Temporal Patterns of Risk Factors for Adjacent Segment Disease After Lumbar Fusion: 5 Years or More and up to 15 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Factors Considered to Cause ASD
2.3. Patient-Related Factors
2.4. Preoperative Lumbar Spinal Factors
2.5. Surgery-Related Factors
2.6. Postoperative Radiological Change-Related Factors
- (1)
- LLA
- (2)
- Correction of LLA: The difference in correction values between preoperative LLA and postoperative LLA.
- (3)
- Fused segment lordotic angle (FSLA): This is measured between the upper and lower endplates of the fused segments.
- (4)
- FSLA per level: This is calculated as the FSLA divided by the number of fused segments.
- (5)
- Pelvic incidence (PI): This is measured on whole-spine standing lateral radiographs.
- (6)
- PI-LL mismatch: This is calculated as the difference between PI and postoperative LLA, with 10° used as the threshold for analysis.
2.7. Criteria for ASD
2.8. Statistical Analysis
2.9. Declaration of Generative AI and AI-Assisted Technologies in Writing Process
3. Results
3.1. ASD
3.2. Incidence of ASD
3.3. Analysis of Causative Factors
3.3.1. Patient-Related Factors
3.3.2. Preoperative Lumbar Spinal Factors
3.3.3. Surgery-Related Factors
3.3.4. Postoperative Radiological Change-Related Factors
3.3.5. Multivariable Cox Proportional Hazards Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Adjacent segment disease |
| E-ASD | Early adjacent segment disease |
| PLF | Posterolateral fusion |
| PLIF | Posterior lumbar interbody fusion |
| LLA | Lumbar lordotic angle |
| FSLA | Fused segment lordotic angle |
| PI-LL | Pelvic incidence–lumbar lordosis |
| BMI | Body mass index |
References
- Aota, Y.; Kumano, K.; Hirabayashi, S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. Clin. Spine Surg. 1995, 8, 464–473. [Google Scholar]
- Kwon, J.W.; Moon, S.H.; Park, S.Y.; Park, S.J.; Park, S.R.; Suk, K.S.; Kim, H.S.; Lee, B.H. Lumbar Spinal Stenosis: Review Update 2022. Asian Spine J. 2022, 16, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Kim, K.N.; Song, K.H.; Lee, J.M. Comparison of posterior lumbar interbody fusion with posterolateral fusion in degenerative lumbar spinal disorders. J. Korean Orthop. Assoc. 2006, 41, 623–629. [Google Scholar] [CrossRef]
