Unexpected Long-Term Survival and Downstaging in Oligometastatic Non-Small Cell Lung Cancer Treated with Multimodal Therapy
Abstract
1. Introduction
- After 12 months of treatment with Nivolumab, PFS increases to 38.1 months.
- Interrupting IT at 6 months in patients without progression or administering IT for 2 years maintains a prolonged progression-free survival (PFS) and long-term survival.
2. Case Presentation
2.1. Systemic Third-Line Therapy (4xDocetaxel) Was Initiated 04–07/2020
2.2. Monitoring 2022: Oncological Commission 1910/16.08.2022
2.3. Oncological Committee 07/25/2023 Recommended
- Consider rescue re-irradiation (SBRT), taking into account the current primary tumor dimensions of 24 × 34.2 mm on PET CT from 06/2023 and also the total dose previously administered (08–09/2020), the previously applied fractionation, the time between the two irradiations, the cellular repair time and the dose constraints for the organs at risk.
- Genetic testing for ROS1, BRAF V600E, NTRK1/2/3, MET Exon14, RET and ERBB2 (HER2) in order to consider the opportunity for targeted therapy.
3. Case Results
4. Case Conclusions
5. Discussion and Literature Review
5.1. Immunotherapy Resistance
5.2. Rechallenge with Immunotherapy
5.3. Progression of Metastatic NSCLC After First Line of Chemo-Immunotherapy
- -
- Combination with angiogenesis inhibitors: Ramucirumab/Pembrolizumab+Docetaxel [38].
- -
- -
- -
5.4. The Role of Chemotherapy in Second-Line and Third-Line Therapies After Progression of NSCLC to IT
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lixi, L.; Tianhao, S.; Di, Z.; Fei, M. Nowcasting and forecasting global aging and cancer burden: Analysis of data from the GLOBOCAN and Global Burden of Disease Study. J. Natl. Cancer Cent. 2024, 4, 223–232. [Google Scholar]
