Bilateral Spontaneous Hemothorax: A Rare Case of Primary Pleural Angiosarcoma and Literature Review †
Abstract
1. Introduction
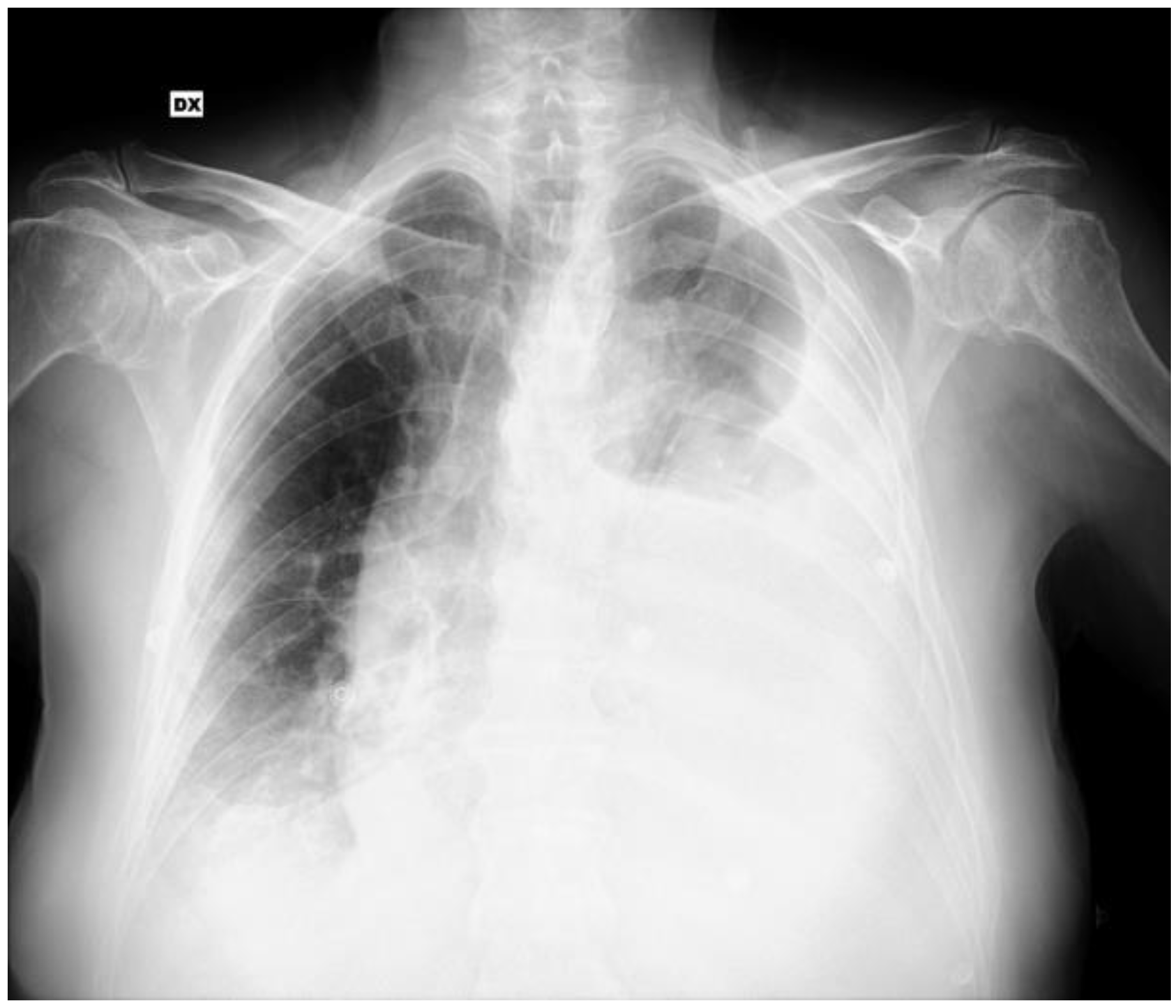
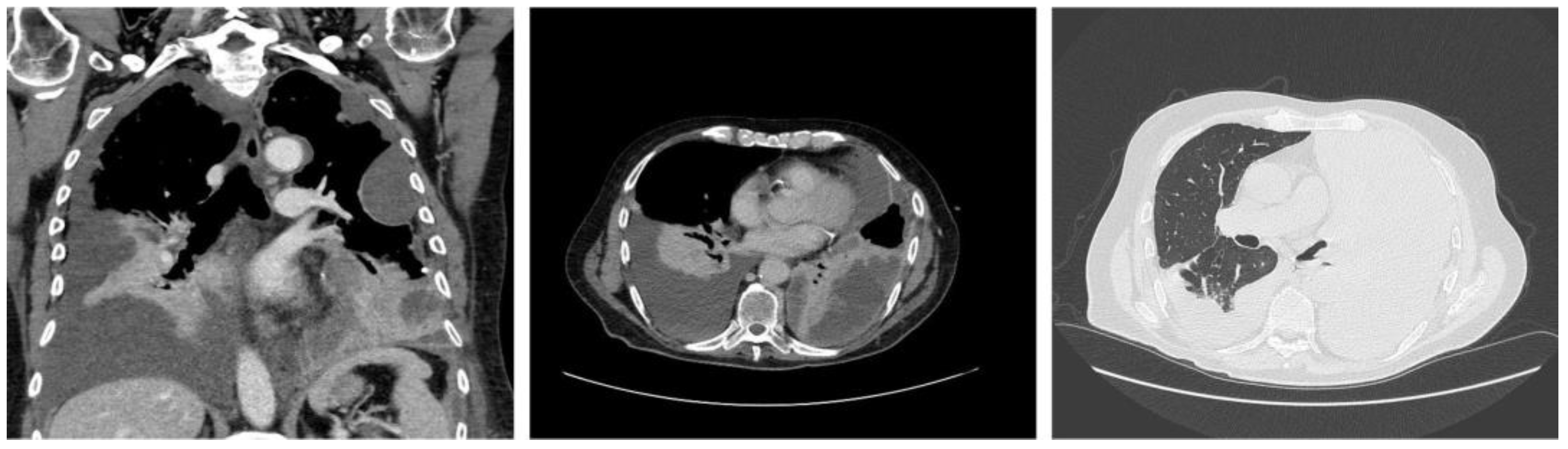
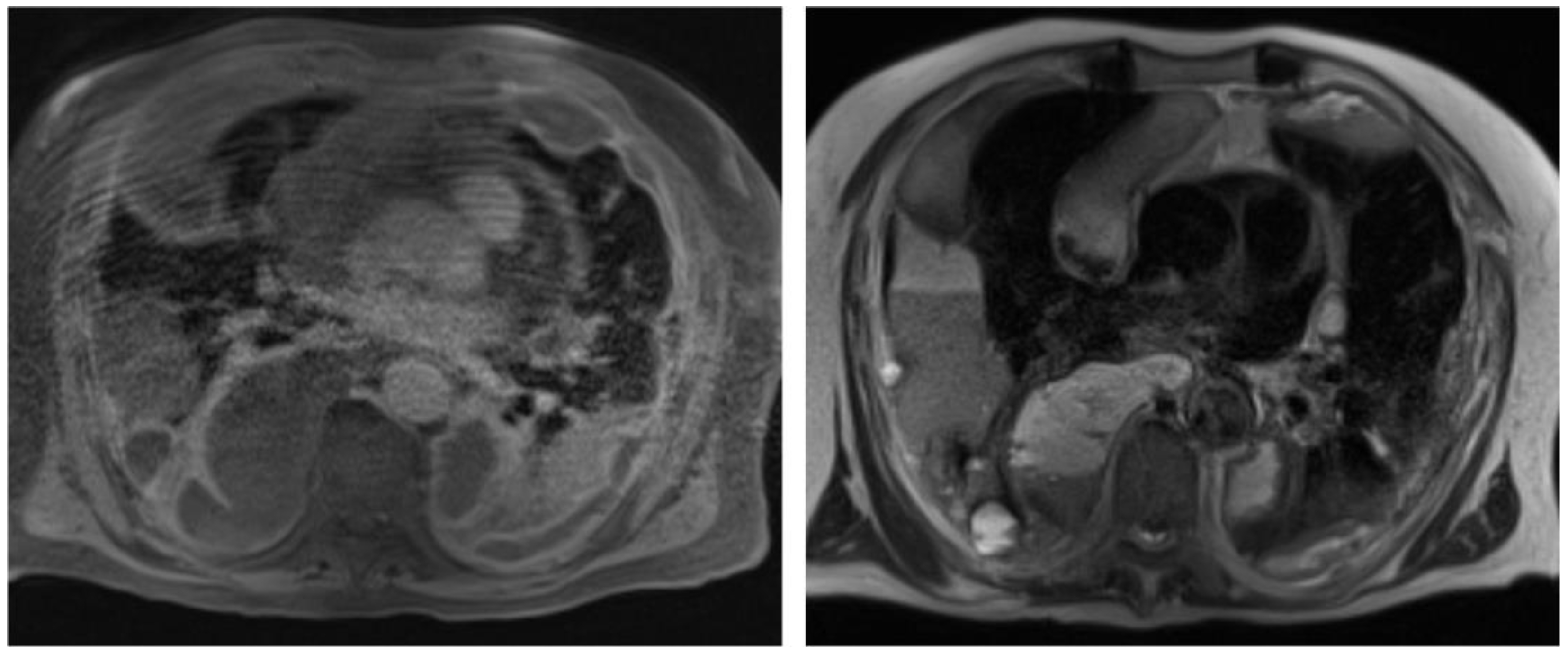
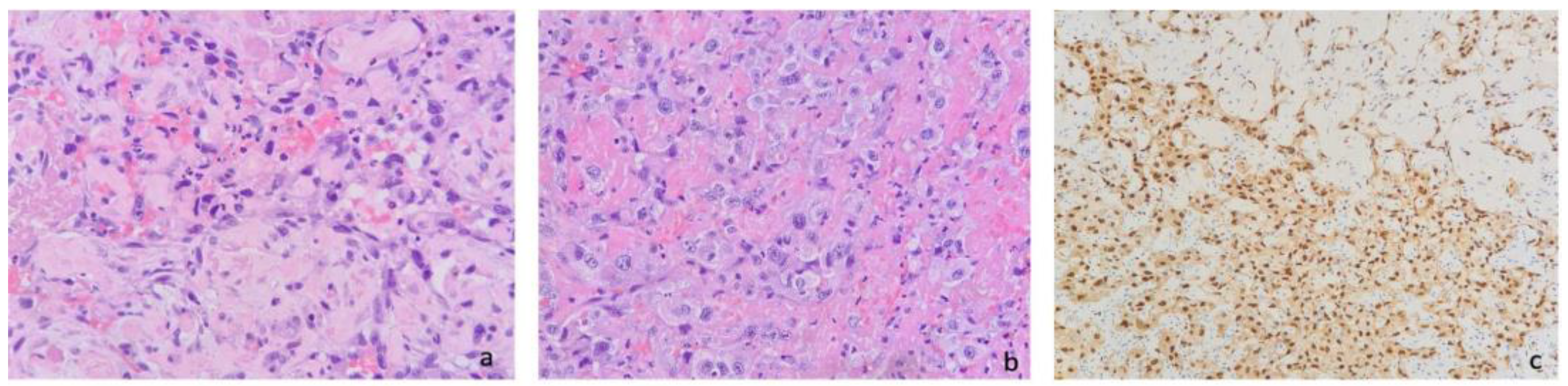
2. Case Report
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPA | primary pleural angiosarcoma |
| VATS | Video-Assisted Thoracoscopy |
References
- Lim, R.M.H.; Lee, J.Y.; Kannan, B.; Ko, T.K.; Chan, J.Y. Molecular and immune pathobiology of human angiosarcoma. Biochim. Biophys. Acta—Rev. Cancer 2024, 1879, 189159. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z.; Luo, Y.; Cai, J.; Wei, J.; Liu, A.; Zeng, Z. Characteristics and outcomes of primary pleural angiosarcoma: A retrospective study of 43 published cases. Medicine 2022, 101, e28785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Szczyrek, M.; Bitkowska, P.; Jutrzenka, M.; Szudy-Szczyrek, A.; Drelich-Zbroja, A.; Milanowski, J. Pleural Neoplasms-What Could MRI Change? Cancers 2023, 15, 3261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gil, B.M.; Chung, M.H.; Lee, K.N.; Jung, J.I.; Yoo, W.J.; Kwon, S.S.; Lee, H. Multidetector CT findings of primary pleural angiosarcoma: A systematic review, an additional cases report. Cancer Imaging 2022, 22, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, S.; Razak, A.A. Overview of the Initial Treatment of Metastatic Soft Tissue Sarcoma. Available online: https://www.uptodate.com/contents/overview-of-the-initial-treatment-of-metastatic-soft-tissue-sarcoma (accessed on 1 January 2025).
