Laryngeal Cancer in the Modern Era: Evolving Trends in Diagnosis, Treatment, and Survival Outcomes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Risk Factors of LC
3.2. Histological Subtypes of LC
3.3. Diagnosis
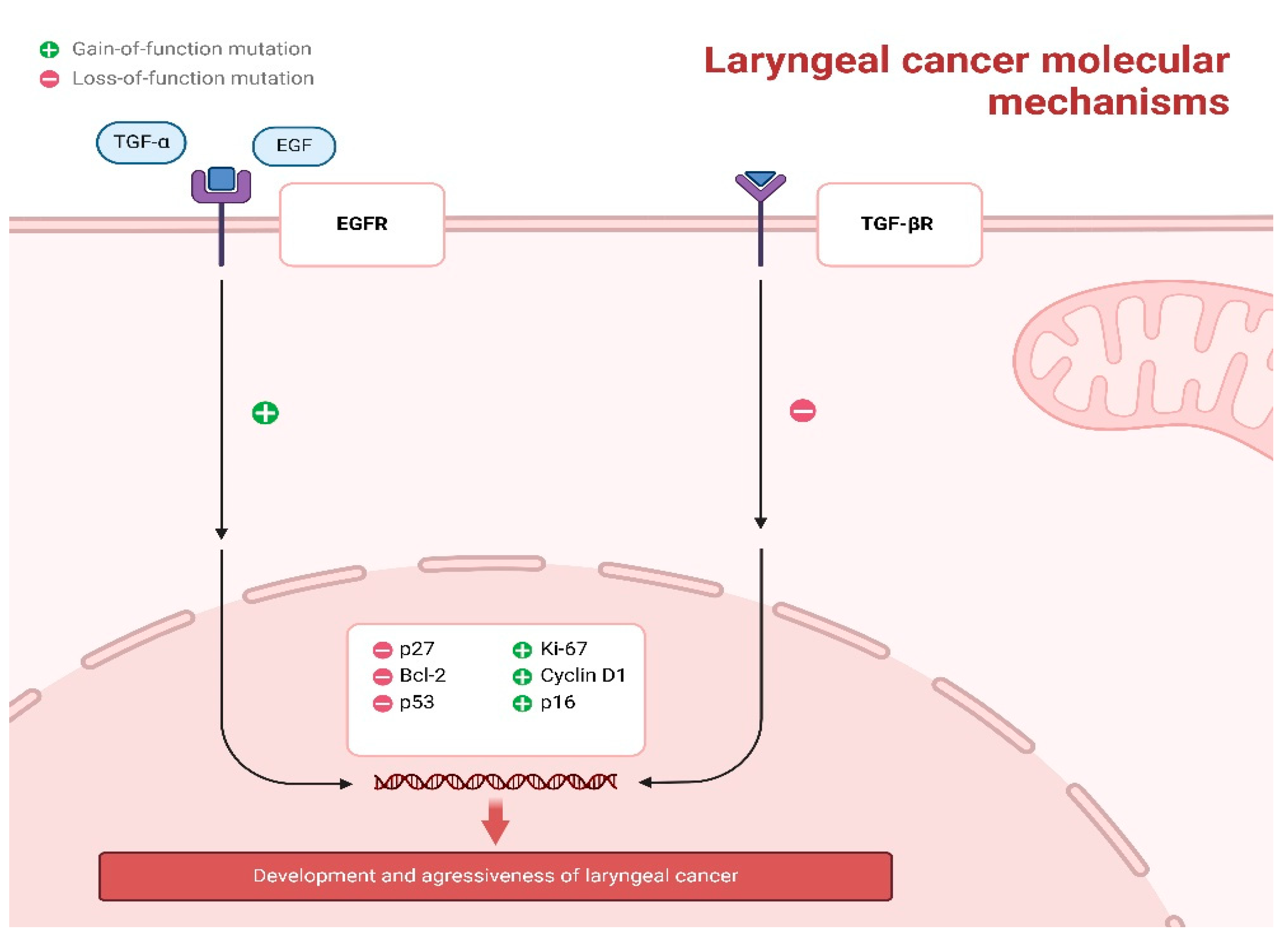
3.4. Molecular Biomarkers in Laryngeal Squamous Cell Carcinoma
3.5. Metastatic Patterns of LC
3.6. Recent Trials Further Investigating Biomarkers in LC
3.7. Treatment
3.8. Survival
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ciolofan, M.S.; Vlăescu, A.N.; Mogoantă, C.-A.; Ioniță, E.; Ioniță, I.; Căpitănescu, A.-N.; Mitroi, M.-R.; Anghelina, F. Clinical, Histological and Immunohistochemical Evaluation of Larynx Cancer. Curr. Health Sci. J. 2017, 43, 367–375. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F.; et al. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/14-larynx-fact-sheet.pdf (accessed on 14 March 2025).
- Zuo, J.-J.; Tao, Z.-Z.; Chen, C.; Hu, Z.-W.; Xu, Y.-X.; Zheng, A.-Y.; Guo, Y. Characteristics of Cigarette Smoking without Alcohol Consumption and Laryngeal Cancer: Overall and Time-Risk Relation. A Meta-Analysis of Observational Studies. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.M.; Watz, C.G.; Motofelea, A.C.; Chiriac, S.; Poenaru, M.; Dehelean, C.A.; Borza, C.; Ionita, I. Challenges of Pharyngeal Cancer Screening in Lower-Income Countries during Economic and Social Transitions: A Population-Based Analysis. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2226–2237. [Google Scholar] [CrossRef]
- Tang, J.A.; Lango, M.N. Diverging Incidence Trends for Larynx and Tonsil Cancer in Low Socioeconomic Regions of the US. Oral. Oncol. 2019, 91, 65–68. [Google Scholar] [CrossRef]
- Liao, L.; Wang, H.; Cui, W.; Zhang, Q.; He, X.; Wang, L.; Xiong, Y.; Jiang, L.; Xie, Y. Global, Regional and National Burden and Trends of Larynx Cancer among Adults Aged 55 and Older from 1990 to 2021: Results from the Global Burden of Disease Study 2021. BMC Public. Health 2025, 25, 906. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Zhang, Q.-W.; Wen, K.; Wang, C.; Ji, X.; Zhang, L. Temporal Trends in Incidence and Mortality Rates of Laryngeal Cancer at the Global, Regional and National Levels, 1990–2017. BMJ Open 2021, 11, e050387. [Google Scholar] [CrossRef]
- Balogun, O.; Rodin, D.; Ngwa, W.; Grover, S.; Longo, J. Challenges and Prospects for Providing Radiation Oncology Services in Africa. Semin. Radiat. Oncol. 2017, 27, 184–188. [Google Scholar] [CrossRef]
- Chang, C.-P.; Chang, S.-C.; Chuang, S.-C.; Berthiller, J.; Ferro, G.; Matsuo, K.; Wünsch-Filho, V.; Toporcov, T.N.; de Carvalho, M.B.; La Vecchia, C. Age at Start of Using Tobacco on the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium (INHANCE). Cancer Epidemiol. 2019, 63, 101615. [Google Scholar] [CrossRef]
- Di Credico, G.; Edefonti, V.; Polesel, J.; Pauli, F.; Torelli, N.; Serraino, D.; Negri, E.; Luce, D.; Stucker, I.; Matsuo, K. Joint Effects of Intensity and Duration of Cigarette Smoking on the Risk of Head and Neck Cancer: A Bivariate Spline Model Approach. Oral. Oncol. 2019, 94, 47–57. [Google Scholar] [CrossRef] [PubMed]
- LoConte, N.K.; Brewster, A.M.; Kaur, J.S.; Merrill, J.K.; Alberg, A.J. Alcohol and Cancer: A Statement of the American Society of Clinical Oncology. J. Clin. Oncol. 2018, 36, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Berdzuli, N.; Ferreira-Borges, C.; Gual, A.; Rehm, J. Alcohol Control Policy in Europe: Overview and Exemplary Countries. Int. J. Environ. Res. Public Health 2020, 17, 8162. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E. Alcohol Consumption and Site-Specific Cancer Risk: A Comprehensive Dose–Response Meta-Analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- He, J.; Meng, H.; Yao, G.; Fu, Y.; Geng, J. New Research Progress of HPV and Larynx Cancer. Progress. Mod. Biomed. 2017, 17, 5989–5992. [Google Scholar]
- Peng, W.; Mi, J.; Jiang, Y. Asbestos Exposure and Laryngeal Cancer Mortality. Laryngoscope 2016, 126, 1169–1174. [Google Scholar] [CrossRef]
- Hall, A.L.; Kromhout, H.; Schüz, J.; Peters, S.; Portengen, L.; Vermeulen, R.; Agudo, A.; Ahrens, W.; Boffetta, P.; Brennan, P. Laryngeal Cancer Risks in Workers Exposed to Lung Carcinogens: Exposure–Effect Analyses Using a Quantitative Job Exposure Matrix. Epidemiology 2020, 31, 145–154. [Google Scholar] [CrossRef]
- Haddad, G.; Sataloff, R.T.; Hamdan, A.-L. Laryngeal Metastatic Lesions: A Literature Review. J. Voice 2024, 38, 1458–1464. [Google Scholar] [CrossRef]
- Santos, A.; Santos, I.C.; dos Reis, P.F.; Rodrigues, V.D.; Peres, W.A.F. Impact of Nutritional Status on Survival in Head and Neck Cancer Patients After Total Laryngectomy. Nutr. Cancer 2022, 74, 1252–1260. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Hoffmann, T.K. Systemic therapy strategies for head-neck carcinomas: Current status. Laryngo Rhino Otol. 2012, 91 (Suppl. 1), S23–S143. [Google Scholar] [CrossRef]
- Baird, B.J.; Sung, C.K.; Beadle, B.M.; Divi, V. Treatment of Early-Stage Laryngeal Cancer: A Comparison of Treatment Options. Oral. Oncol. 2018, 87, 8–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Fu, Q.; Han, Z.; Wang, D.; Umar Shinge, S.A.; Muluh, T.A.; Lu, X. Combined Immunotherapy and Targeted Therapies for Cancer Treatment: Recent Advances and Future Perspectives. Curr. Cancer Drug Targets 2023, 23, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.R.; Ferlito, A.; Devaney, K.O.; Mäkitie, A.A.; Rinaldo, A. Prognostic Factors in Laryngeal Squamous Cell Carcinoma. Laryngoscope Investig. Otolaryngol. 2020, 5, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Sauer, A.G.; Siegel, R.L.; Miller, K.D.; Islami, F.; Fedewa, S.A.; Jacobs, E.J.; Jemal, A. State-Level Cancer Mortality Attributable to Cigarette Smoking in the United States. JAMA Intern. Med. 2016, 176, 1792–1798. [Google Scholar] [CrossRef]
- Permitasari, A.L.; Satibi, S.; Kristina, S.A. National Burden of Cancers Attributable to Secondhand Smoking in Indonesia. Asian Pac. J. Cancer Prev. 2018, 19, 1951–1955. [Google Scholar]
- Allegra, E.; Bianco, M.R.; Ralli, M.; Greco, A.; Angeletti, D.; De Vincentiis, M. Role of Clinical-Demographic Data in Survival Rates of Advanced Laryngeal Cancer. Medicina 2021, 57, 267. [Google Scholar] [CrossRef]
- Voltzke, K.J.; Lee, Y.-C.A.; Zhang, Z.-F.; Zevallos, J.P.; Yu, G.-P.; Winn, D.M.; Vaughan, T.L.; Sturgis, E.M.; Smith, E.; Schwartz, S.M. Racial Differences in the Relationship between Tobacco, Alcohol, and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the INHANCE Consortium. Cancer Causes Control 2018, 29, 619–630. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global Alcohol Exposure between 1990 and 2017 and Forecasts until 2030: A Modelling Study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef]
- Grevers, X.; Ruan, Y.; Poirier, A.E.; Walter, S.D.; Villeneuve, P.J.; Friedenreich, C.M.; Brenner, D.R.; Team, C.S. Estimates of the Current and Future Burden of Cancer Attributable to Alcohol Consumption in Canada. Prev. Med. 2019, 122, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Szukalska, M.; Szyfter, K.; Florek, E.; Rodrigo, J.P.; Rinaldo, A.; Mäkitie, A.A.; Strojan, P.; Takes, R.P.; Suárez, C.; Saba, N.F.; et al. Electronic Cigarettes and Head and Neck Cancer Risk-Current State of Art. Cancers 2020, 12, 3274. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-H.; Shin, H.-S. Measurement of Aldehydes in Replacement Liquids of Electronic Cigarettes by Headspace Gas Chromatography-Mass Spectrometry. Bull. Korean Chem. Soc. 2013, 34, 2691–2696. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking; IARC: Lyon, France, 2004; ISBN 9283212835. [Google Scholar]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-Related Carcinogenesis in Head and Neck Cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef]