- Rahnea-Nita, R.A.; Stoian, A.R.; Anghel, R.M.; Rebegea, L.F.; Ciuhu, A.N.; Bacinschi, X.E.; Zgura, A.F.; Trifanescu, O.G.; Toma, R.V.; Constantin, G.B. The Efficacy of Immunotherapy in Long-Term Survival in Non-Small Cell Lung Cancer (NSCLC) Associated with the Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH). Life 2023, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Silva Nicolau, J.; Mendoza Lopez, R.V.; Terra de Moraes, L.C.; Braga Ribeiro, K.; Aparecida, R.; Maistro, S.; Hirata, M.L.; Mendonça, N.R.J.; de Castro, G.; Neto, J.E.; et al. Survival analysis of young adults from a Brazilian cohort of non-small cell lung cancer patients. Ecancermedicalscience 2021, 15, 1279. [Google Scholar]
- Garinet, S.; Wang, P.; Mansuet-Lupo, A.; Fournel, L.; Wislez, M.; Blons, H. Updated Prognostic Factors in Localized NSCLC. Cancers 2022, 14, 1400. [Google Scholar] [CrossRef]
- De Castro, J.; Insa, A.; Collado-Borrell, R.; Escudero-Vilaplana, V.; Martínez, A.; Fernandez, E.; Sullivan, I.; Arrabal, N.; Carcedo, D.; Manzaneque, A. Economic burden of locoregional and metastatic relapses in resectable early-stage non-small cell lung cancer in Spain. BMC Pulm. Med. 2023, 23, 69. [Google Scholar] [CrossRef]
- LoPiccolo, J.; Gusev, A.; Christiani, D.C.; Jänne, P.A. Lung cancer in patients who have never smoked—An emerging disease. Nat. Rev. Clin. Oncol. 2024, 21, 121–146. [Google Scholar] [CrossRef]
- David Palma, A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.; Haasbeek, C.; Mulroy, L. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The Abscopal Effect: A Review of Pre-Clinical and Clinical Advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nabrinsky, E.; Macklis, J.; Bitran, J. A Review of the Abscopal Effect in the Era of Immunotherapy. Cureus 2022, 14, e29620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moscardini-Martelli, J.; Rodríguez-Camacho, A.; Torres-Ríos, J.A.; Meraz-Soto, J.M.; Flores-Vázquez, J.G.; Hernández-Sánchez, L.C.; Lozano-Ruiz, F.J.; Maldonado-Magos, F.; Cid-Sánchez, D.; Flores-Balcázar, C.H.; et al. A Comprehensive Revision of Radiation Immunotherapy and the Abscopal Effect in Central Nervous System Metastases: Reassessing the Frontier. Curr. Issues Mol. Biol. 2024, 46, 11075–11085. [Google Scholar] [CrossRef] [PubMed]