- Hassan, M.; Touman, A.A.; Grabczak, E.M.; Skaarup, S.H.; Faber, K.; Blyth, K.G.; Pochepnia, S. Imaging of pleural disease. Breathe 2024, 20, 230172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu-Zaid, A.; Mohammed, S. Primary pleural angiosarcoma in a 63-year-old gentleman. Case Rep. Pulmonol. 2013, 2013, 974567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ai, L.; Li, J.; Wang, W.; Li, Y. Medical thoracoscopy-diagnosed pleural angiosarcoma after complete resection of brain meningioma in an adolescent male. Asian J. Surg. 2022, 45, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Clelland, C.A.; Robinson, D.; Morgan, W.E. Primary angiosarcomas of the chest wall and pleura. Eur. J. Cardio-Thorac. Surg. 1998, 14, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Azzakhmam, M.; Elktaibi, A.; El Ochi, M.R.; Allaoui, M.; Albouzidi, A.; Oukabli, M. Primary epitheloid angiosarcoma of the pleura: An exceptional tumor location. Pan. Afr. Med. J. 2019, 33, 327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baisi, A.; Raveglia, F.; De Simone, M.; Cioffi, U. Primary multifocal angiosarcoma of the pleura. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Cabibi, D.; Pipitone, G.; Porcasi, R.; Ingrao, S.; Benza, I.; Porrello, C.; Cajozzo, M.; Giannone, A.G. Pleural epithelioid angiosarcoma with lymphatic differentiation arisen after radiometabolic therapy for thyroid carcinoma: Immunohistochemical findings and review of the literature. Diagn. Pathol. 2017, 12, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.Y.; Wu, Y.C.; Chou, T.Y.; Yang, K.Y. Pleural angiosarcoma mimicking pleural haematoma. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 886–888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Shih, H.J.; Seguerra, E., Jr.; Lin, J.H. Pathologic quiz case: A 39-year-old man with diffuse pleural thickening and massive hemothorax. Epithelioid angiosarcoma of pleura. Arch. Pathol. Lab. Med. 2004, 128, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Dainese, E.; Pozzi, B.; Milani, M.; Rossi, G.; Pezzotta, M.G.; Vertemati, G.; Tricomi, P.; Sessa, F. Primary pleural epithelioid angiosarcoma. A case report and review of the literature. Pathol. Res. Pract. 2010, 206, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Durani, U.; Gallo de Moraes, A.; Beachey, J.; Nelson, D.; Robinson, S.; Anavekar, N.S. Epithelioid angiosarcoma: A rare cause of pericarditis and pleural effusion. Respir. Med. Case Rep. 2018, 24, 77–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falconieri, G.; Bussani, R.; Mirra, M.; Zanella, M. Pseudomesotheliomatous angiosarcoma: A pleuropulmonary lesion simulating malignant pleural mesothelioma. Histopathology 1997, 30, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Kapetanakis, E.I.; Spiliopoulos, S.; Kostopanagiotou, K.; Tomos, P.; Kelekis, A. Bleeding Remission with Microwave Ablation in a Transfusion-Dependent Patient with Hemorrhaging Angiosarcoma of the Pleura. J. Vasc. Interv. Radiol. 2018, 29, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Chow, J.M.; Wang, K.M.; Fang, C.L.; Chu, J.S.; Chen, C.L. Primary pleural angiosarcoma as a mimicker of mesothelioma: A case report. Diagn. Pathol. 2011, 6, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimura, M.; Ito, H.; Furuta, T.; Tsumoto, T.; Hayashi, S. Pyothorax-associated angiosarcoma of the pleura with metastasis to the brain. Pathol. Int. 2003, 53, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.E.; Serra, S.; Duclos, B.; Brolly, F.; Dufour, P.; Bergerat, J.P. Diffuse primary angiosarcoma of the pleura: A case report and review of the literature. Sarcoma 2000, 8, 103–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levi, G.; Orzes, N.; Uccelli, S.; Cettolo, F.; Arici, M.; Ciarfaglia, M.; Fisogni, S.; Marchetti, G.; Rocchetti, C. Spontaneous synchronous bilateral hemothorax as the only finding in primary pleural angiosarcoma: A case report and a literature review. Monaldi Arch. Chest Dis. 2021, 91, 1520. [Google Scholar] [CrossRef] [PubMed]