- Khariwala, S.S.; Carmella, S.G.; Stepanov, I.; Fernandes, P.; Lassig, A.A.; Yueh, B.; Hatsukami, D.; Hecht, S.S. Elevated Levels of 1-hydroxypyrene and N′-nitrosonornicotine in Smokers with Head and Neck Cancer: A Matched Control Study. Head Neck 2013, 35, 1096–1100. [Google Scholar] [CrossRef]
- Salturk, Z.; Çakır, Ç.; Sünnetçi, G.; Atar, Y.; Kumral, T.L.; Yıldırım, G.; Berkiten, G.; Uyar, Y. Effects of Electronic Nicotine Delivery System on Larynx: Experimental Study. J. Voice 2015, 29, 560–563. [Google Scholar] [CrossRef]
- Sheikh, M.; Shakeri, R.; Poustchi, H.; Pourshams, A.; Etemadi, A.; Islami, F.; Khoshnia, M.; Gharavi, A.; Roshandel, G.; Khademi, H. Opium Use and Subsequent Incidence of Cancer: Results from the Golestan Cohort Study. Lancet Glob. Health 2020, 8, e649–e660. [Google Scholar] [CrossRef]
- Mohebbi, E.; Hadji, M.; Rashidian, H.; Rezaianzadeh, A.; Marzban, M.; Haghdoost, A.A.; Naghibzadeh Tahami, A.; Moradi, A.; Gholipour, M.; Najafi, F. Opium Use and the Risk of Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2021, 148, 1066–1076. [Google Scholar] [CrossRef]
- Tsimplaki, E.; Argyri, E.; Sakellaridis, A.; Kyrodimos, E.; Xesfyngi, D.; Panotopoulou, E. Oropharyngeal and Laryngeal but Not Oral Cancers Are Strongly Associated with High-risk Human Papillomavirus in 172 Greek Patients. J. Med. Virol. 2017, 89, 170–176. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Nahet, A.; Boublenza, L.; Hassaine, H.; Masdoua, N.; Pretet, J.-L.; Belglaiaa, E.; Mougin, C. HPV DNA Genotyping: A Study of Anogenital, Head and Neck and Skin Cancers in a Population from West Algerian. HPV Detection in Different Cancers from an Algerian Population. Bull. Cancer 2016, 103, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kariche, N.; Hortal, M.T.; Benyahia, S.; Alemany, L.; Moulaï, N.; Clavero, O.; Muñoz, M.; Ouahioune, W.; Djennaoui, D.; Touil-Boukoffa, C. Comparative Assessment of HPV, Alcohol and Tobacco Etiological Fractions in Algerian Patients with Laryngeal Squamous Cell Carcinoma. Infect. Agents Cancer 2018, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chuang, H.-C.; Lin, Y.-T.; Huang, C.-C.; Chien, C.-Y. Clinical Impact of Human Papillomavirus in Laryngeal Squamous Cell Carcinoma: A Retrospective Study. PeerJ 2017, 5, e3395. [Google Scholar] [CrossRef]
- De Lima, M.A.P.; Silva, Á.D.L.; do Nascimento Filho, A.C.S.; Cordeiro, T.L.; Bezerra, J.P.d.S.; Rocha, M.A.B.; Pinheiro, S.d.F.L.; Pinheiro, R.F.F., Jr.; Gadelha, M.d.S.V.; da Silva, C.G.L. Epstein-Barr Virus-Associated Carcinoma of the Larynx: A Systematic Review with Meta-Analysis. Pathogens 2021, 10, 1429. [Google Scholar] [CrossRef]
- Vazquez-Guillen, J.M.; Palacios-Saucedo, G.C.; Alanis-Valdez, A.Y.; Huerta-Escobedo, A.; Zavala-Pompa, A.; Rivera-Morales, L.G.; Martinez-Torres, A.C.; Gonzalez-Villasana, V.; Serna-Hernandez, J.C.; Hernandez-Martinez, S.J. p16INK4a and pRb Expression in Laryngeal Squamous Cell Carcinoma with and without Infection by EBV or Different Genotypes of HPV: A Retrospective Study. Infect. Agents Cancer 2023, 18, 43. [Google Scholar] [CrossRef]
- Igissin, N.; Zatonskikh, V.; Telmanova, Z.; Tulebaev, R.; Moore, M. Laryngeal Cancer: Epidemiology, Etiology, and Prevention: A Narrative Review. Iran. J. Public Health 2023, 52, 2248–2259. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Yen, C.-J.; Hsiao, J.-R.; Ou, C.-Y.; Huang, J.-S.; Wong, T.-Y.; Tsai, S.-T.; Huang, C.-C.; Lee, W.-T.; Chen, K.-C. A Comprehensive Analysis on the Association between Tobacco-Free Betel Quid and Risk of Head and Neck Cancer in Taiwanese Men. PLoS ONE 2016, 11, e0164937. [Google Scholar] [CrossRef]
- Chang, C.; Siwakoti, B.; Sapkota, A.; Gautam, D.K.; Lee, Y.A.; Monroe, M.; Hashibe, M. Tobacco Smoking, Chewing Habits, Alcohol Drinking and the Risk of Head and Neck Cancer in Nepal. Int. J. Cancer 2020, 147, 866–875. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Yang, Y.; Zhou, L.; Tao, L. Association between Helicobacter Pylori Infection and Carcinoma of the Larynx or Pharynx. Head Neck 2016, 38, E2291–E2296. [Google Scholar] [CrossRef]
- Eells, A.C.; Mackintosh, C.; Marks, L.; Marino, M.J. Gastroesophageal Reflux Disease and Head and Neck Cancers: A Systematic Review and Meta-Analysis. Am. J. Otolaryngol. 2020, 41, 102653. [Google Scholar] [CrossRef]
- Parsel, S.M.; Wu, E.L.; Riley, C.A.; McCoul, E.D. Gastroesophageal and Laryngopharyngeal Reflux Associated with Laryngeal Malignancy: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Sereg-Bahar, M.; Jerin, A.; Hocevar-Boltezar, I. Higher Levels of Total Pepsin and Bile Acids in the Saliva as a Possible Risk Factor for Early Laryngeal Cancer. Radiol. Oncol. 2015, 49, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Han, K.; Joo, Y.-H. Metabolic Syndrome and Incidence of Laryngeal Cancer: A Nationwide Cohort Study. Sci. Rep. 2019, 9, 667. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kim, G.-J.; Han, K.; Joo, Y.-H. Changes in Metabolic Syndrome Status and Risk of Laryngeal Cancer: A Nationwide Cohort Study. PLoS ONE 2021, 16, e0252872. [Google Scholar] [CrossRef]
- Gong, H.-L.; Shi, Y.; Zhou, L.; Wu, C.-P.; Cao, P.-Y.; Tao, L.; Xu, C.; Hou, D.-S.; Wang, Y.-Z. The Composition of Microbiome in Larynx and the Throat Biodiversity between Laryngeal Squamous Cell Carcinoma Patients and Control Population. PLoS ONE 2013, 8, e66476. [Google Scholar] [CrossRef]
- Yu, S.; Chen, J.; Zhao, Y.; Yan, F.; Fan, Y.; Xia, X.; Shan, G.; Zhang, P.; Chen, X. Oral-Microbiome-Derived Signatures Enable Non-Invasive Diagnosis of Laryngeal Cancers. J. Transl. Med. 2023, 21, 438. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S.; et al. Composition and Function of Oral Microbiota between Gingival Squamous Cell Carcinoma and Periodontitis. Oral. Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Kelly, D.; Mulder, I.E. Microbiome and Immunological Interactions. Nutr. Rev. 2012, 70, S18–S30. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed]
- McMaughan, D.J.; Oloruntoba, O.; Smith, M.L. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front. Public Health 2020, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Fullmer, T.; Wilde, D.C.; Shi, J.W.; Wagner, T.; Skinner, H.; Eicher, S.A.; Sandulache, V.C.; Hernandez, D.J. Demographic and Tumor Characteristic Impact on Laryngeal Cancer Outcomes in a Minority Underserved Patient Population. Otolaryngol. Head Neck Surg. 2020, 162, 888–896. [Google Scholar] [CrossRef]
- Lu, Y.; Li, P.; Luo, G.; Liu, D.; Zou, H. Cancer Attributable to Human Papillomavirus Infection in China: Burden and Trends. Cancer 2020, 126, 3719–3732. [Google Scholar] [CrossRef]
- Buttmann-Schweiger, N.; Deleré, Y.; Klug, S.J.; Kraywinkel, K. Cancer Incidence in Germany Attributable to Human Papillomavirus in 2013. BMC Cancer 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Senkomago, V. Human Papillomavirus–Attributable Cancers—United States, 2012–2016. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef]