- Kodet, O.; Němejcova, K.; Strnadová, K.; Havlínová, A.; Dundr, P.; Krajsová, I.; Štork, J.; Smetana, K., Jr.; Lacina, L. The Abscopal Effect in the Era of Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 7204. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, L.; Moreau Bachelard, C.; Renaud, E.; Dhamelincourt, E.; Lucia, F. The abscopal effect of immune-radiation therapy in recurrent and metastatic cervical cancer: A narrative review. Front. Immunol. 2023, 14, 1201675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Liu, X.; Li, N.; Zhao, A.; Sun, Z.; Wang, M.; Luo, J. Radiotherapy combined with immunotherapy could improve the immune infiltration of melanoma in mice and enhance the abscopal effect. Radiat. Oncol. J. 2023, 41, 129–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez, J.C.; Nuñez-García, B.; Garitaonaindia, Y.; Calvo, V.; Blanco, M.; Ramos Martín-Vegue, A.; Royuela, A.; Manso, M.; Cantos, B.; Méndez, M.; et al. Quality of care assessment for non-small cell lung cancer patients: Transforming routine care data into a continuous improvement system. Clin. Transl. Oncol. 2024, 27, 1047–1061. [Google Scholar] [CrossRef]
- Ghinescu, M.; Olaroiu, M.; Aurelian, S.; Halfens, R.J.; Dumitrescu, L.; Schols, J.M.; Rahnea-Nita, G.; Curaj, A.; Alexa, I.; van den Heuvel, W.J. Assessment of care problems in Romania: Feasibility and exploration. J. Am. Med. Dir. Assoc. 2015, 16, 86.e9–86.e12. [Google Scholar] [CrossRef]
- Prapa, P.; Papathanasiou, I.V.; Bakalis, V.; Malli, F.; Papagiannis, D.; Fradelos, E.C. Quality of Life and Psychological Distress of Lung Cancer Patients Undergoing Chemotherapy. World J. Oncol. 2021, 12, 61–66. [Google Scholar] [CrossRef]