- Tan, L.; Du, X.; Tang, R.; Zhang, L. Preoperative Adjacent Facet Joint Osteoarthritis Is Associated with the Incidence of Adjacent Segment Degeneration and Low Back Pain after Lumbar Interbody Fusion. Asian Spine J. 2024, 18, 21–31. [Google Scholar] [CrossRef]
- Chow, D.H.; Luk, K.D.; Evans, J.H.; Leong, J.C. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine 1996, 21, 549–555. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ha, K.Y.; Kim, Y.S.; Kim, K.W.; Rhyu, K.W.; Park, J.B.; Shin, J.H.; Kim, Y.Y.; Lee, J.S.; Park, H.Y.; et al. Lumbar Interbody Fusion and Osteobiologics for Lumbar Fusion. Asian Spine J. 2022, 16, 1022–1033. [Google Scholar] [CrossRef]
- Throckmorton, T.W.; Hilibrand, A.S.; Mencio, G.A.; Hodge, A.; Spengler, D.M. The impact of adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine 2003, 28, 2546–2550. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Jönsson, B.; Strömqvist, B. Lumbar spine surgery in the elderly. Complications and surgical results. Spine 1994, 19, 1431–1435. [Google Scholar] [CrossRef]
- Kettler, A.; Wilke, H.J.; Haid, C.; Claes, L. Effects of specimen length on the monosegmental motion behavior of the lumbar spine. Spine 2000, 25, 543–550. [Google Scholar] [CrossRef]
- Booth, K.C.; Bridwell, K.H.; Eisenberg, B.A.; Baldus, C.R.; Lenke, L.G. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine 1999, 24, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.Y.; Kim, Y.H.; Kang, K.S. Surgery for adjacent segment changes after lumbosacral fusion. J. Korean Soc. Spine Surg. 2002, 9, 332–340. [Google Scholar] [CrossRef]
- Kumar, M.N.; Baklanov, A.; Chopin, D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur. Spine J. 2001, 10, 314–319. [Google Scholar] [CrossRef]
- Etebar, S.; Cahill, D.W. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J. Neurosurg. 1999, 90, 163–169. [Google Scholar] [CrossRef]
- Ou, C.Y.; Lee, T.C.; Lee, T.H.; Huang, Y.H. Impact of body mass index on adjacent segment disease after lumbar fusion for degenerative spine disease. Neurosurgery 2015, 76, 396–401. [Google Scholar] [CrossRef]
- Weinhoffer, S.L.; Guyer, R.D.; Herbert, M.; Griffith, S.L. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine 1995, 20, 526–531. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, Y.; Soh, J.W.; Shin, B.J. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: Comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine 2014, 39, E339–E345. [Google Scholar] [CrossRef]