- Wang, N.; Lv, H.; Huang, M. Impact of Gender on Survival in Patients with Laryngeal Squamous Cell Carcinoma: A Propensity Score Matching Analysis. Int. J. Clin. Exp. Pathol. 2020, 13, 573–581. [Google Scholar]
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and Neck Cancer Prevention: From Primary Prevention to Impact of Clinicians on Reducing Burden. Ann. Oncol. 2019, 30, 744–756. [Google Scholar] [CrossRef]
- Andersson, T.M.L.; Engholm, G.; Pukkala, E.; Stenbeck, M.; Tryggvadottir, L.; Storm, H.; Weiderpass, E. Avoidable Cancers in the Nordic Countries—The Impact of Alcohol Consumption. Eur. J. Cancer 2018, 103, 299–307. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Zhu, Q.; Zhou, E.; Zhang, J.; Song, F.; Xu, C.; Shen, Y.; Zou, J.; Zhu, H.; et al. Global Trends and Risk Factors of Laryngeal Cancer: A Systematic Analysis for the Global Burden of Disease Study (1990–2021). BMC Cancer 2025, 25, 296. [Google Scholar] [CrossRef]
- Liberale, C.; Soloperto, D.; Marchioni, A.; Monzani, D.; Sacchetto, L. Updates on Larynx Cancer: Risk Factors and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 12913. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Husain, Q.; Kam, D.; Dubal, P.M.; Baredes, S.; Eloy, J.A. Laryngeal Papillary Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2015, 153, 54–59. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S.D. Non-Squamous Laryngeal Cancer. Otolaryngol. Clin. N. Am. 2023, 56, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Dubal, P.M.; Marchiano, E.; Kam, D.; Dutta, R.; Kalyoussef, E.; Baredes, S.; Eloy, J.A. Laryngeal Spindle Cell Carcinoma: A Population-Based Analysis of Incidence and Survival. Laryngoscope 2015, 125, 2709–2714. [Google Scholar] [CrossRef]
- Gerry, D.; Fritsch, V.A.; Lentsch, E.J. Spindle Cell Carcinoma of the Upper Aerodigestive Tract. Ann. Otol. Rhinol. Laryngol. 2014, 123, 576–583. [Google Scholar] [CrossRef]
- Fritsch, V.A.; Lentsch, E.J. Basaloid Squamous Cell Carcinoma of the Larynx: Analysis of 145 Cases with Comparison to Conventional Squamous Cell Carcinoma. Head Neck 2013, 36, 164–170. [Google Scholar] [CrossRef]
- Kass, J.I.; Lee, S.C.; Abberbock, S.; Seethala, R.R.; Duvvuri, U. Adenosquamous Carcinoma of the Head and Neck: Molecular Analysis Using CRTC-MAML FISH and Survival Comparison with Paired Conventional Squamous Cell Carcinoma. Laryngoscope 2015, 125, E371–E376. [Google Scholar] [CrossRef]
- Kusafuka, K.; Muramatsu, K.; Iida, Y.; Mori, K.; Miki, T.; Suda, T.; Fuke, T.; Kamijo, T.; Onitsuka, T.; Nakajima, T. MUC Expression in Adenosquamous Carcinoma of the Head and Neck Regions of Japanese Patients: Immunohistochemical Analysis. Pathol. Int. 2014, 64, 104–114. [Google Scholar] [CrossRef]
- Schick, U.; Pusztaszeri, M.; Betz, M.; Ghadjar, P.; Demiroz, C.; Kaanders, J.H.A.M.; Ozsahin, M. Adenosquamous Carcinoma of the Head and Neck: Report of 20 Cases and Review of the Literature. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 116, 313–320. [Google Scholar] [CrossRef]
- Jones, T.M.; De, M.; Foran, B.; Harrington, K.; Mortimore, S. Laryngeal Cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S75–S82. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Fawaz, S.A.; Sabri, S.M.; Sweed, A.; Rabie, H. Sensitivity and Specificity of Stroboscopy in Preoperative Differentiation of Dysplasia from Early Invasive Glottic Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Master, Z.; Kannan, S.; Agarwal, J.P.; Ghsoh-Laskar, S.; Rangarajan, V.; Murthy, V.; Budrukkar, A. Diagnostic Performance of Post-Treatment FDG PET or FDG PET/CT Imaging in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Sharma, N.; Raghavan, A. Role of Computed Tomography, Magnetic Resonance Imaging, and Endoscopy in Pretherapeutic Evaluation of Laryngeal Tumors. Astrocyte 2016, 2, 172. Available online: https://go.gale.com/ps/i.do?id=GALE%7CA466753779&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=23490977&p=AONE&sw=w&userGroupName=anon%7Ec07fdcb8&aty=open-web-entry (accessed on 14 March 2025). [CrossRef]
- De Vito, A.; Meccariello, G.; Vicini, C. Narrow Band Imaging as Screening Test for Early Detection of Laryngeal Cancer: A Prospective Study. Clin. Otolaryngol. 2016, 42, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, Plasma and Saliva Biomarkers for Head and Neck Cancer. Expert. Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined Circulating Tumor DNA and Protein Biomarker-Based Liquid Biopsy for the Earlier Detection of Pancreatic Cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Correction to: Liquid Biopsy and Tumor Heterogeneity in Metastatic Solid Tumors: The Potentiality of Blood Samples. J. Exp. Clin. Cancer Res. 2020, 39, 120. [Google Scholar] [CrossRef]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The Systemic Inflammation-Based Neutrophil–Lymphocyte Ratio: Experience in Patients with Cancer. Crit. Rev. Oncol./Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Iacob, R.; Mandea, M.; Iacob, S.; Pietrosanu, C.; Paul, D.; Hainarosie, R.; Gheorghe, C. Liquid Biopsy in Squamous Cell Carcinoma of the Esophagus and of the Head and Neck. Front. Med. 2022, 9, 827297. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Y.; Feng, L.-F.; Meng, X.; Chen, R.; Xu, W.-H.; Hou, J.; Xu, T.; Zhang, L. Liquid Biopsy in Head and Neck Squamous Cell Carcinoma: Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes. Expert. Rev. Mol. Diagn. 2020, 20, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Spruce, R.; Beggs, A.; Sharma, N.; Kong, A.; Martin, T.; Parmar, S.; Praveen, P.; Nankivell, P.; Mehanna, H. Circulating Tumor DNA as a Biomarker and Liquid Biopsy in Head and Neck Squamous Cell Carcinoma. Head Neck 2018, 40, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Vrba, L.; Oshiro, M.M.; Kim, S.S.; Garland, L.L.; Placencia, C.; Mahadevan, D.; Nelson, M.A.; Futscher, B.W. DNA Methylation Biomarkers Discovered in Silico Detect Cancer in Liquid Biopsies from Non-Small Cell Lung Cancer Patients. Epigenetics 2020, 15, 419–430. [Google Scholar] [CrossRef]
- Ooki, A.; Maleki, Z.; Tsay, J.-C.J.; Goparaju, C.; Brait, M.; Turaga, N.; Nam, H.-S.; Rom, W.N.; Pass, H.I.; Sidransky, D. A Panel of Novel Detection and Prognostic Methylated DNA Markers in Primary Non–Small Cell Lung Cancer and Serum DNA. Clin. Cancer Res. 2017, 23, 7141–7152. [Google Scholar] [CrossRef]
- Schröck, A.; Leisse, A.; de Vos, L.; Gevensleben, H.; Dröge, F.; Franzen, A.; Wachendörfer, M.; Schröck, F.; Ellinger, J.; Teschke, M. Free-Circulating Methylated DNA in Blood for Diagnosis, Staging, Prognosis, and Monitoring of Head and Neck Squamous Cell Carcinoma Patients: An Observational Prospective Cohort Study. Clin. Chem. 2017, 63, 1288–1296. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M.; Esteller, M.; Wu, L.; Nawroz-Danish, H.; Yoo, G.H.; Koch, W.M.; Jen, J.; Herman, J.G.; Sidransky, D. Gene Promoter Hypermethylation in Tumors and Serum of Head and Neck Cancer Patients. Cancer Res. 2000, 60, 892–895. [Google Scholar]
- Kawada, T.; Takahashi, H.; Sakakura, K.; Ida, S.; Mito, I.; Toyoda, M.; Chikamatsu, K. Circulating Tumor Cells in Patients with Head and Neck Squamous Cell Carcinoma: Feasibility of Detection and Quantitation. Head Neck 2017, 39, 2180–2186. [Google Scholar] [CrossRef]