- Wang, Y.; Safi, M.; Hirsch, F.R.; Lu, S.; Peters, S.; Govindan, R.; Rosell, R.; Park, K.; Zhang, J.J. Immunotherapy for advanced-stage squamous cell lung cancer: The state of the art and outstanding questions. Nat. Rev. Clin. Oncol. 2025, 22, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Joshi, A.; Bhaskar, N.; Pearson, J.D. Neuroendocrine Transformation as a Mechanism of Resistance to Targeted Lung Cancer Therapies: Emerging Mechanisms and Their Therapeutic Implications. Cancers 2025, 17, 260. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Minari, R.; Mazzaschi, G.; Gnetti, L.; La Monica, S.; Alfieri, R.; Campanini, N.; Verzè, M.; Olivani, A.; Ventura, L.; et al. Small Cell Lung Cancer Transformation as a Resistance Mechanism to Osimertinib in Epidermal Growth Factor Receptor-Mutated Lung Adenocarcinoma: Case Report and Literature Review. Front. Oncol. 2021, 11, 642190. [Google Scholar] [CrossRef]
- Shiba-Ishii, A.; Takemura, N.; Kawai, H.; Matsubara, D. Histologic transformation of non-small-cell lung cancer in response to tyrosine kinase inhibitors: Current knowledge of genetic changes and molecular mechanisms. Cancer Sci. 2024, 115, 2138–2146. [Google Scholar] [CrossRef]
- Yang, M.H.; Yu, J.; Cai, C.L.; Li, W. Small cell lung cancer transformation and tumor heterogeneity after sequential targeted therapy and immunotherapy in EGFR-mutant nonsmall cell lung cancer: A case report. Front. Oncol. 2022, 12, 1029282. [Google Scholar]
- Rebegea, L.; Stefan, A.M.; Firescu, D.; Miron, D.; Romila, A. Paraneoplastic pemphigus associated with a hypopharynx squamous cell carcinoma. Case report. Acta Medica Mediterr. 2018, 34, 1265. [Google Scholar]
- Sato, Y.; Saito, G.; Fujimoto, D. Histologic transformation in lung cancer: When one door shuts, another opens. Ther. Adv. Med. Oncol. 2022, 14, 17588359221130503. [Google Scholar] [CrossRef]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Perez-Gracia, J.L.; Han, J.Y.; Arvis, C.D.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Long-Term Outcomes and Retreatment Among Patients with Previously Treated, Programmed Death-Ligand 1-Positive, Advanced Non-Small-Cell Lung Cancer in the KEYNOTE-010 Study. J. Clin. Oncol. 