- Kim, H.T.; Kang, D.W.; Yoo, C.H.; Jeoung, J.H.; Chang, S.A. Late changes at the adjacent segments to lumbar spine. J. Korean Soc. Spine Surg. 1996, 3, 1–10. [Google Scholar]
- Cho, J.L.; Park, Y.S.; Han, J.H.; Lee, C.H.; Roh, W.I. The changes of adjacent segments after spinal fusion, follow up more than three years after spinal fusion. J. Korean Soc. Spine Surg. 1998, 5, 239–246. [Google Scholar]
- Rohlmann, A.; Neller, S.; Bergmann, G.; Graichen, F.; Claes, L.; Wilke, H.J. Effect of an internal fixator and a bone graft on intersegmental spinal motion and intradiscal pressure in the adjacent regions. Eur. Spine J. 2001, 10, 301–308. [Google Scholar] [CrossRef]
- Ha, K.Y.; Kim, K.W.; Park, S.J.; Lee, Y.H. Changes of the adjacent unfused mobile segment after instrumental lumbar fusion. More than 5 years follow up. J. Korean Soc. Spine Surg. 1998, 5, 205–214. [Google Scholar]
- Grouw, A.V.; Nadel, C.I.; Weierman, R.J.; Lowell, H.A. Long term follow-up of patients with idiopathic scoliosis treated surgically: A preliminary subjective study. Clin. Orthop. Relat. Res. 1976, 117, 197–201. [Google Scholar] [CrossRef]
- Chung, J.Y.; Seo, H.Y.; Jung, J.W. Surgical Treatment of Adjacent Degenerative Segment after Lumbar Fusion—Preliminary report. J. Korean Soc. Spine Surg. 2000, 7, 264–270. [Google Scholar]
- Herkowitz, H.N.; Kurz, L.T. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J. Bone Jt. Surg. 1991, 73, 802–808. [Google Scholar] [CrossRef]
- Ahn, D.K.; Lee, S.; Jeong, K.W.; Park, J.S.; Cha, S.K.; Park, H.S. Adjacent segment failure after lumbar spine fusion—Controlled study for risk factors. J. Korean Orthop. Assoc. 2005, 40, 203–208. [Google Scholar] [CrossRef]
- Soh, J.; Lee, J.C.; Shin, B.J. Analysis of risk factors for adjacent segment degeneration occurring more than 5 years after fusion with pedicle screw fixation for degenerative lumbar spine. Asian Spine J. 2013, 7, 273–281. [Google Scholar] [CrossRef]
- Rothenfluh, D.A.; Mueller, D.A.; Rothenfluh, E.; Min, K. Pelvic incidence-lumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur. Spine J. 2015, 24, 1251–1258. [Google Scholar] [CrossRef]