- Nichols, A.C.; Lowes, L.E.; Szeto, C.C.T.; Basmaji, J.; Dhaliwal, S.; Chapeskie, C.; Todorovic, B.; Read, N.; Venkatesan, V.; Hammond, A.; et al. Detection of Circulating Tumor Cells in Advanced Head and Neck Cancer Using the Cellsearch System. Head Neck 2011, 34, 1440–1444. [Google Scholar] [CrossRef]
- Rizzo, M.I.; Ralli, M.; Nicolazzo, C.; Gradilone, A.; Carletti, R.; Di Gioia, C.; De Vincentiis, M.; Greco, A. Detection of Circulating Tumor Cells in Patients with Laryngeal Cancer Using ScreenCell: Comparative Pre- and Post-Operative Analysis and Association with Prognosis. Oncol. Lett. 2020, 19, 4183–4188. [Google Scholar] [CrossRef]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. miRNA-130a Significantly Improves Accuracy of SGA Nutritional Assessment Tool in Prediction of Malnutrition and Cachexia in Radiotherapy-Treated Head and Neck Cancer Patients. Cancers 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-C.; Song, L.-Q.; Xu, W.-W.; Qi, J.-J.; Wang, X.-Y.; Su, Y. Serum miR-632 Is a Potential Marker for the Diagnosis and Prognosis in Laryngeal Squamous Cell Carcinoma. Acta Oto-Laryngol. 2020, 140, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wang, X.; Yang, D.; Shi, W.J. The Expression of MicroRNA-155 in Plasma and Tissue Is Matched in Human Laryngeal Squamous Cell Carcinoma. Yonsei Med. J. 2016, 57, 298–305. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Lu, J.; Sun, Y.; Xiao, H.; Liu, M.; Tian, L. Combined Detection of Serum Exosomal miR-21 and HOTAIR as Diagnostic and Prognostic Biomarkers for Laryngeal Squamous Cell Carcinoma. Med. Oncol. 2014, 31, 148. [Google Scholar] [CrossRef]
- Cabanero, M.; Tsao, M.S. Circulating Tumour DNA in EGFR-Mutant Non-Small-Cell Lung Cancer. Curr. Oncol. 2018, 25, 38–44. [Google Scholar] [CrossRef]
- Mahmood, H.; Shaban, M.; Rajpoot, N.; Khurram, S.A. Artificial Intelligence-Based Methods in Head and Neck Cancer Diagnosis: An Overview. Br. J. Cancer 2021, 124, 1934–1940. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Zheng, B.; Su, L.; Chen, Y.; Ma, S.; Hu, Q.; Zou, X.; Yao, L.; Yang, Y.; et al. Rapid Histology of Laryngeal Squamous Cell Carcinoma with Deep-Learning Based Stimulated Raman Scattering Microscopy. Theranostics 2019, 9, 2541–2554. [Google Scholar] [CrossRef]
- Moccia, S.; De Momi, E.; Guarnaschelli, M.; Savazzi, M.; Laborai, A.; Guastini, L.; Peretti, G.; Mattos, L.S. Confident Texture-Based Laryngeal Tissue Classification for Early Stage Diagnosis Support. J. Med. Imaging (Bellingham Wash.) 2017, 4, 34502. [Google Scholar] [CrossRef]
- Choi, N.; Kim, J.; Yi, H.; Kim, H.; Kim, T.H.; Chung, M.J.; Ji, M.; Kim, Z.; Son, Y.-I. The Use of Artificial Intelligence Models to Predict Survival in Patients with Laryngeal Squamous Cell Carcinoma. Sci. Rep. 2023, 13, 9734. [Google Scholar] [CrossRef]
- Brandstorp-Boesen, J.; Sørum Falk, R.; Boysen, M.; Brøndbo, K. Impact of Stage, Management and Recurrence on Survival Rates in Laryngeal Cancer. PLoS ONE 2017, 12, e0179371. [Google Scholar] [CrossRef]
- Mulcahy, C.F.; Mohamed, A.S.R.; Kanwar, A.; Hutcheson, K.A.; Ghosh, A.; Vock, D.; Weber, R.S.; Lai, S.Y.; Gunn, G.B. Age-adjusted Comorbidity and Survival in Locally Advanced Laryngeal Cancer. Head Neck 2018, 40, 2060–2069. [Google Scholar] [PubMed]
- Egelmeer, A.G.T.M.; Velazquez, E.R.; de Jong, J.M.A.; Oberije, C.; Geussens, Y.; Nuyts, S.; Kremer, B.; Rietveld, D.; René Leemans, C.; de Jong, M.C.; et al. Development and Validation of a Nomogram for Prediction of Survival and Local Control in Laryngeal Carcinoma Patients Treated with Radiotherapy Alone: A Cohort Study Based on 994 Patients. Radiother. Oncol. 2011, 100, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Datema, F.R.; Ferrier, M.B.; Vergouwe, Y.; Moya, A.; Molenaar, J.; Piccirillo, J.F.; Baatenburg de Jong, R.J. Update and External Validation of a Head and Neck Cancer Prognostic Model. Head Neck 2012, 35, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, M.; Bisogno, A.; Scarpa, A.; D’Urso, A.; Marra, P.; Colacurcio, V.; De Luca, P.; Ralli, M.; Cassandro, E.; Cassandro, C. Biomarkers of Laryngeal Squamous Cell Carcinoma: A Review. Ann. Diagn. Pathol. 2021, 54, 151787. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Rubini, C.; Magliulo, G.; Re, M. Prognostic Value of Bcl-2 Expression in Squamous Cell Carcinoma of the Larynx: A Systematic Review. Int. J. Biol. Markers 2015, 30, 155–160. [Google Scholar] [CrossRef]
- Rashad, U.M.; Hussein, M.R.; Algizawy, S.M. Alterations of P53 and Bcl-2 Protein Expression in the Recurrent Laryngeal and Pharyngeal Squamous Cell Carcinoma. Am. J. Otolaryngol. 2011, 32, 210–214. [Google Scholar] [CrossRef]
- Yildirim, S.; Cermik, H.; Işitmangil, T.; Baloglu, H.; Gungor, A.; Pekkafali, Z. Significance of P53 and Bcl-2 Immunoexpression in the Prognosis of Laryngeal Squamous Cell Carcinoma. J. Int. Med. Res. 2002, 30, 597–600. [Google Scholar] [CrossRef]
- Ozdek, A.; Sarac, S.; Akyol, M.U.; Sungur, A.; Yilmaz, T. C-Myc and Bcl-2 Expression In Supraglottic Squamous Cell Carcinoma of the Larynx. Otolaryngol. Head Neck Surg. 2004, 131, 77–83. [Google Scholar] [CrossRef]
- Jakstas, T.; Bartnykaite, A.; Padervinskis, E.; Vegiene, A.; Juozaityte, E.; Uloza, V.; Ugenskiene, R. The Association of TP53, BCL2, BAX and NOXA SNPs and Laryngeal Squamous Cell Carcinoma Development. Int. J. Mol. Sci. 2024, 25, 11849. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 Expression in Head and Neck Cancer: A Systemic Review and Meta-Analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef]
- Trapasso, S.; Allegra, E. Role of CD44 as a Marker of Cancer Stem Cells in Head and Neck Cancer. Biol. Targets Ther. 2012, 6, 379–383. [Google Scholar] [CrossRef][Green Version]
- Joshua, B.; Kaplan, M.J.; Doweck, I.; Pai, R.; Weissman, I.L.; Prince, M.E.; Ailles, L.E. Frequency of Cells Expressing CD44, a Head and Neck Cancer Stem Cell Marker: Correlation with Tumor Aggressiveness. Head Neck 2011, 34, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Gioacchini, F.M.; Scarpa, A.; Cassandro, C.; Tulli, M.; Cassandro, E. The Prognostic Significance of E-Cadherin Expression in Laryngeal Squamous-Cell Carcinoma: A Systematic Review. Acta Otorhinolaryngol. Ital. 2018, 38, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Yang, X.; Li, S.; Liu, X.; Yang, Q.; Li, Y.; Ye, J. Reduced E-Cadherin Expression Is Associated with Lymph Node Metastases in Laryngeal Squamous Cell Carcinoma. Auris Nasus Larynx 2012, 39, 186–192. [Google Scholar] [CrossRef]
- Akdeniz, O.; Akduman, D.; Haksever, M.; Ozkarakas, H.; Müezzinoglu, B. Relationships between Clinical Behavior of Laryngeal Squamous Cell Carcinomas and Expression of VEGF, MMP-9 and E-Cadherin. Asian Pac. J. Cancer Prev. 2013, 14, 5301–5310. [Google Scholar] [CrossRef]
- Cappellesso, R.; Marioni, G.; Crescenzi, M.; Giacomelli, L.; Guzzardo, V.; Mussato, A.; Staffieri, A.; Martini, A.; Blandamura, S.; Fassina, A. The Prognostic Role of the Epithelial-Mesenchymal Transition Markers E-Cadherin and Slug in Laryngeal Squamous Cell Carcinoma. Histopathology 2015, 67, 491–500. [Google Scholar] [CrossRef]
- CDKN2A Gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/cdkn2a/ (accessed on 8 May 2025).