- Lorentziadis, M.; Sourlas, A. Primary de novo angiosarcoma of the pleura. Ann. Thorac. Surg. 2012, 93, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Maglaras, G.C.; Katsenos, S.; Kakadelis, J.; Katsanos, C.; Metafratzi, Z.; Stefanou, D.G.; Vassiliou, M.P.; Constantopoulos, S.H. Primary angiosarcoma of the lung and pleura. Monaldi Arch. Chest Dis. 2004, 61, 234–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, K.; Yamaryo, T.; Akazawa, Y.; Kawakami, K.; Nakashima, M. Primary pleural angiosarcoma associated with pneumoconiosis: An autopsy case. Pathol. Int. 2015, 65, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Mudambi, L.; Vial, M.R.; Kalhor, N.; Grosu, H.B. Radiation-induced Angiosarcoma as a Cause of Pleural Effusion. Am. J. Respir. Crit. Care Med. 2017, 196, e10–e11. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Goto, A.; Hino, R.; Ota, S.; Okudaira, R.; Murakawa, T.; Nakajima, J.; Fukayama, M. Pleural cavity angiosarcoma arising in chronic expanding hematoma after pneumonectomy. Hum. Pathol. 2011, 42, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Watanabe, K.; Nishimura, T.; Yoshida, K.; Fukumoto, K.; Hiyama, N.; Masuda, Y.; Morikawa, T.; Matsumoto, J.; Usui, K. Primary Pleural Angiosarcoma Treated with Nivolumab and Ipilimumab: A Case Report. Case Rep. Oncol. 2023, 16, 81–87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogino, H.; Tobiume, M.; Kagawa, K.; Kawano, H.; Sakaguchi, S.; Saijo, A.; Matsumoto, D.; Takizawa, H.; Morikawa, Y.; Bando, Y.; et al. Radiation-associated Angiosarcoma Presenting as Massive Pleural Effusion. Intern. Med. 2022, 61, 1393–1397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onur, S.T.; Günlüoglu, Z.; Dalar, L.; Sökücü, S.; Altin, S.; Dinçer, I. Pleural angiosarcoma: A rare cause of spontaneous haemothorax. J. Pak. Med. Assoc. 2013, 63, 265–267. [Google Scholar] [PubMed]
- Otsubo, S.; Tanaka, S.; Kamiryo, Y.; Okumur, K.; Shimokama, T.; Kinjo, M. A case of primary pleural angiosarcoma metastasing to the bladder. Nihon Hinyokika Gakkai Zasshi 2011, 102, 686–690. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Ott, R.A.; Eugene, J.; Kollin, J.; Kanas, R.J.; Conston, D.E.; Chi, J.C. Primary pulmonary angiosarcoma associated with multiple synchronous neoplasms. J. Surg. Oncol. 1987, 35, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Munzer, K.; Dougherty, M.; Williams, P.; Loiselle, A. Pleural Myiasis Associated with Pleural Angiosarcoma. Chest 2016, 149, e157–e160. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Quesada, J.; Khalil, K.; Ferguson, E.C.; Brown, R.E. Morphoproteomic study of primary pleural angiosarcoma of lymphangioendothelial lineage: A case report. Ann. Clin. Lab. Sci. 2013, 43, 317–322. [Google Scholar] [PubMed]
- Roh, M.S.; Seo, J.Y.; Hong, S.H. Epithelioid angiosarcoma of the pleura: A case report. J. Korean Med. Sci. 2001, 16, 792–795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saitou, M.; Niitsuma, K. A case of pyothorax-associated pleural angiosarcoma diagnosed by autopsy. Kekkaku 2009, 84, 531–534. (In Japanese) [Google Scholar] [PubMed]
- Sedhai, Y.R.; Basnyat, S.; Golamari, R.; Koirala, A.; Yuan, M. Primary pleural angiosarcoma: Case report and literature review. SAGE Open Med. Case Rep. 2020, 8, 2050313X20904595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinkler, M.A.; Ullah, A.; Wehrle, C.J.; Ibrahim, M.A.; White, J. Primary Pleural Epithelioid Angiosarcoma With Extensive and Rapid Metastasis to Brain and Bilateral Adrenal Glands. Cureus 2020, 12, e9982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsubouchi, K.; Yoshioka, H.; Ishida, T. Significant response to gemcitabine monotherapy in primary pleural epithelioid angiosarcoma. J. Thorac. Oncol. 2012, 7, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, J.; Zeng, Z.; Cai, J.; Lu, Z.; Liu, A. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: A rare case report. Medicine 2021, 100, e27132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, J.; Gong, L.; Huang, X. A case description of primary pleural epithelioid angiosarcoma in an instance of sudden massive hemoptysis in a 52-year-old man. Quant. Imaging Med. Surg. 2021, 12, 2575–2578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woo, J.J.; Kim, Y.; An, J.K.; Lee, H. Primary pleural epithelioid angiosarcoma manifesting as a loculated hemothorax: A case report and literature review focusing on CT findings. Radiol. Case Rep. 2021, 16, 3072–3075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, J.; Liu, M.; Yuan, H.; Liu, Z.; Zhu, D. Case Report: Primary Pleural Angiosarcoma in a Patient with Klippel-Trenaunay Syndrome. Front. Genet. 2022, 13, 792466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaguchi, K.; Yoshino, K.; Imafuku, K.; Tsuboi, S.; Ohara, K. Case of primary pleural angiosarcoma with malignant seeding along the pleural tap tract. J. Dermatol. 2016, 44, e75–e76. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, H.; Tekneci, A.K.; Akcam, T.I.; Turhan, K.; Akalın, T. Pleural angiosarcoma presenting with spontaneous hemothorax. Indian J. Thorac. Cardiovasc. Surg. 2023, 39, 543–546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; Zheng, Y.; Liu, W.; Yu, X. Primary epithelioid angiosarcoma of the pleura: A case report and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 2153–2158. [Google Scholar] [PubMed] [PubMed Central]
- Zhu, C.; Yang, N.; Yao, J.; Du, X. Primary Pleural Epithelioid Angiosarcoma with Lung and Bone Metastases: A Case Report. Case Rep. Oncol. 2024, 17, 101–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Age (Mean ± SD) | 61.8 ± 15.3 years |
| Sex | Males: 39 Females: 10 |
| Symptoms | Dyspnea: 22 Chest pain: 15 Hemoptysis: 6 Cough: 5 No respiratory symptoms: 2 (Total described cases: 39) |
| Pleural effusion | No:11 (23.9%) Monolateral: 26 (56.5%) Bilateral: 9 (19.6%) Hematic effusion: 17 (48.6%) Non-hematic effusion: 18 (51.4%) |
| Pleural effusion cytology, when performed | Diagnostic: 3 (11.1%) Not diagnostic: 24 (88.9%) (Total described cases: 27) |
| CT specific findings, when performed (pleural masses or nodules) | Yes: 24 (57.1%) No: 18 (42.9%) (Total described cases: 42) |
| Diagnostic procedure | Cytology: 0 (0%) Thoracoscopy and pleural biopsy: 33 (71.7%) Autopsy: 7 (15.3%) CT guided biopsy: 6 (13%) |
| Treatment | No therapy: 11 (27.5%) Chemotherapy: 18 (45%) Radiotherapy: 3 (75%) Surgery: 7 (17.5%) Palliative care, including pleurodesis: 11 (27.5%) (Total described cases: 40) |
| Survival from diagnosis (mean ± SD) | 20 ± 29.2 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piamonti, D.; Giannone, S.; D’Antoni, L.; Sanna, A.; Landini, N.; Pernazza, A.; Bassi, M.; Carillo, C.; Diso, D.; Venuta, F.; et al. Bilateral Spontaneous Hemothorax: A Rare Case of Primary Pleural Angiosarcoma and Literature Review. J. Clin. Med. 2025, 14, 3377. https://doi.org/10.3390/jcm14103377
Piamonti D, Giannone S, D’Antoni L, Sanna A, Landini N, Pernazza A, Bassi M, Carillo C, Diso D, Venuta F, et al. Bilateral Spontaneous Hemothorax: A Rare Case of Primary Pleural Angiosarcoma and Literature Review. Journal of Clinical Medicine. 2025; 14(10):3377. https://doi.org/10.3390/jcm14103377
Chicago/Turabian StylePiamonti, Daniel, Silvia Giannone, Letizia D’Antoni, Arianna Sanna, Nicholas Landini, Angelina Pernazza, Massimiliano Bassi, Carolina Carillo, Daniele Diso, Federico Venuta, and et al. 2025. "Bilateral Spontaneous Hemothorax: A Rare Case of Primary Pleural Angiosarcoma and Literature Review" Journal of Clinical Medicine 14, no. 10: 3377. https://doi.org/10.3390/jcm14103377
APA StylePiamonti, D., Giannone, S., D’Antoni, L., Sanna, A., Landini, N., Pernazza, A., Bassi, M., Carillo, C., Diso, D., Venuta, F., Graziano, P., Pignatelli, P., Corbetta, L., Bonini, M., & Palange, P. (2025). Bilateral Spontaneous Hemothorax: A Rare Case of Primary Pleural Angiosarcoma and Literature Review. Journal of Clinical Medicine, 14(10), 3377. https://doi.org/10.3390/jcm14103377