- Aiki, H.; Ohwada, O.; Kobayashi, H.; Hayakawa, M.; Kawaguchi, S.; Takebayashi, T.; Yamashita, T. Adjacent segment stenosis after lumbar fusion requiring second operation. J. Orthop. Sci. 2005, 10, 490–495. [Google Scholar] [CrossRef]
- Gillet, P. The fate of the adjacent motion segments after lumbar fusion. Spine 2003, 28, 338–345. [Google Scholar] [CrossRef]
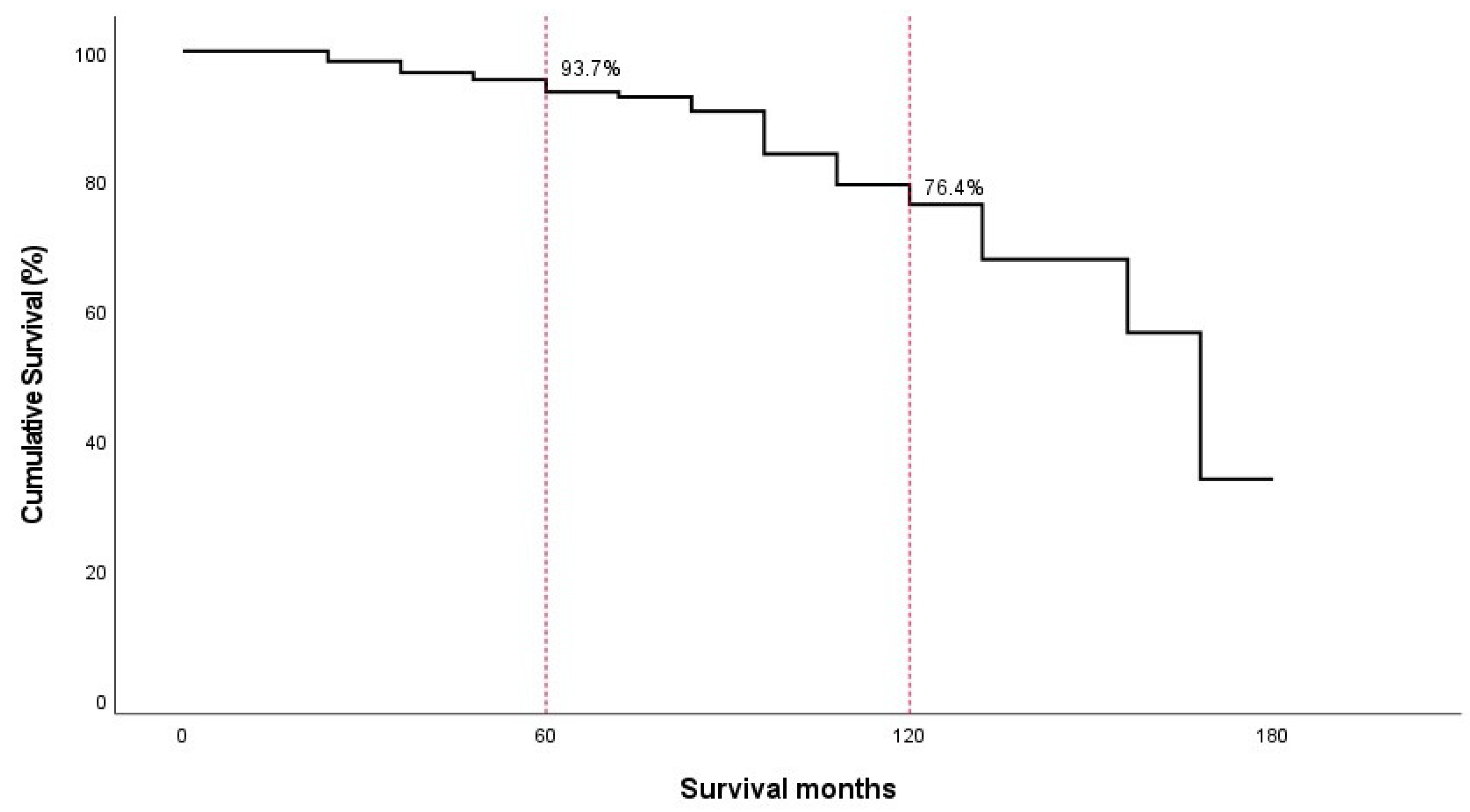
| Months | No. of Patients Entered | No. of Patients Withdrawing | No. of Occurrences of ASD | Annual Incidence (%) (95% CI, %) | Cumulated Survival Rate (95% CI, %) |
|---|---|---|---|---|---|
| 0~12 | 457 | 85 | 0 | 0 | 100.0 |
| 12~24 | 372 | 114 | 5 | 1.3 (0.5–2.2) | 98.4 (97.7–99.1) |
| 24~36 | 253 | 51 | 4 | 1.6 (0.5–2.6) | 96.7 (95.6–97.8) |
| 36~48 | 198 | 36 | 2 | 1.0 (0–2.2) | 95.6 (94.3–96.9) |
| 48~60 | 160 | 18 | 3 | 1.9 (0.5–3.2) | 93.7 (92.0–95.4) |
| 60~72 | 139 | 36 | 1 | 0.7 (0–2.1) | 92.9 (91.1–94.8) |
| 72~84 | 102 | 34 | 2 | 2.0 (0.3–3.6) | 90.7 (88.4–93.1) |
| 84~96 | 66 | 22 | 4 | 6.1 (4.0–8.1) | 84.1 (80.3–88.0) |
| 96~108 | 40 | 9 | 2 | 5.0 (2.3–7.7) | 79.4 (74.5–84.3) |
| 108~120 | 29 | 6 | 1 | 3.4 (0.3–6.6) | 76.4 (70.8–81.9) |
| 120~132 | 22 | 8 | 2 | 9.1 (5.5–12.7) | 67.9 (60.4–75.4) |
| 132~144 | 12 | 4 | 0 | 0 | 67.9 (60.4–75.4) |
| 144~156 | 8 | 4 | 1 | 12.5 (6.5–18.5) | 56.6 (44.5–68.6) |
| 156~168 | 3 | 1 | 1 | 33.3 (23.6–43.1) | 33.9 (15.0–52.9) |
| 168~180 | 1 | 1 | 0 | 0 | 33.9 (15.0–52.9) |
| Risk Factors | Patients | ASD (+) (E-ASD) | ASD (−) | p-Value | ||
|---|---|---|---|---|---|---|
| ASD | E-ASD | |||||
| Patient-related factors | ||||||
| Sex | Male | 55 | 12 (8) | 43 | 0.690 | 0.156 |
| Female | 84 | 16 (6) | 68 | |||
| Age | <65 years | 124 | 24 (11) | 100 | 0.505 | 0.176 |
| ≥65 years | 15 | 4 (3) | 11 | |||
| BMI | <25 kg/m2 | 71 | 13 (6) | 58 | 0.582 | 0.516 |
| ≥25 kg/m2 | 68 | 15 (8) | 53 | |||
| Preoperative lumbar factors | ||||||
| Preoperative spinal diagnosis | Degenerative | 74 | 16 (9) | 58 | 0.643 | 0.382 |
| Instability | 65 | 12 (5) | 53 | |||
| Preoperative LLA | <35° | 65 | 16 (8) | 49 | 0.218 | 0.412 |
| ≥35° | 74 | 12 (6) | 62 | |||
| Preoperative Pfirrmann grade | <grade 3 | 115 | 21 (9) | 94 | 0.226 | 0.054 |
| ≥grade 3 | 24 | 7 (5) | 17 | |||