- Nowosad, A.; Besson, A. CDKN1B/P27 Regulates Autophagy via the Control of Ragulator and MTOR Activity in Amino Acid-Deprived Cells. Autophagy 2020, 16, 2297–2298. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Kaleci, S.; Magliulo, G.; Presutti, L.; Re, M. The Prognostic Value of Cyclin D1 Expression in Head and Neck Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2014, 273, 801–809. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Lin, J.; Hu, Y.; Luo, G.; Luo, Z.; Cheng, C.; Wang, P. Clinicopathologic and Prognostic Significance of Human Epidermal Growth Factor Receptor in Patients with Gastric Cancer: An Updated Meta-Analysis. Oncotarget 2017, 8, 17202–17215. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Magliulo, G.; Rubini, C.; Presutti, L.; Re, M. The Clinical Relevance of Ki-67 Expression in Laryngeal Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2014, 272, 1569–1576. [Google Scholar] [CrossRef]
- Mirza, S.; Jeannon, J.P.; Soames, J.; Wilson, J.A. Is Ki67 a Marker for the Transformation of Laryngeal Dysplasia to Carcinoma? Acta Oto-Laryngol. 2006, 126, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Acikalin, M.F.; Öner, Ü.; Tel, N.; Paşaoğlu, Ö.; Çakli, H.; Çolak, E. Prognostic Significance of Ki-67 Expression for Patients with Laryngeal Squamous Cell Carcinoma Primarily Treated by Total Laryngectomy. Eur. Arch. Oto-Rhino-Laryngol. 2003, 261, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Zizzi, A.; Ferrante, L.; Stramazzotti, D.; Goteri, G.; Gioacchini, F.M.; Olivieri, F.; Magliulo, G.; Rubini, C. P63 and Ki-67 Immunostainings in Laryngeal Squamous Cell Carcinoma Are Related to Survival. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. In The Enzymes; Academic Press: Cambridge, MA, USA, 2016; Volume 39, pp. 231–254. [Google Scholar]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The Role of Osteopontin in the Progression of Solid Organ Tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Bradford, C.R.; Kumar, B.; Bellile, E.; Lee, J.; Taylor, J.; D’Silva, N.; Cordell, K.; Kleer, C.; Kupfer, R.; Kumar, P.; et al. Biomarkers in Advanced Larynx Cancer. Laryngoscope 2014, 124, 179–187. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Teknos, T.N.; Cox, C.; Yoo, S.; Chepeha, D.B.; Wolf, G.T.; Bradford, C.R.; Carey, T.E.; Fisher, S.G. Elevated Serum Vascular Endothelial Growth Factor and Decreased Survival in Advanced Laryngeal Carcinoma. Head Neck 2002, 24, 1004–1011. [Google Scholar] [CrossRef]
- Parikh, R.R.; Yang, Q.; Haffty, B.G. Prognostic Significance of Vascular Endothelial Growth Factor Protein Levels in T1-2 N0 Laryngeal Cancer Treated with Primary Radiation Therapy. Cancer 2007, 109, 566–573. [Google Scholar] [CrossRef]
- Neuchrist, C.; Quint, C.; Pammer, A.; Burian, M. Vascular Endothelial Growth Factor (VEGF) and Microvessel Density in Squamous Cell Carcinomas of the Larynx: An Immunohistochemical Study. Acta Otolaryngol. 1999, 119, 732–738. [Google Scholar] [CrossRef]
- Cossu, A.M.; Mosca, L.; Zappavigna, S.; Misso, G.; Bocchetti, M.; De Micco, F.; Quagliuolo, L.; Porcelli, M.; Caraglia, M.; Boccellino, M. Long Non-Coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. Int. J. Mol. Sci. 2019, 20, 3444. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.M.; Johnson, A.M. Exploring the Mechanisms behind Long Noncoding RNAs and Cancer. Non-Coding RNA Res. 2018, 3, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, K.K.; Zhang, J.; Xiao, B.; Huang, Z.; Ju, C.; Sun, J.; Zhang, F.; Lv, X.-B.; Huang, G. The Decade of Exosomal Long RNA Species: An Emerging Cancer Antagonist. Mol. Cancer 2018, 17, 75. [Google Scholar] [CrossRef]
- Zvrko, E.; Mikic, A.; Vuckovic, L. CD105 Expression as a Measure of Microvessel Density in Supraglottic Laryngeal Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1971–1976. [Google Scholar] [CrossRef]
- Zvrko, E.; Mikic, A.; Vuckovic, L. Clinicopathologic Significance of CD105-Assessed Microvessel Density in Glottic Laryngeal Squamous Cell Carcinoma. Auris Nasus Larynx 2010, 37, 77–83. [Google Scholar] [CrossRef]
- Marioni, G.; Blandamura, S.; Loreggian, L.; Koussis, H.; Lionello, M.; Giacomelli, L.; Fasanaro, E.; Lovato, A.; Staffieri, A. Laryngeal Carcinoma Prognosis after Postoperative Radiotherapy Correlates with CD105 Expression, but Not with Angiogenin or EGFR Expression. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1779–1787. [Google Scholar] [CrossRef]
- Villaronga, M.Á.; Hermida-Prado, F.; Granda-Díaz, R.; Menéndez, S.T.; Álvarez-Teijeiro, S.; Quer, M.; Vilaseca, I.; Allonca, E.; Garzón-Arango, M.; Sanz-Moreno, V.; et al. Immunohistochemical Expression of Cortactin and Focal Adhesion Kinase Predicts Recurrence Risk and Laryngeal Cancer Risk Beyond Histologic Grading. Cancer Epidemiol. Biomark. Prev. 2018, 27, 805–813. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Álvarez-Alija, G.; Menéndez, S.T.; Mancebo, G.; Allonca, E.; García-Carracedo, D.; Fresno, M.F.; Suárez, C.; García-Pedrero, J.M. Cortactin and Focal Adhesion Kinase as Predictors of Cancer Risk in Patients with Laryngeal Premalignancy. Cancer Prev. Res. 2011, 4, 1333–1341. [Google Scholar] [CrossRef]
- Marioni, G.; Lionello, M.; Marchese-Ragona, R.; Fasanaro, E.; Valentini, E.; Zanoletti, E.; Stritoni, P.; Ramacciotti, G.; Guzzardo, V.; Giacomelli, L.; et al. Cortactin and Phosphorylated Cortactin Tyr421 and Tyr466 Expression in Supraglottic Laryngeal Carcinomas and Lymph Node Metastases. Int. J. Biol. Markers 2017, 33, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gibcus, J.H.; Mastik, M.F.; Menkema, L.; de Bock, G.H.; Kluin, P.M.; Schuuring, E.; van der Wal, J.E. Cortactin Expression Predicts Poor Survival in Laryngeal Carcinoma. Br. J. Cancer 2008, 98, 950–955. [Google Scholar] [CrossRef]
- Fei, M.; Zhang, J.; Zhou, J.; Xu, Y.; Wang, J. Sex-Related Hormone Receptor in Laryngeal Squamous Cell Carcinoma: Correlation with Androgen Estrogen-α and Prolactin Receptor Expression and Influence of Prognosis. Acta Oto-Laryngol. 2017, 138, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kalyi, V.V.; Goncharuk-Khomyn, M.Y. Analysis of the Role and Impact of Sex Hormones in Diagnostics and Assessment of Clinical Progress of Laryngeal Cancer. Morphologia 2016, 10, 41–45. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, Evolution, and Mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Kretz, M. The More the Merrier-Complexity in Long Non-Coding RNA Loci. Front. Endocrinol. 2017, 8, 90. [Google Scholar] [CrossRef]
- Ahmed, W.A.; Suzuki, K.; Imaeda, Y.; Horibe, Y. Ki-67, P53 and Epidermal Growth Factor Receptor Expression in Early Glottic Cancer Involving the Anterior Commissure Treated with Radiotherapy. Auris Nasus Larynx 2008, 35, 213–219. [Google Scholar] [CrossRef]
- Aras, S.; Ozkanli, S.; Erdem, E.; Gokalp, S.; Erdogan, C.E. Investigation of Low and High Dose Rate X-Ray Effects on Histopathological Changes and Prognostic Importance of Ki-67 in Laryngeal Cancer Radiotherapy. Appl. Radiat. Isot. 2023, 197, 110823. [Google Scholar] [CrossRef]
- Sakata, K.; Oouchi, A.; Nagakura, H.; Akiba, H.; Tamakawa, M.; Koito, K.; Hareyama, M.; Asakura, K.; Satoh, M.; Ohtani, S. Accelerated Radiotherapy for T1, 2 Glottic Carcinoma: Analysis of Results with KI-67 Index. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 81–88. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hanjing, S.; Zhang, B.-H.; Luo, X.-Y. Expression of P16 and Ki67 in Laryngeal Squamous Cell Carcinoma and Their Clinical Significance. Front. Oncol. 2024, 14, 1430830. [Google Scholar] [CrossRef]
- DE Almeida, M.R.; Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Somoza-Martín, J.M.; García-García, A. P27(Kip1) Expression as a Prognostic Marker for Squamous Cell Carcinoma of the Head and Neck. Oncol. Lett. 2015, 10, 2675–2682. [Google Scholar] [CrossRef]
- Allegra, E.; Bianco, M.R.; Mignogna, C.; Caltabiano, R.; Grasso, M.; Puzzo, L. Role of P16 Expression in the Prognosis of Patients with Laryngeal Cancer: A Single Retrospective Analysis. Cancer Control 2021, 28, 10732748211033544. [Google Scholar] [CrossRef]
- Lee, L.-A.; Fang, T.-J.; Li, H.-Y.; Chuang, H.-H.; Kang, C.-J.; Chang, K.-P.; Liao, C.-T.; Chen, T.-C.; Huang, C.-G.; Yen, T.-C. Effects of Epstein-Barr Virus Infection on the Risk and Prognosis of Primary Laryngeal Squamous Cell Carcinoma: A Hospital-Based Case-Control Study in Taiwan. Cancers 2021, 13, 1741. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.V.e.; Caponio, V.C.A.; Camolesi, G.C.V.; Padín-Iruegas, M.E.; Lorenzo-Pouso, A.I.; Lima, K.C.; Vieira, S.L.S.; Chamorro-Petronacci, C.M.; Suaréz-Peñaranda, J.M.; Pérez-Sayáns, M. Correlation of Bcl-2 Expression with Prognosis and Survival in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol./Hematol. 2023, 187, 104021. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.K.; Zhen, G.; Agrawal, N. The Role of Tumor DNA as a Diagnostic Tool for Head and Neck Squamous Cell Carcinoma. Semin. Cancer Biol. 2019, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, A.; Mathe, E.; Kato, S.; Ishioka, C.; Tavtigian, S.V.; Hainaut, P.; Olivier, M. Impact of Mutant P53 Functional Properties on TP53 Mutation Patterns and Tumor Phenotype: Lessons from Recent Developments in the IARC TP53 Database. Human. Mutat. 2007, 28, 622–629. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The Molecular Biology of Head and Neck Cancer. Nat. Rev. Cancer 2010, 11, 9–22. [Google Scholar] [CrossRef]