2020, 38, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Macioch, T.; Krzakowski, M.; Gołębiewska, K. Pembrolizumab monotherapy survival benefits in metastatic non-small-cell lung cancer: A systematic review of real-world data. Discov. Onc. 2024, 15, 303. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Bilger, G.; Girard, N.; Doubre, H.; Levra, M.G.; Giroux-Leprieur, E.; Giraud, F.; Decroisette, C.; Carton, M.; Massiani, M.A. Discontinuation of immune checkpoint inhibitor (ICI) above 18 months of treatment in real-life patients with advanced non-small cell lung cancer (NSCLC): INTEPI, a multicentric retrospective study. Cancer Immunol. Immunother. 2022, 71, 1719–1731. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Li, R.; Ning, F.; Tian, L. Efficacy and Mechanism of Combining Radiotherapy and Immunotherapy in Stage IV Non-Small Cell Lung Cancer. Curr. Treat Options Oncol. 2024, 25, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.N.; Xie, J.; Jin, M.M.; Ma, X.T.; Dou, Z.; Wang, J.P.; Li, J.L. Efficacy and prognostic factors of stereotactic body radiotherapy combined with immunotherapy for pulmonary oligometastases: A preliminary retrospective cohort study. Transl. Lung Cancer Res. 2024, 13, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, Y.; Yan, Y.; Li, M.; Wu, S.; Cao, L.; Chen, W.; Luo, H.; He, Y. Safety and Efficacy of Rechallenge with Immune Checkpoint Inhibitors in Advanced Solid Tumor: A Systematic Review and Meta-Analysis. Cancer Med. 2024, 13, e70324. [Google Scholar] [CrossRef]
- Boyero, L.; Sánchez-Gastaldo, A.; Alonso, M.; Noguera-Uclés, J.F.; Molina-Pinelo, S.; Bernabé-Caro, R. Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy. Cancers 2020, 12, 3729. [Google Scholar] [CrossRef]
- Moliner, L.; Spurgeon, L.; Califano, R. Controversies in NSCLC: Which second-line strategy after chemo-immunotherapy? ESMO Open. 2023, 8, 100879. [Google Scholar] [CrossRef]