| Surgery-related factors | ||||||
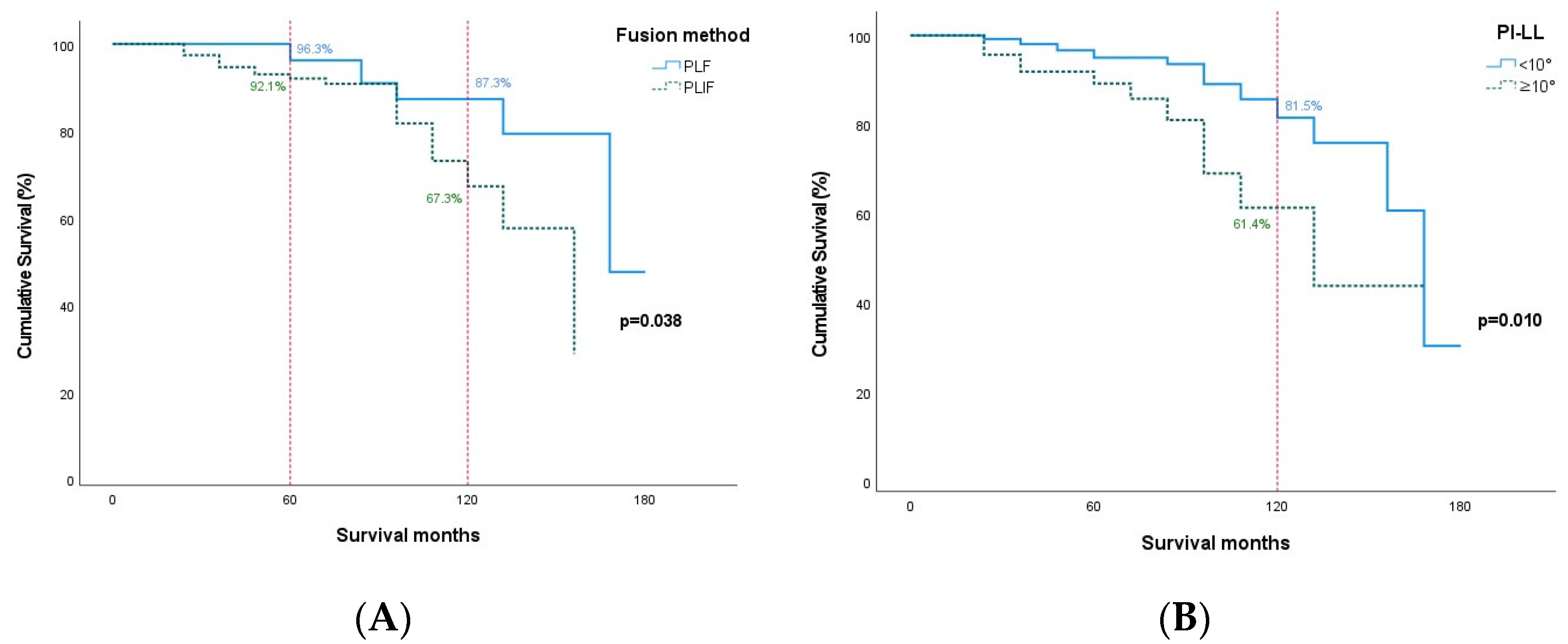
| Fusion method | PLF | 51 | 7 (2) | 44 | 0.151 | 0.067 |
| PLIF | 88 | 21 (12) | 67 | |||
| Number of fused segments | Single level | 83 | 15 (8) | 68 | 0.458 | 0.836 |
| 2 or 3 levels | 56 | 13 (6) | 43 | |||
| Postoperative radiologic changes | ||||||
| Postoperative LLA | <38° | 71 | 17 (9) | 54 | 0.254 | 0.297 |
| ≥38° | 68 | 11 (5) | 57 | |||
| Correction of LLA | <8° | 87 | 16 (7) | 71 | 0.505 | 0.305 |
| ≥8° | 52 | 12 (7) | 40 | |||
| Postoperative FSLA per level | <14° | 70 | 14 (7) | 56 | 0.966 | 0.977 |
| ≥14° | 69 | 14 (7) | 55 | |||
| Postoperative PI-LL | <10° | 108 | 16 (8) | 92 | 0.003 * | 0.051 |
| ≥10° | 31 | 12 (6) | 19 | |||
| Patients | Hazard Ratio | 95% Confidence Interval | p-Value | ||||
|---|---|---|---|---|---|---|---|
| ASD | E-ASD | ASD | E-ASD | ASD | E-ASD | ||
| Sex | 0.904 | 0.155 | |||||
| Male | 55 | Reference group | Reference group | ||||
| Female | 84 | 0.951 | 0.421 | 0.421–2.148 | 0.128–1.387 | ||
| Age | 0.783 | 0.756 | |||||
| <65 years | 124 | 0.843 | 0.747 | 0.249–2.845 | 0.118–4.716 | ||
| ≥65 years | 15 | Reference group | Reference group | ||||
| BMI | 0.332 | 0.116 | |||||
| <25 kg/m2 | 71 | Reference group | Reference group | ||||
| ≥25 kg/m2 | 68 | 1.512 | 2.674 | 0.656–3.487 | 0.783–9.126 | ||
| Preoperative spinal diagnosis | 0.448 | 0.419 | |||||
| Degenerative | 74 | Reference group | Reference group | ||||
| Instability | 65 | 0.722 | 0.613 | 0.311–1.674 | 0.187–2.009 | ||
| Preoperative LLA | 0.357 | 0.373 | |||||
| <35° | 71 | Reference group | Reference group | ||||
| ≥35° | 68 | 0.648 | 0.552 | 0.258–1.630 | 0.150–2.037 | ||
| Preoperative Pfirrmann grade | 0.924 | 0.306 | |||||
| <grade 3 | 115 | Reference group | Reference group | ||||
| ≥grade 3 | 24 | 0.948 | 2.073 | 0.318–2.829 | 0.513–8.370 | ||
| Fusion method | * 0.005 | * 0.038 | |||||
| PLF | 51 | Reference group | Reference group | ||||
| PLIF | 88 | * 4.442 | * 5.490 | 1.576–12.517 | 1.100–27.393 | ||
| Number of fused segments | 0.121 | 0.824 | |||||
| Single level | 83 | Reference group | Reference group | ||||
| 2 or 3 levels | 56 | 2.002 | 1.154 | 0.832–4.816 | 0.325–4.093 | ||
| Postoperative LLA | 0.105 | 0.343 | |||||