- Poeta, M.L.; Manola, J.; Goldwasser, M.A.; Forastiere, A.; Benoit, N.; Califano, J.A.; Ridge, J.A.; Goodwin, J.; Kenady, D.; Saunders, J.; et al. TP53 Mutations and Survival in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2007, 357, 2552–2561. [Google Scholar] [CrossRef]
- Ioachim, E. Expression Patterns of Cyclins D1, E and Cyclin-Dependent Kinase Inhibitors P21waf1/Cip1, P27kip1 in Colorectal Carcinoma: Correlation with Other Cell Cycle Regulators (pRb, P53 and Ki-67 and PCNA) and Clinicopathological Features. Int. J. Clin. Pract. 2008, 62, 1736–1743. [Google Scholar] [CrossRef]
- Basyuni, S.; Nugent, G.; Ferro, A.; Barker, E.; Reddin, I.; Jones, O.; Lechner, M.; O’Leary, B.; Jones, T.; Masterson, L.; et al. Value of P53 Sequencing in the Prognostication of Head and Neck Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 20776. [Google Scholar] [CrossRef]
- Tan, A.; Eskiizmir, G.; Kamiloglu, U.; Sarioglu, S. P53 and PTEN Expression Evaluation with Molecular Evident Recent Criteria in Laryngeal Carcinoma. Medicine 2023, 102, e33676. [Google Scholar] [CrossRef]
- Chong, C.R.; Jänne, P.A. The Quest to Overcome Resistance to EGFR-Targeted Therapies in Cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef]
- Lionello, M.; Staffieri, A.; Marioni, G. Potential Prognostic and Therapeutic Role for Angiogenesis Markers in Laryngeal Carcinoma. Acta Oto-Laryngol. 2012, 132, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kontić, M.; Čolović, Z.; Paladin, I.; Gabelica, M.; Barić, A.; Pešutić-Pisac, V. Association between EGFR Expression and Clinical Outcome of Laryngeal HPV Squamous Cell Carcinoma. Acta Oto-Laryngol. 2019, 139, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Marijić, B.; Braut, T.; Babarović, E.; Krstulja, M.; Maržić, D.; Avirović, M.; Kujundžić, M.; Hadžisejdić, I. Nuclear EGFR Expression Is Associated with Poor Survival in Laryngeal Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.; Strzalka, B.; Kowalczyk, M.; Jurzak, M.; Mazurek, U.; Gierek, T.; Paluch, J.; Markowski, J.; Swiatkowska, L.; Weglarz, L. Transforming Growth Factor Beta Isoforms (TGF-Beta1, TGF-Beta2, TGF-Beta3) Messenger RNA Expression in Laryngeal Cancer. Am. J. Otolaryngol. 2008, 29, 233–237. [Google Scholar] [CrossRef]
- Mosch, B.; Reissenweber, B.; Neuber, C.; Pietzsch, J. Eph Receptors and Ephrin Ligands: Important Players in Angiogenesis and Tumor Angiogenesis. J. Oncol. 2010, 2010, 135285. [Google Scholar] [CrossRef]
- Shigyo, H.; Nonaka, S.; Katada, A.; Bandoh, N.; Ogino, T.; Katayama, A.; Takahara, M.; Hayashi, T.; Harabuchi, Y. Inducible Nitric Oxide Synthase Expression in Various Laryngeal Lesions in Relation to Carcinogenesis, Angiogenesis, and Patients’ Prognosis. Acta Oto-Laryngol. 2007, 127, 970–979. [Google Scholar] [CrossRef]
- Rueda, A.; Cazorla, O.; Pérez, L.; Álvarez, M.; Redondo, M.; Gallego, E.; Sáez, M.; Medina, J.A.; Solano, J.; Matilla, A. Vascular Endothelial Growth Factor and Vascular Endothelial Growth Factor Receptor-2 Tumor Expression in Patients with Advanced Laryngeal Cancer after Induction Chemotherapy for Organ Preservation. Head Neck 2010, 33, 808–816. [Google Scholar] [CrossRef]
- Bonhin, R.G.; Rocha, V.B.C.; Carvalho, G.M.d.; Guimarães, A.C.; Crespo, A.N.; Chone, C.T.; Amstalden, E.M.I. Correlation between Vascular Endothelial Growth Factor Expression and Presence of Lymph Node Metastasis in Advanced Squamous Cell Carcinoma of the Larynx. Braz. J. Otorhinolaryngol. 2015, 81, 58–62. [Google Scholar] [CrossRef]
- Yoshioka, N.; Wang, L.; Kishimoto, K.; Tsuji, T.; Hu, G. A Therapeutic Target for Prostate Cancer Based on Angiogenin-Stimulated Angiogenesis and Cancer Cell Proliferation. Proc. Natl. Acad. Sci. USA 2006, 103, 14519–14524. [Google Scholar] [CrossRef]
- Marioni, G.; Marino, F.; Blandamura, S.; D’Alessandro, E.; Giacomelli, L.; Guzzardo, V.; Lionello, M.; De Filippis, C.; Staffieri, A. Neoangiogenesis in Laryngeal Carcinoma: Angiogenin and CD105 Expression Is Related to Carcinoma Recurrence Rate and Disease-free Survival. Histopathology 2010, 57, 535–543. [Google Scholar] [CrossRef]
- Carico, E.; Radici, M.; Bucci, B.; Firrisi, L.; Fabiano, A.; Salerno, G.; Giovagnoli, M.R.; Vecchione, A. p16INK4/Ki-67 Dual-Staining Expression as a Prognostic Indicator in Laryngeal Cancer. J. Cancer Prev. Curr. Res. 2014, 1, 68–72. [Google Scholar] [CrossRef][Green Version]
- Verma, A.; Schwartz, N.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Estrogen Signaling and Estrogen Receptors as Prognostic Indicators in Laryngeal Cancer. Steroids 2019, 152, 108498. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Verma, A.; Muktipaty, C.; Bivens, C.; Schwartz, Z.; Boyan, B.D. Estradiol Receptor Profile and Estrogen Responsiveness in Laryngeal Cancer and Clinical Outcomes. Steroids 2019, 142, 34–42. [Google Scholar] [CrossRef]
- Ou, D.; Adam, J.; Garberis, I.; Blanchard, P.; Nguyen, F.; Levy, A.; Casiraghi, O.; Gorphe, P.; Breuskin, I.; Janot, F.; et al. Clinical Relevance of Tumor Infiltrating Lymphocytes, PD-L1 Expression and Correlation with HPV/P16 in Head and Neck Cancer Treated with Bio- or Chemo-Radiotherapy. Oncoimmunology 2017, 6, e1341030. [Google Scholar] [CrossRef]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Song, X.; Zeng, W.; Wang, S.; Chen, F.; Ding, H. The Prognostic Value of Systemic and Local Inflammation in Patients with Laryngeal Squamous Cell Carcinoma. OncoTargets Ther. 2016, 9, 7177–7185. [Google Scholar] [CrossRef]
- Tudor, F.; Marijić, B.; Babarović, E.; Hadžisejdić, I. Significance of PD-L1 and Tumor Microenvironment in Laryngeal Squamous Cell Cancer. Cancers 2024, 16, 2645. [Google Scholar] [CrossRef]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-Novo and Acquired Resistance to Immune Checkpoint Targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Ritprajak, P.; Azuma, M. Intrinsic and Extrinsic Control of Expression of the Immunoregulatory Molecule PD-L1 in Epithelial Cells and Squamous Cell Carcinoma. Oral. Oncol. 2015, 51, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; Fadous-Khalifé, M.C.; Tannous, R.; Fakhreddine, S.; Massoud, M.; Hadchity, J.; Aftimos, G.; Hadchity, E. Role of Krüppel-like Factor 4 and Heat Shock Protein 27 in Cancer of the Larynx. Mol. Clin. Oncol. 2017, 7, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Kaigorodova, E.V.; Zavyalova, M.V.; Bychkov, V.A.; Perelmuter, V.M.; Choynzonov, E.L. Functional State of the Hsp27 Chaperone as a Molecular Marker of an Unfavorable Course of Larynx Cancer. Cancer Biomark. 2016, 17, 145–153. [Google Scholar] [CrossRef]
- Lee, J.-H.; Sun, D.; Cho, K.-J.; Kim, M.-S.; Hong, M.-H.; Kim, I.-K.; Lee, J.-S.; Lee, J.-H. Overexpression of Human 27 kDa Heat Shock Protein in Laryngeal Cancer Cells Confers Chemoresistance Associated with Cell Growth Delay. J. Cancer Res. Clin. Oncol. 2006, 133, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; Conway de Macario, E.; et al. Quantitative Patterns of Hsps in Tubular Adenoma Compared with Normal and Tumor Tissues Reveal the Value of Hsp10 and Hsp60 in Early Diagnosis of Large Bowel Cancer. Cell Stress Chaperones 2016, 21, 927–933. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zhang, X.; Qiu, Y.; Huang, D.; Li, W.; Xiao, X.; Tian, Y. HSP70: A Promising Target for Laryngeal Carcinoma Radiaotherapy by Inhibiting Cleavage and Degradation of Nucleolin. J. Exp. Clin. Cancer Res. 2010, 29, 106. [Google Scholar] [CrossRef]
- Orasan, A.; Negru, M.-C.; Morgovan, A.I.; Fleser, R.C.; Sandu, D.; Sitaru, A.M.; Motofelea, A.-C.; Balica, N.C. Strategies to Mitigate Cisplatin-Induced Ototoxicity: A Literature Review of Protective Agents, Mechanisms, and Clinical Gaps. Audiol. Res. 2025, 15, 22. [Google Scholar] [CrossRef]
- Song, X.; Liao, Z.; Zhou, C.; Lin, R.; Lu, J.; Cai, L.; Tan, X.; Zeng, W.; Lu, X.; Zheng, W.; et al. HSP47 Is Associated with the Prognosis of Laryngeal Squamous Cell Carcinoma by Inhibiting Cell Viability and Invasion and Promoting Apoptosis. Oncol. Rep. 2017, 38, 2444–2452. [Google Scholar] [CrossRef][Green Version]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Ioachim, E.; Assimakopoulos, D.; Peschos, D.; Zissi, A.; Skevas, A.; Agnantis, N.J. Immunohistochemical Expression of Metallothionein in Benign Premalignant and Malignant Epithelium of the Larynx: Correlation with P53 and Proliferative Cell Nuclear Antigen. Pathol.—Res. Pract. 1999, 195, 809–814. [Google Scholar] [CrossRef]