- Messori, A.; Ossato, A.; Gasperoni, L.; Del Bono, L.; Inno, A.; Damuzzo, V. Treatment Options for Advanced Non-Small Cell Lung Cancer After Failure of Previous Immune Checkpoint Inhibitors and Chemotherapy: Meta-Analysis of Five Randomized Controlled Trials. Curr. Oncol. 2025, 32, 46. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 384, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Martínez-Pérez, A.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Chemo-Immunotherapy: A New Trend in Cancer Treatment. Cancers 2023, 15, 2912. [Google Scholar] [CrossRef]
- Osta, B.E.; Carlisle, J.; Steuer, C.; Pakkala, S.; Leal, T.; Dhodapkar, M.; Liu, Y.; Chen, Z.; Owonikoko, T.; Ramalingam, S. A Phase 2 Study of Docetaxel, Ramucirumab, and Pembrolizumab for Patients with Metastatic or Recurrent Non-Small-Cell Lung Cancer (NSCLC) who Progressed on Platinum-Doublet and PD-1/PD-L1 Blockade. Clin. Lung Cancer 2022, 23, e400–e404. [Google Scholar] [CrossRef]
- Matusz-Fisher, A.; Tan, A.R. Sacituzumab govitecan and other antibody-drug conjugates targeting trophoblast cell-surface antigen 2 (Trop-2) in breast cancer. Ann. Transl. Med. 2022, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Yuca, E.; Evans, K.W.; Zhao, M.; Maejima, T.; Karibe, T.; Raso, M.G.; Tang, X.; Zheng, X.; Rizvi, Y.Q.; et al. Antitumor Activity and Biomarker Analysis for TROP2 Antibody-Drug Conjugate Datopotamab Deruxtecan in Patient-Derived Breast Cancer Xenograft Models. Clin. Cancer Res. 2025, 31, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Bedard, P.L.; Bang, Y.J.; Vieito, M.; Ryu, M.H.; Fagniez, N.; Chadjaa, M.; Soufflet, C.; Masson, N.; Gazzah, A. Tusamitamab Ravtansine in Patients with Advanced Solid Tumors: Phase I Study of Safety, Pharmacokinetics, and Antitumor Activity Using Alternative Dosing Regimens. Cancer Res. Commun. 2023, 3, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Kim, T.M.; Vicente, D. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: Results from an expansion cohort of a phase 1 trial. J. Thorac. Oncol. 2020, 15, 1210–1222. [Google Scholar] [CrossRef]