| <38° | 71 | Reference group | Reference group | ||||
| ≥38° | 68 | 0.449 | 0.522 | 0.170–1.182 | 0.136–1.998 | ||
| Correction of LLA | 0.137 | 0.116 | |||||
| <8° | 87 | Reference group | Reference group | ||||
| ≥8° | 52 | 1.993 | 2.663 | 0.803–4.946 | 0.786–9.026 | ||
| Postoperative FSLA per level | 0.343 | 0.771 | |||||
| <14° | 70 | Reference group | Reference group | ||||
| ≥14° | 69 | 1.560 | 1.214 | 0.623–3.909 | 0.330–4.456 | ||
| Postoperative PI-LL | * 0.004 | 0.052 | |||||
| <10° | 108 | Reference group | Reference group | ||||
| ≥10° | 31 | * 3.653 | 3.621 | 1.502–8.884 | 0.987–13.289 | ||
| Group | Months | No. of Patients Entered | No. of Patients Withdrawing | No. of Occurrences of ASP | Cumulated Survival Rate (95% CI, %) |
|---|---|---|---|---|---|
| PLF | 0~12 | 178 | 32 | 0 | 100.0 |
| 12~24 | 146 | 49 | 0 | 100.0 | |
| 24~36 | 97 | 23 | 0 | 100.0 | |
| 36~48 | 74 | 18 | 0 | 100.0 | |
| 48~60 | 56 | 5 | 2 | 96.3 (93.7–98.9) | |
| 60~72 | 49 | 8 | 0 | 96.3 (93.7–98.9) | |
| 72~84 | 41 | 9 | 2 | 91.0 (86.6–95.4) | |
| 84~96 | 30 | 10 | 1 | 87.3 (81.8–92.9) | |
| 96~108 | 19 | 5 | 0 | 87.3 (81.8–92.9) | |
| 108~120 | 14 | 1 | 0 | 87.3 (81.8–92.9) | |
| 120~132 | 13 | 4 | 1 | 79.4 (70.3–88.5) | |
| 132~144 | 8 | 3 | 0 | 79.4 (70.3–88.5) | |
| 144~156 | 5 | 2 | 0 | 79.4 (70.3–88.5) | |
| 156~168 | 3 | 1 | 1 | 47.6 (22.4–72.8) | |
| 168–180 | 1 | 1 | 0 | 47.6 (22.4–72.8) | |
| PLIF | 0~12 | 279 | 53 | 0 | 100.0 |
| 12~24 | 226 | 65 | 5 | 97.4 (96.3–98.6) | |
| 24~36 | 156 | 28 | 4 | 94.7 (92.9–96.4) | |
| 36~48 | 124 | 18 | 2 | 93.0 (91.0–95.1) | |
| 48~60 | 104 | 13 | 1 | 92.1 (89.8–94.3) | |
| 60~72 | 90 | 28 | 1 | 90.9 (88.3–93.4) | |
| 72~84 | 61 | 25 | 0 | 90.9 (88.3–93.4) | |
| 84~96 | 36 | 12 | 3 | 81.8 (76.3–87.2) | |
| 96~108 | 21 | 4 | 2 | 73.2 (65.6–80.7) | |
| 108~120 | 15 | 5 | 1 | 67.3 (58.4–76.3) | |
| 120~132 | 9 | 4 | 1 | 57.7 (46.0–69.4) | |
| 132~144 | 4 | 1 | 0 | 57.7 (46.0–69.4) | |
| 144~156 | 3 | 2 | 1 | 28.8 (7.6–50.1) |
| Group | Months | No. of Patients Entered | No. of Patients Withdrawing | No. of Occurrences of ASP | Cumulated Survival Rate (95% CI, %) |
|---|---|---|---|---|---|
| PI-LL | 0~12 | 362 | 71 | 0 | 100.0 |
| <10° | 12~24 | 291 | 91 | 2 | 99.2 (98.6–99.8) |
| 24~36 | 198 | 42 | 2 | 98.1 (97.1–99.0) | |