- Nowinska, K.; Chmielewska, M.; Piotrowska, A.; Pula, B.; Pastuszewski, W.; Krecicki, T.; Podhorska-Okołow, M.; Zabel, M.; Dziegiel, P. Correlation between Levels of Expression of Minichromosome Maintenance Proteins, Ki-67 Proliferation Antigen and Metallothionein I/II in Laryngeal Squamous Cell Cancer. Int. J. Oncol. 2015, 48, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Krześlak, A.; Forma, E.; Olszewski, J.; Lewy-Trenda, I.; Osuch-Wójcikiewicz, E.; Bryś, M. Genetic Polymorphism of Metallothionein 2A and Risk of Laryngeal Cancer in a Polish Population. Med. Oncol. 2014, 31, 75. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; Celesia, A.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Giuliano, M. The Good and Bad of Nrf2: An Update in Cancer and New Perspectives in COVID-19. Int. J. Mol. Sci. 2021, 22, 7963. [Google Scholar] [CrossRef]
- Namani, A.; Matiur Rahaman, M.; Chen, M.; Tang, X. Gene-Expression Signature Regulated by the KEAP1-NRF2-CUL3 Axis Is Associated with a Poor Prognosis in Head and Neck Squamous Cell Cancer. BMC Cancer 2018, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, X.-R.; Ma, Y.-L.; Zhao, Z.-J.; Zhao, R.; Wang, Y.-Y. Nrf2 Promotes Esophageal Squamous Cell Carcinoma (ESCC) Resistance to Radiotherapy through the CaMKIIα-Associated Activation of Autophagy. Cell Biosci. 2020, 10, 90. [Google Scholar] [CrossRef]
- Sheth, S.; Farquhar, D.R.; Schrank, T.P.; Stepp, W.; Mazul, A.; Hayward, M.; Lenze, N.; Little, P.; Jo, H.; Major, M.B.; et al. Correlation of Alterations in the KEAP1/CUL3/NFE2L2 Pathway with Radiation Failure in Larynx Squamous Cell Carcinoma. Laryngoscope Investig. Otolaryngol. 2021, 6, 699–707. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Wang, S.; Zhu, J. Expression and Correlation of NRF2, KEAP1, NQO-1 and HO-1 in Advanced Squamous Cell Carcinoma of the Larynx and Their Association with Clinicopathologic Features. Mol. Med. Rep. 2016, 14, 5171–5179. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Bao, X.; Shi, J.; Liu, B.; Chen, Y.; Li, J. Nuclear Nrf2 Activity in Laryngeal Carcinoma Is Regulated by SENP3 After Cisplatin-Induced Reactive Oxygen Species Stress. J. Cancer 2019, 10, 3427–3434. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-Inflammatory Agents: An Update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Wei, Y.; Xu, H. Prognostic and Clinical Significance of Cyclooxygenase-2 Overexpression in Endometrial Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 1202. [Google Scholar] [CrossRef]
- Kourelis, K.; Vandoros, G.; Kourelis, T.; Papadas, T.; Goumas, P.; Sotiropoulou-Bonikou, G. Low COX2 in Tumor and Upregulation in Stroma Mark Laryngeal Squamous Cell Carcinoma Progression. Laryngoscope 2009, 119, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Luo, R.-Z.; Li, Y.; Cui, B.-K.; Song, M.; Yang, A.-K.; Chen, W.-K. High Expression Levels of COX-2 and P300 Are Associated with Unfavorable Survival in Laryngeal Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2013, 270, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Feng, J.; Luo, D.; Peng, L. Prognostic and Clinical Significance of COX-2 Overexpression in Laryngeal Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 854946. [Google Scholar] [CrossRef] [PubMed]
- Sackett, M.K.; Bairati, I.; Meyer, F.; Jobin, E.; Lussier, S.; Fortin, A.; Gélinas, M.; Nabid, A.; Brochet, F.; Têtu, B. Prognostic Significance of Cyclooxygenase-2 Overexpression in Glottic Cancer. Clin. Cancer Res. 2008, 14, 67–73. [Google Scholar] [CrossRef]
- Klatka, J.; Grywalska, E.; Hymos, A.; Guz, M.; Polberg, K.; Roliński, J.; Stepulak, A. Cyclooxygenase-2 Inhibition Enhances Proliferation of NKT Cells Derived from Patients with Laryngeal Cancer. Anticancer Res. 2017, 37, 4059–4066. [Google Scholar] [CrossRef][Green Version]
- Kinoshita, T.; Hanazawa, T.; Nohata, N.; Kikkawa, N.; Enokida, H.; Yoshino, H.; Yamasaki, T.; Hidaka, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumor Suppressive microRNA-218 Inhibits Cancer Cell Migration and Invasion through Targeting Laminin-332 in Head and Neck Squamous Cell Carcinoma. Oncotarget 2012, 3, 1386–1400. [Google Scholar] [CrossRef]
- Bruzgielewicz, A.; Osuch-Wojcikiewicz, E.; Niemczyk, K.; Sieniawska-Buccella, O.; Siwak, M.; Walczak, A.; Nowak, A.; Majsterek, I. Altered Expression of miRNAs Is Related to Larynx Cancer TNM Stage and Patients’ Smoking Status. DNA Cell Biol. 2017, 36, 581–588. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.-P.; Yang, D.; Zhang, G.; Zhou, H.-F. Clinical Significance of miR-149 in the Survival of Patients with Laryngeal Squamous Cell Carcinoma. BioMed Res. Int. 2016, 2016, 8561251. [Google Scholar] [CrossRef]
- Karatas, O.F. Antiproliferative Potential of miR-33a in Laryngeal Cancer Hep-2 Cells via Targeting PIM1. Head Neck 2018, 40, 2455–2461. [Google Scholar] [CrossRef]
- Chen, H.; Cai, X.; Du, B.; Cai, J.; Luo, Z. MicroRNA-150-5p Inhibits the Proliferation and Invasion of Human Larynx Epidermiod Cancer Cells Though Regulating Peptidyl-Prolyl Cis/Trans Isomerase. Braz. J. Otorhinolaryngol. 2023, 89, 383–392. [Google Scholar] [CrossRef]
- Luo, M.; Sun, G.; Sun, J. MiR-196b Affects the Progression and Prognosis of Human LSCC through Targeting PCDH-17. Auris Nasus Larynx 2019, 46, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, H. MicroRNA-143-3p Suppresses Cell Growth and Invasion in Laryngeal Squamous Cell Carcinoma via Targeting the k-Ras/Raf/MEK/ERK Signaling Pathway. Int. J. Oncol. 2018, 54, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.; de Carvalho, A.C.; Horst, M.A.; Carvalho, A.L.; Scapulatempo-Neto, C.; Vettore, A.L. Expression of miR-296-5p as Predictive Marker for Radiotherapy Resistance in Early-Stage Laryngeal Carcinoma. J. Transl. Med. 2015, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Oztürkcan, S.; Katilmiş, H.; Ozdemir, I.; Tuna, B.; Güvenç, I.A.; Dündar, R. Occult Contralateral Nodal Metastases in Supraglottic Laryngeal Cancer Crossing the Midline. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 117–120. [Google Scholar] [CrossRef]
- Koroulakis, A.; Agarwal, M. Laryngeal Cancer. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Sharbel, D.D.; Abkemeier, M.; Groves, M.W.; Albergotti, W.G.; Byrd, J.K.; Reyes-Gelves, C. Occult Metastasis in Laryngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Ann. Otol. Rhinol. Laryngol. 2021, 130, 67–77. [Google Scholar] [CrossRef]
- Khan, U.; MacKay, C.; Rigby, M.; Trites, J.; Corsten, M.; Taylor, S.M. Management of Positive Resection Margins Following Transoral Laser Microsurgery for Glottic Cancer. Laryngoscope Investig. Otolaryngol. 2023, 8, 1579–1583. [Google Scholar] [CrossRef]
- Kjems, J.; Zukauskaite, R.; Johansen, J.; Eriksen, J.G.; Lassen, P.; Andersen, E.; Andersen, M.; Farhadi, M.; Overgaard, J.; Vogelius, I.R. Distant Metastases in Squamous Cell Carcinoma of the Pharynx and Larynx: A Population-Based DAHANCA Study. Acta Oncol. 2021, 60, 1472–1480. [Google Scholar] [CrossRef]
- Van den Bovenkamp, K.; van der Vegt, B.; Halmos, G.B.; Slagter-Menkema, L.; Langendijk, J.A.; van Dijk, B.A.C.; Schuuring, E.; van der Laan, B.F.A.M. The Relation between Hypoxia and Proliferation Biomarkers with Radiosensitivity in Locally Advanced Laryngeal Cancer. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 3801–3809. [Google Scholar] [CrossRef]
- Jotic, A.; Milovanovic, J.; Savic-Vujovic, K.; Radin, Z.; Medic, B.; Folic, M.; Pavlovic, B.; Vujovic, A.; Dundjerovic, D. Immune Cell and Biochemical Biomarkers in Advanced Laryngeal Cancer. Dose Response 2022, 20, 15593258221115537. [Google Scholar] [CrossRef]
- Koca, T.; Cetmi, D.A.; Aksoy, R.; Korcum, A.F. The Predictive Role of Inflammatory Biomarkers in Patients with Larynx Cancer Undergoing Definitive Radiotherapy. Technol. Cancer Res. Treat. 2024, 23, 15330338241280433. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients with Locally Advanced Larynx Cancer. JCO 2013, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Harris, J.; Garden, A.S.; Chao, C.K.S.; Straube, W.; Harari, P.M. Multi-Institutional Trial of Accelerated Hypofractionated Intensity-Modulated Radiation Therapy for Early-Stage Oropharyngeal Cancer (RTOG 00-22). Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus Cetuximab or Cisplatin in Human Papillomavirus-Positive Oropharyngeal Cancer (NRG Oncology RTOG 1016): A Randomised, Multicentre, Non-Inferiority Trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; Castro, G. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- NCI. FDA Approves Nivolumab for Head and Neck Cancer. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2016/fda-nivolumab-scchn (accessed on 4 May 2025).