- Assi, H.I.; Zerdan, M.B.; Hodroj, M.; Khoury, M.; Naji, N.S.; Amhaz, G.; Zeidane, R.A.; El Karak, F. Value of chemotherapy post immunotherapy in stage IV non-small cell lung cancer (NSCLC). Oncotarget 2023, 14, 517–525. [Google Scholar] [CrossRef]
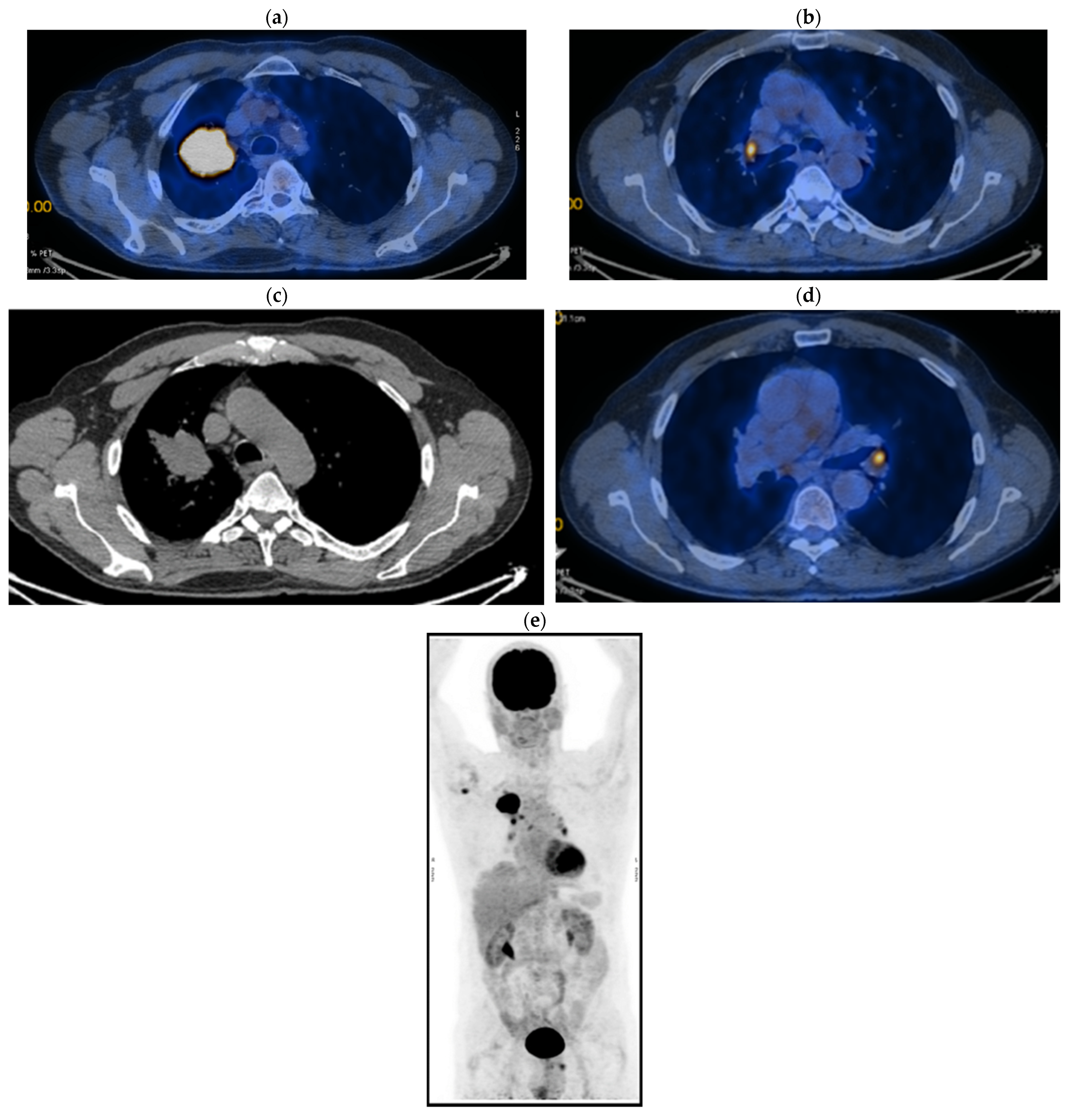
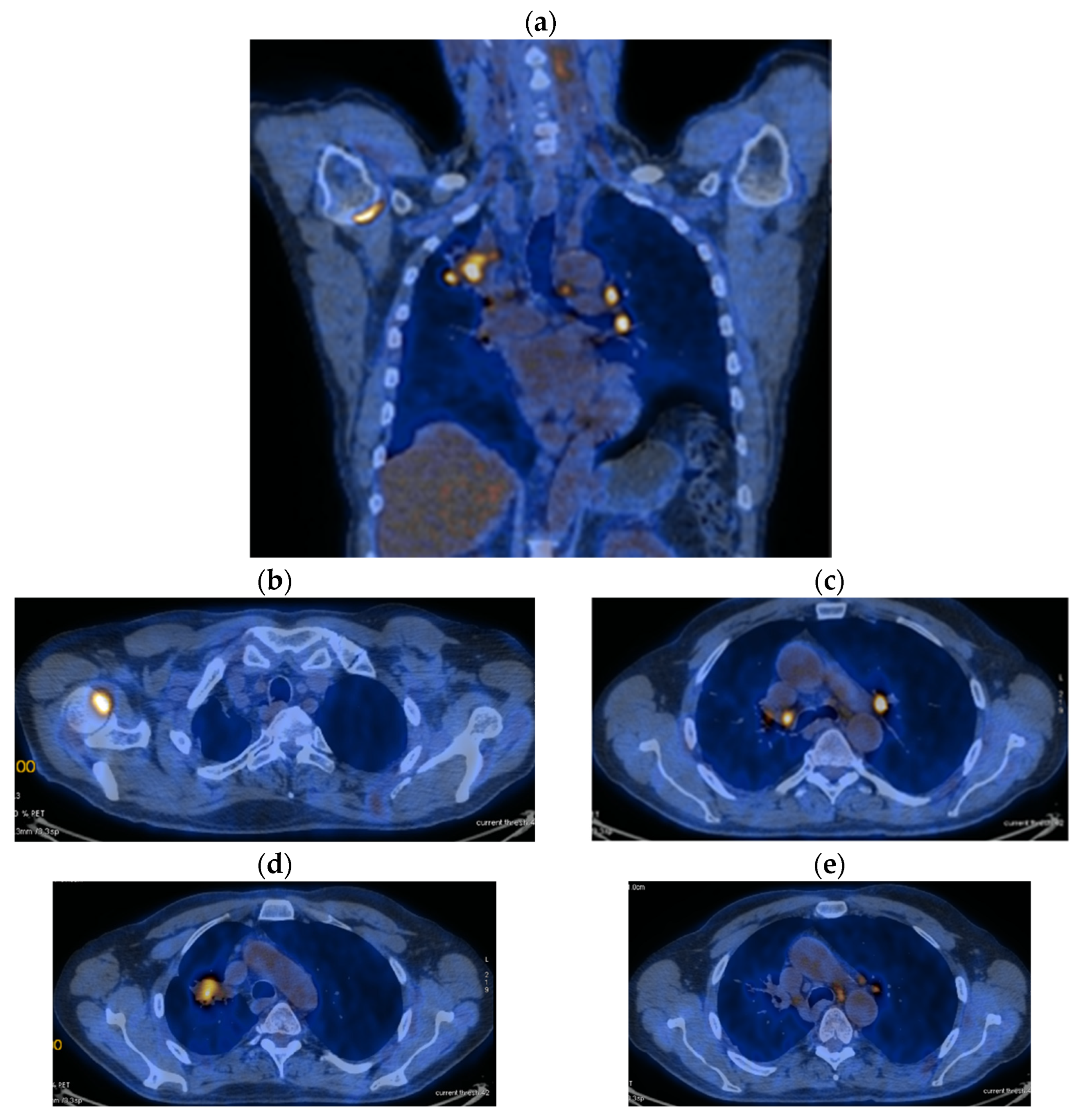
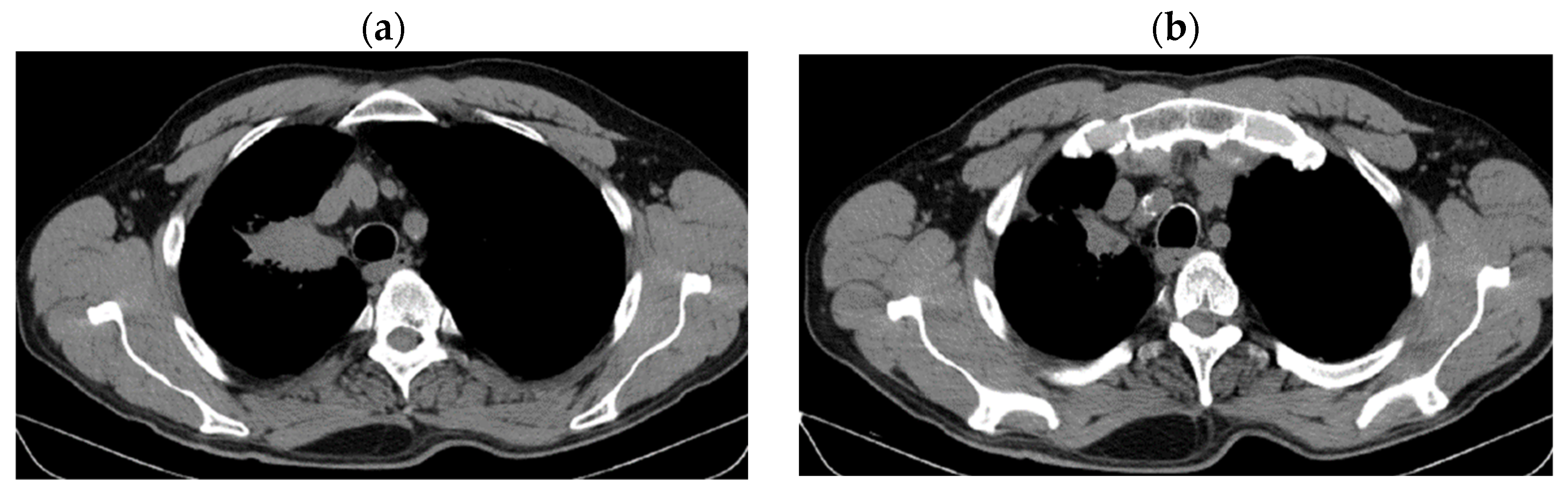
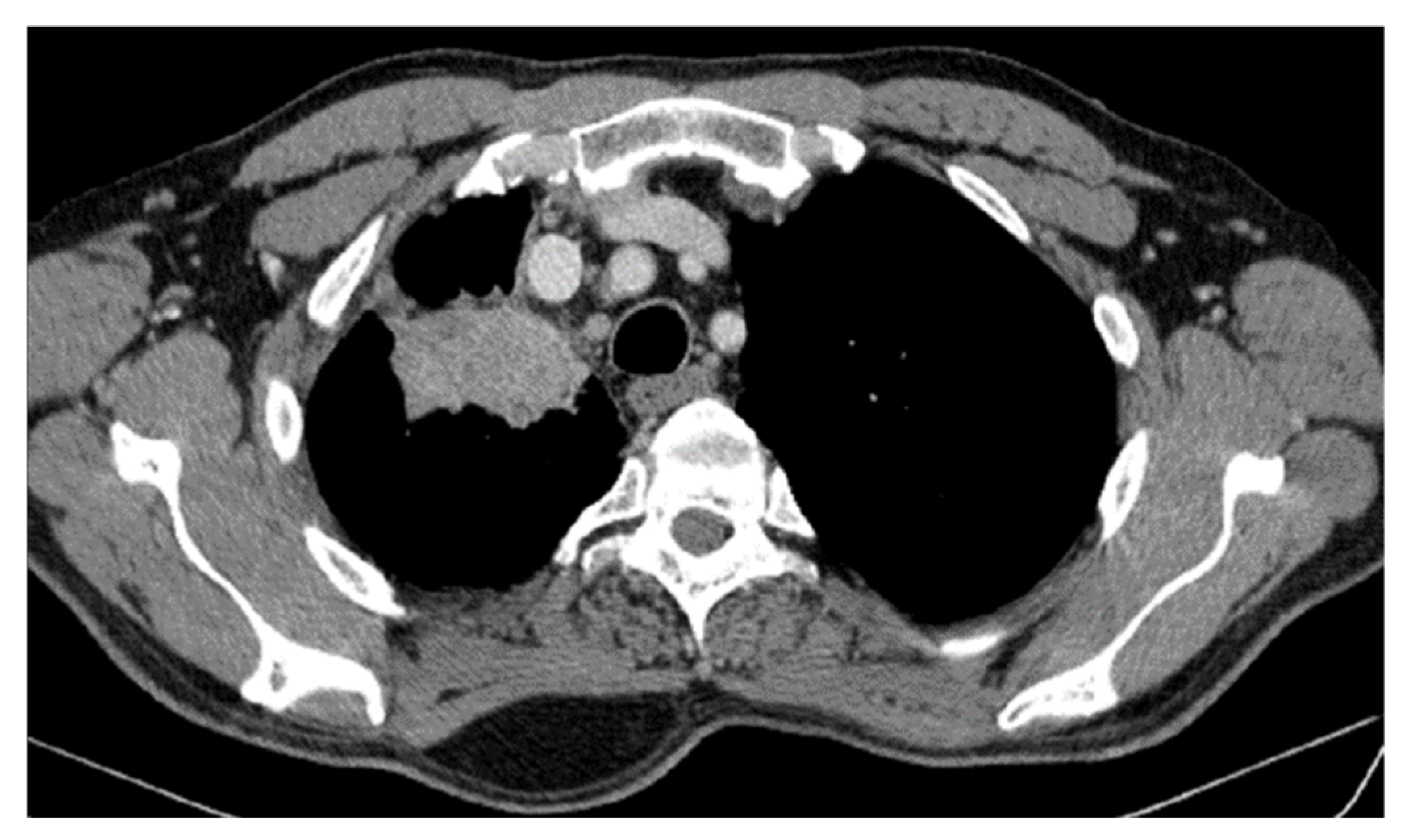
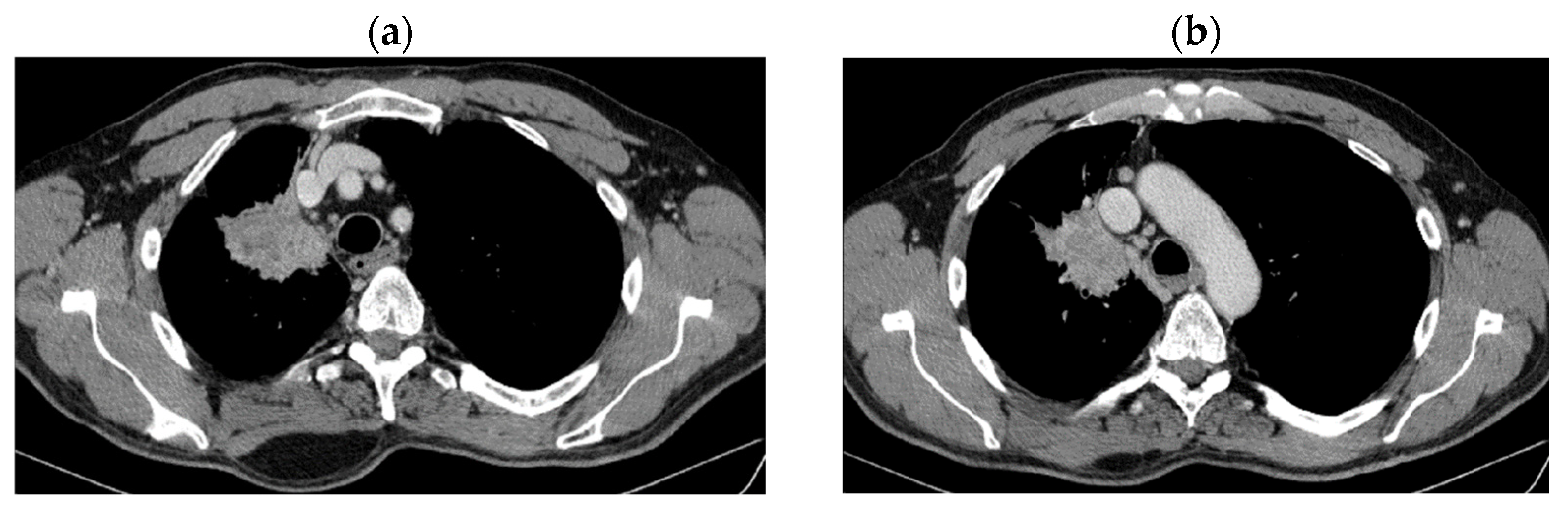
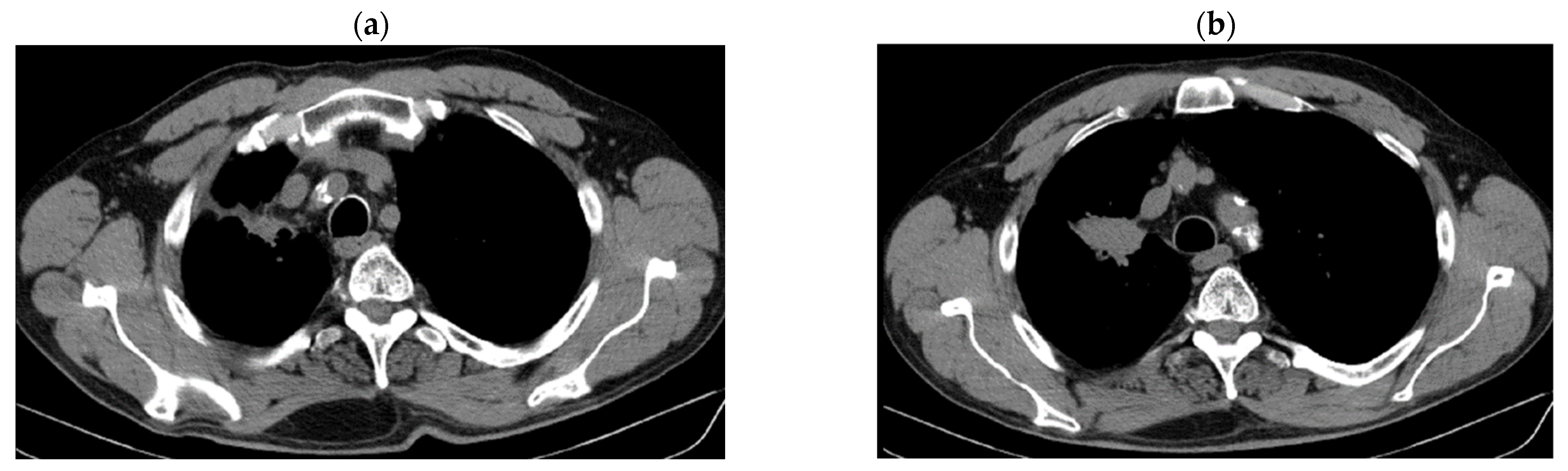
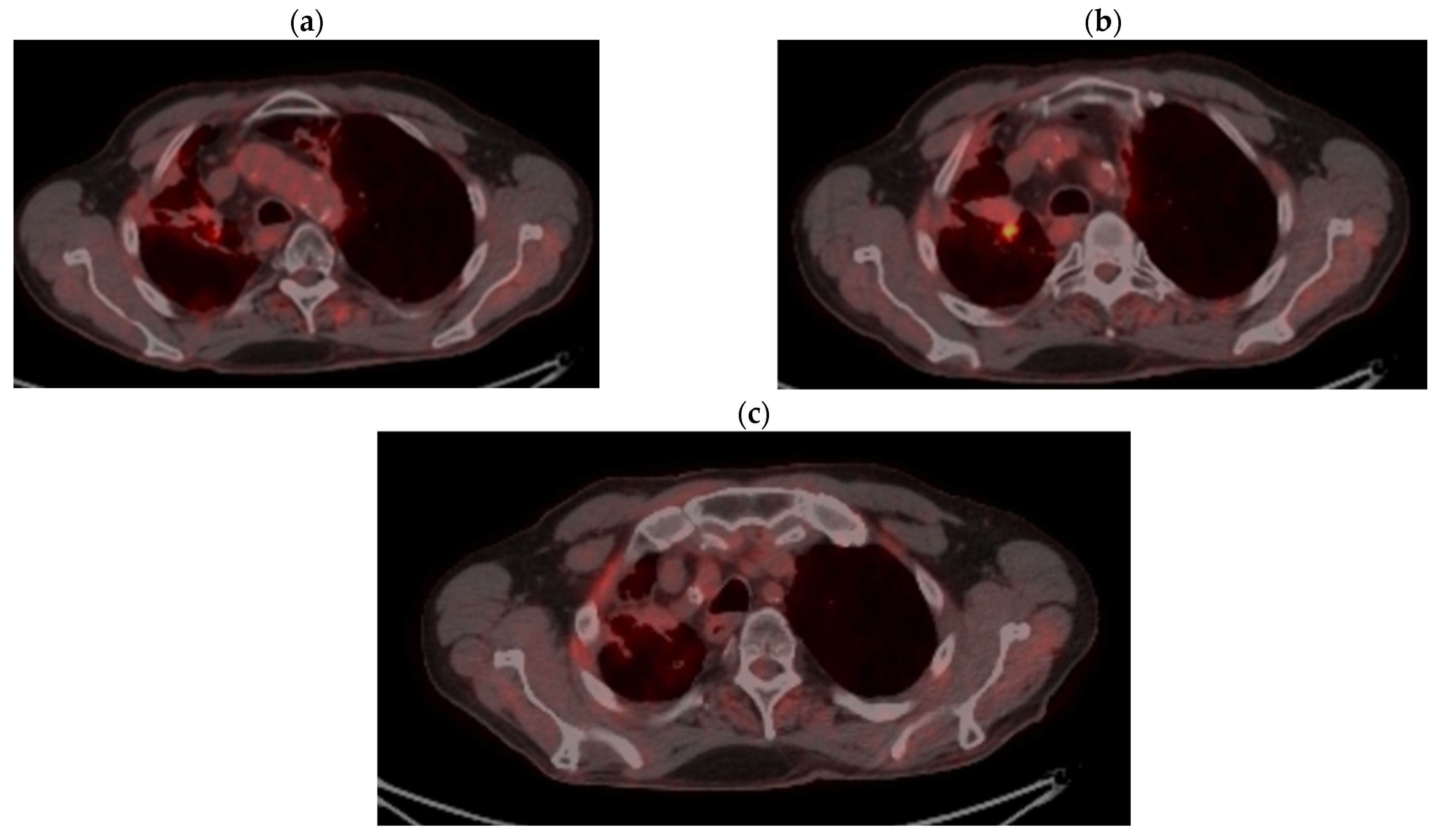
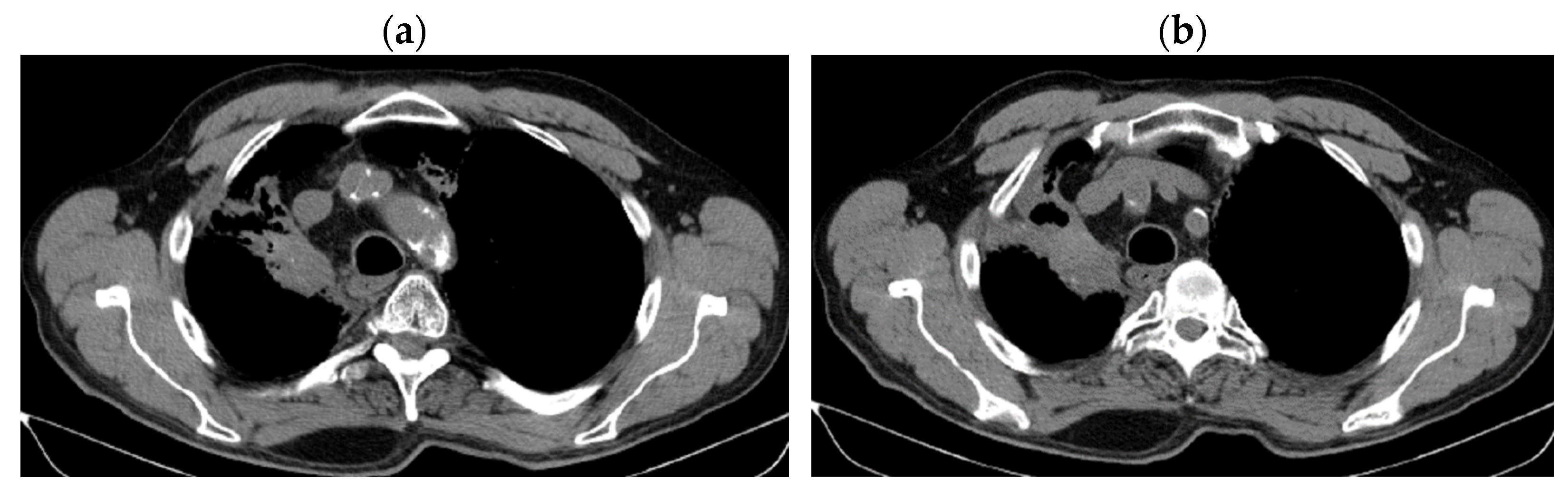
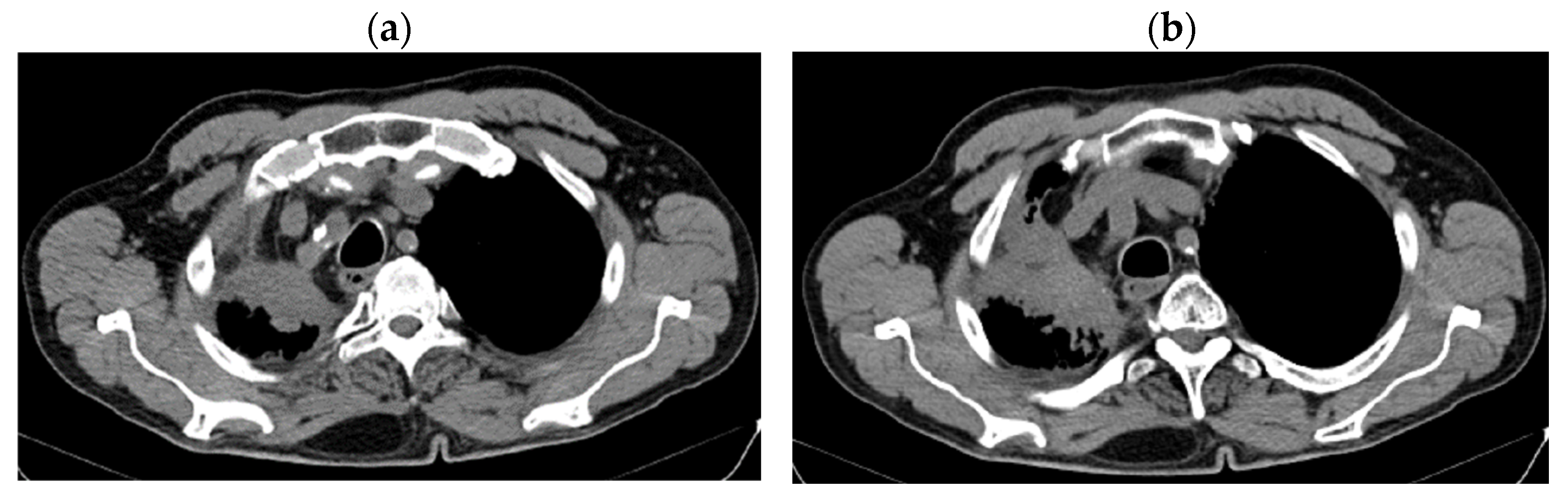
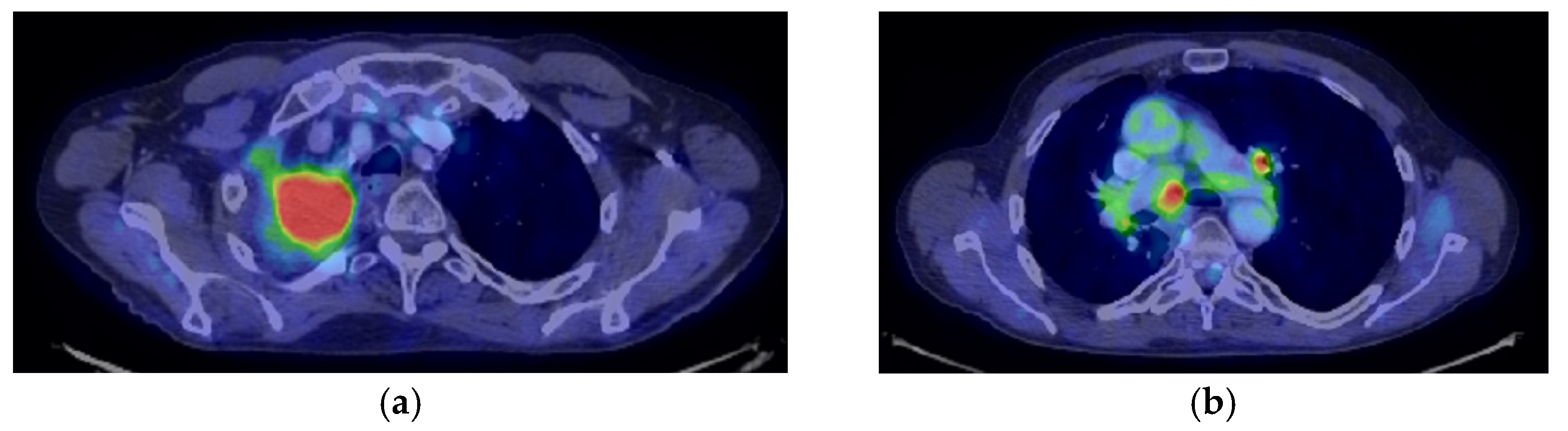
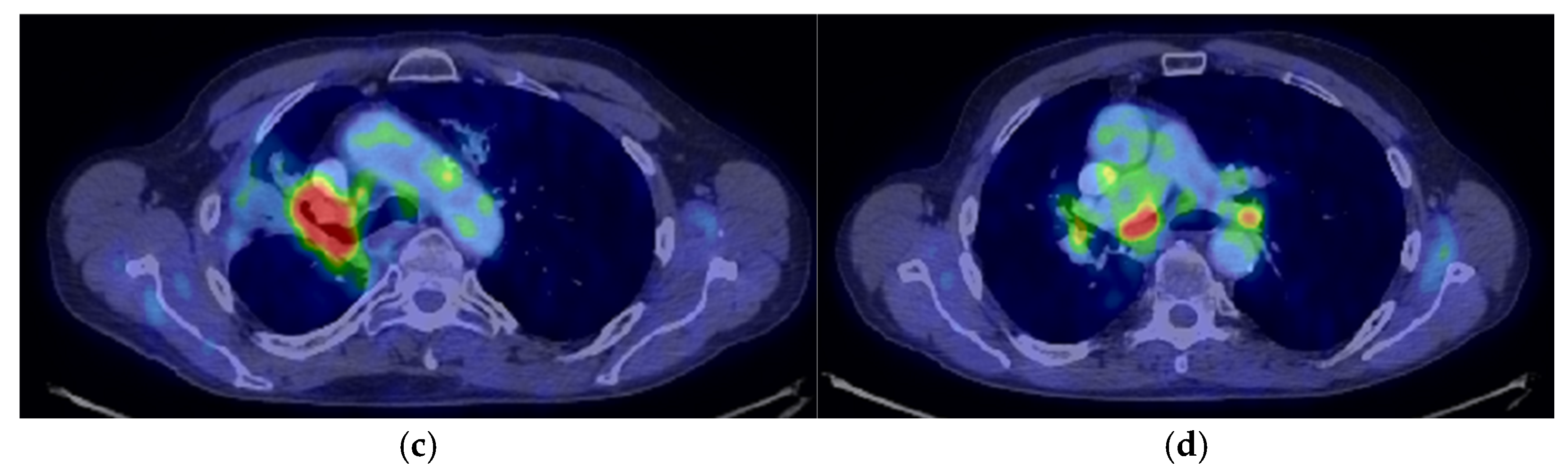
| Period of Time | Treatment | Regimen |
|---|---|---|
| 08–12/2017 | First-line therapy | 6xCis/Carbo + Gem |
| 04–05/2018 | Palliative RTE (right humeral head) | DT = 30 Gy |
| 05/2018–04/2020 | Second-line therapy | |
| 04–07/2020 | Third-line therapy | 4xDoce |
| 05.08–18.09.2020 | Palliative RTE (primary tumor + regional lymph node areas) | DT = 61.2 Gy |
| 01–04/2021 | Fourth-line therapy | 5xCarbo/Pacli |
| No oncological treatment. Stable disease | ||
| 11.2023–02.2025 | Fifth-line therapy | Pemetrexed |
| Results | Details | Time | Period |
|---|---|---|---|
| Progression of disease on IT | Secondary resistance at NIVO | At 23 months (1 year and 11 months) of NIVO | 05/2018–04/2020 |
| Survival without progression | Stable disease | 28 months (2 years and 4 months) | 11/2020–03/2023 |
| Progression after CHT + IT | From Stop NIVO | At 35 months (2 years and 11 months) | 04/2020–03/2023 |
| From finishing Line IV | At 23 months (1 year and 11 months) | 04/2021–03/2023 | |
| Without oncological treatment | 1 year and 7 months | 04/2021–11/2023 | |
