassano P, et al. Neurometabolite Changes After Transcranial Photobiomodulation in Major Depressive Disorder: A Randomized Controlled Trial Investigating Dose-Dependent Effects. Journal of Clinical Medicine. 2025; 14(10):3402. https://doi.org/10.3390/jcm14103402
Chicago/Turabian StyleCoelho, David R. A., Ümit Tural, Aura Maria Hurtado Puerto, Katherine Anne Collins, Maia Beth Gersten, Zamfira Parincu, Kari Siu, Dan Vlad Iosifescu, Eva-Maria Ratai, Paolo Cassano, and et al. 2025. "Neurometabolite Changes After Transcranial Photobiomodulation in Major Depressive Disorder: A Randomized Controlled Trial Investigating Dose-Dependent Effects" Journal of Clinical Medicine 14, no. 10: 3402. https://doi.org/10.3390/jcm14103402
APA StyleCoelho, D. R. A., Tural, Ü., Hurtado Puerto, A. M., Collins, K. A., Gersten, M. B., Parincu, Z., Siu, K., Iosifescu, D. V., Ratai, E.-M., Cassano, P., & Weerasekera, A. (2025). Neurometabolite Changes After Transcranial Photobiomodulation in Major Depressive Disorder: A Randomized Controlled Trial Investigating Dose-Dependent Effects. Journal of Clinical Medicine, 14(10), 3402. https://doi.org/10.3390/jcm14103402