| 36~48 | 154 | 29 | 2 | 96.7 (95.3–98.0) | |
| 48~60 | 123 | 13 | 2 | 95.0 (93.2–96.8) | |
| 60~72 | 108 | 26 | 0 | 95.0 (93.2–96.8) | |
| 72~84 | 82 | 30 | 1 | 93.6 (91.3–95.8) | |
| 84~96 | 51 | 19 | 2 | 89.1 (85.3–92.8) | |
| 96~108 | 30 | 7 | 1 | 85.7 (80.8–90.6) | |
| 108~120 | 22 | 3 | 1 | 81.5 (75.3–87.7) | |
| 120~132 | 18 | 7 | 1 | 75.9 (68.0–83.8) | |
| 132~144 | 10 | 3 | 0 | 75.9 (68.0–83.8) | |
| 144~156 | 7 | 4 | 1 | 60.7 (45.7–75.7) | |
| 156~168 | 2 | 0 | 1 | 30.4 (7.6–53.1) | |
| 168–180 | 1 | 1 | 0 | 30.4 (7.6–53.1) | |
| PI-LL | 0~12 | 95 | 14 | 0 | 100.0 |
| ≥10° | 12~24 | 81 | 23 | 3 | 95.7 (93.2–98.1) |
| 24~36 | 55 | 9 | 2 | 91.9 (88.4–95.4) | |
| 36~48 | 44 | 7 | 0 | 91.9 (88.4–95.4) | |
| 48~60 | 37 | 5 | 1 | 89.2 (84.9–93.5) | |
| 60~72 | 31 | 10 | 1 | 85.8 (80.5–91.1) | |
| 72~84 | 20 | 4 | 1 | 81.0 (74.2–87.9) | |
| 84~96 | 15 | 3 | 2 | 69.0 (59.3–78.8) | |
| 96~108 | 10 | 2 | 1 | 61.4 (50.1–72.7) | |
| 108~120 | 7 | 3 | 0 | 61.4 (50.1–72.7) | |
| 120~132 | 4 | 1 | 1 | 43.8 (27.0–60.7) | |
| 132~144 | 2 | 1 | 0 | 43.8 (27.0–60.7) | |
| 144~156 | 1 | 0 | 0 | 43.8 (27.0–60.7) | |
| 156~168 | 1 | 1 | 0 | 43.8 (27.0–60.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soh, J.; Jang, H.-D.; Lee, J.C.; Jeong, T.; Shin, B.-J. Temporal Patterns of Risk Factors for Adjacent Segment Disease After Lumbar Fusion: 5 Years or More and up to 15 Years. J. Clin. Med. 2025, 14, 3400. https://doi.org/10.3390/jcm14103400
Soh J, Jang H-D, Lee JC, Jeong T, Shin B-J. Temporal Patterns of Risk Factors for Adjacent Segment Disease After Lumbar Fusion: 5 Years or More and up to 15 Years. Journal of Clinical Medicine. 2025; 14(10):3400. https://doi.org/10.3390/jcm14103400
Chicago/Turabian StyleSoh, Jaewan, Hae-Dong Jang, Jae Chul Lee, Taejong Jeong, and Byung-Joon Shin. 2025. "Temporal Patterns of Risk Factors for Adjacent Segment Disease After Lumbar Fusion: 5 Years or More and up to 15 Years" Journal of Clinical Medicine 14, no. 10: 3400. https://doi.org/10.3390/jcm14103400
APA StyleSoh, J., Jang, H.-D., Lee, J. C., Jeong, T., & Shin, B.-J. (2025). Temporal Patterns of Risk Factors for Adjacent Segment Disease After Lumbar Fusion: 5 Years or More and up to 15 Years. Journal of Clinical Medicine, 14(10), 3400. https://doi.org/10.3390/jcm14103400