| Survival | Since starting NIVO | 6 years and 9 months | 05/2018–02/2025 |
| Since stopping NIVO | 4 years and 10 months | 04/2020–02/2025 | |
| After Line IV (CHT) | 4 years and 1 month | 04/2021–02//2025 | |
| After Line V (CHT) | 1 year and 3 months | 11/202302/2025 | |
| General survival | 7 years and 7 months | 07/2017–02/2025 | |
| IP 0 (ECOG) | Continues professional activity | 02/2025 | |
| DOWNSTAGING from metastatic to locoregional stage | From cT3N3M1b(OSS) stage IVA | In cT2bN3M0, stage IIIB (without M1OSS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahnea-Nita, G.; Corobcean, N.; Constantin, G.B.; Nechifor, A.; Maier, A.-C.; Rahnea-Nita, R.-A.; Firescu, D.; Rebegea, L.-F. Unexpected Long-Term Survival and Downstaging in Oligometastatic Non-Small Cell Lung Cancer Treated with Multimodal Therapy. J. Clin. Med. 2025, 14, 3394. https://doi.org/10.3390/jcm14103394
Rahnea-Nita G, Corobcean N, Constantin GB, Nechifor A, Maier A-C, Rahnea-Nita R-A, Firescu D, Rebegea L-F. Unexpected Long-Term Survival and Downstaging in Oligometastatic Non-Small Cell Lung Cancer Treated with Multimodal Therapy. Journal of Clinical Medicine. 2025; 14(10):3394. https://doi.org/10.3390/jcm14103394
Chicago/Turabian StyleRahnea-Nita, Gabriela, Nadejda Corobcean, Georgiana Bianca Constantin, Alexandru Nechifor, Adrian-Cornel Maier, Roxana-Andreea Rahnea-Nita, Dorel Firescu, and Laura-Florentina Rebegea. 2025. "Unexpected Long-Term Survival and Downstaging in Oligometastatic Non-Small Cell Lung Cancer Treated with Multimodal Therapy" Journal of Clinical Medicine 14, no. 10: 3394. https://doi.org/10.3390/jcm14103394
APA StyleRahnea-Nita, G., Corobcean, N., Constantin, G. B., Nechifor, A., Maier, A.-C., Rahnea-Nita, R.-A., Firescu, D., & Rebegea, L.-F. (2025). Unexpected Long-Term Survival and Downstaging in Oligometastatic Non-Small Cell Lung Cancer Treated with Multimodal Therapy. Journal of Clinical Medicine, 14(10), 3394. https://doi.org/10.3390/jcm14103394