- Yen, C.-J.; Kiyota, N.; Hanai, N.; Takahashi, S.; Yokota, T.; Iwae, S.; Shimizu, Y.; Hong, R.-L.; Goto, M.; Kang, J.-H.; et al. Two-Year Follow-up of a Randomized Phase III Clinical Trial of Nivolumab vs. the Investigator’s Choice of Therapy in the Asian Population for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (CheckMate 141). Head Neck 2020, 42, 2852–2862. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.H.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus Standard-of-Care Chemoradiotherapy versus Chemoradiotherapy Alone in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomised, Double-Blind, Placebo-Controlled, Multicentre, Phase 3 Trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, N.Y. JAVELIN Head and Neck 100: A Phase III Trial of Avelumab and Chemoradiation for Locally Advanced Head and Neck Cancer. Future Oncol. 2019, 15, 687–694. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Tao, Y.; Licitra, L.; Burtness, B.; Tahara, M.; Rischin, D.; Alves, G.; Lima, I.P.F.; Hughes, B.G.M.; Pointreau, Y.; et al. Pembrolizumab plus Concurrent Chemoradiotherapy versus Placebo plus Concurrent Chemoradiotherapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-412): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2024, 25, 572–587. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2023, 41, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Wildsmith, S.; Fayette, J.; Harrington, K.; Gillison, M.; Ahn, M.-J.; Takahashi, S.; Weiss, J.; Machiels, J.-P.; Baxi, S.; et al. Outcomes in Biomarker-Selected Subgroups from the KESTREL Study of Durvalumab and Tremelimumab in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Immunother. 2024, 73, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, A.; Cohen, M.A.; Sherman, E.J.; Lee, N.Y. Past, Present and Future of Proton Therapy for Head and Neck Cancer. Oral. Oncol. 2020, 110, 104879. [Google Scholar] [CrossRef]
- Kang, B.-H.; Yu, T.; Kim, J.H.; Park, J.M.; Kim, J.-I.; Chung, E.-J.; Kwon, S.K.; Kim, J.H.; Wu, H.G. Early Closure of a Phase 1 Clinical Trial for SABR in Early-Stage Glottic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 104–109. [Google Scholar] [CrossRef]
- Chotipanich, A. Total Laryngectomy: A Review of Surgical Techniques. Cureus 2021, 13, e18181. [Google Scholar] [CrossRef]
- Campbell, G.; Glazer, T.A.; Kimple, R.J.; Bruce, J.Y. Advances in Organ Preservation for Laryngeal Cancer. Curr. Treat. Options Oncol. 2022, 23, 594–608. [Google Scholar] [CrossRef]
- Remacle, M.; Van Haverbeke, C.; Eckel, H.; Bradley, P.; Chevalier, D.; Djukic, V.; de Vicentiis, M.; Friedrich, G.; Olofsson, J.; Peretti, G.; et al. Proposal for Revision of the European Laryngological Society Classification of Endoscopic Cordectomies. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 709. [Google Scholar] [CrossRef]
- Canis, M.; Ihler, F.; Martin, A.; Wolff, H.A.; Matthias, C.; Steiner, W. Organ Preservation in T4a Laryngeal Cancer: Is Transoral Laser Microsurgery an Option? Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2719–2727. [Google Scholar] [CrossRef]
- Peretti, G.; Piazza, C.; Mora, F.; Garofolo, S.; Guastini, L. Reasonable Limits for Transoral Laser Microsurgery in Laryngeal Cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 135–139. [Google Scholar] [CrossRef]
- Day, A.T.; Sinha, P.; Nussenbaum, B.; Kallogjeri, D.; Haughey, B.H. Management of Primary T1–T4 Glottic Squamous Cell Carcinoma by Transoral Laser Microsurgery. Laryngoscope 2016, 127, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.G.; Ihler, F.; Wolff, H.A.; Schneider, S.; Canis, M.; Steiner, W.; Welz, C. Transoral Laser Microsurgery for Treatment for Hypopharyngeal Cancer in 211 Patients. Head Neck 2017, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Martin, A.; Ihler, F.; Wolff, H.A.; Kron, M.; Matthias, C.; Steiner, W. Results of Transoral Laser Microsurgery for Supraglottic Carcinoma in 277 Patients. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Cornu, N.; Hans, S.; Sadoughi, B.; Badoual, C.; Brasnu, D. Early Glottic Cancer Involving the Anterior Commissure Treated by Transoral Laser Cordectomy. Laryngoscope 2015, 126, 1817–1822. [Google Scholar] [CrossRef]
- Breda, E.; Catarino, R.; Monteiro, E. Transoral Laser Microsurgery for Laryngeal Carcinoma: Survival Analysis in a Hospital-based Population. Head Neck 2014, 37, 1181–1186. [Google Scholar] [CrossRef]
- Vilaseca, I.; Aviles-Jurado, F.X.; Valduvieco, I.; Berenguer, J.; Grau, J.J.; Baste, N.; Muxí, Á.; Castillo, P.; Lehrer, E.; Jordana, M. Transoral Laser Microsurgery in Locally Advanced Laryngeal Cancer: Prognostic Impact of Anterior versus Posterior Compartments. Head Neck 2021, 43, 3832–3842. [Google Scholar] [CrossRef]
- Hans, S.; Chekkoury-Idrissi, Y.; Circiu, M.P.; Distinguin, L.; Crevier-Buchman, L.; Lechien, J.R. Surgical, Oncological, and Functional Outcomes of Transoral Robotic Supraglottic Laryngectomy. Laryngoscope 2020, 131, 1060–1065. [Google Scholar] [CrossRef]
- Lechien, J.R.; Fakhry, N.; Saussez, S.; Chiesa-Estomba, C.-M.; Chekkoury-Idrissi, Y.; Cammaroto, G.; Melkane, A.E.; Barillari, M.R.; Crevier-Buchman, L.; Ayad, T.; et al. Surgical, Clinical and Functional Outcomes of Transoral Robotic Surgery for Supraglottic Laryngeal Cancers: A Systematic Review. Oral. Oncol. 2020, 109, 104848. [Google Scholar] [CrossRef]
- Lechien, J.R.; Baudouin, R.; Circiu, M.P.; Chiesa-Estomba, C.M.; Crevier-Buchman, L.; Hans, S. Transoral Robotic Cordectomy for Glottic Carcinoma: A Rapid Review. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 5449–5456. [Google Scholar] [CrossRef]
- Remacle, M.; Prasad, V.M.N. Preliminary Experience in Transoral Laryngeal Surgery with a Flexible Robotic System for Benign Lesions of the Vocal Folds. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 761–765. [Google Scholar] [CrossRef]
- Hans, S.; Chebib, E.; Lisan, Q.; Chekkoury-Idrissi, Y.; Distinguin, L.; Circiu, M.P.; Crevier-Buchman, L.; Lechien, J.R. Oncological, Surgical and Functional Outcomes of Transoral Robotic Cordectomy for Early Glottic Carcinoma. J. Voice 2023, 37, 801.e3–801.e7. [Google Scholar] [CrossRef] [PubMed]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An Update on Larynx Cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Zhao, S.; Eskander, A.; Rygalski, C.; Brock, G.; Parikh, A.S.; Haring, C.T.; Swendseid, B.; Zhan, K.Y.; Bradford, C.R. Stage Migration and Survival Trends in Laryngeal Cancer. Ann. Surg. Oncol. 2021, 28, 7300–7309. [Google Scholar] [CrossRef]
- Patel, T.R.; Eggerstedt, M.; Toor, J.; Tajudeen, B.A.; Husain, I.; Stenson, K.; Al-Khudari, S. Occult Lymph Node Metastasis in Early-stage Glottic Cancer in the National Cancer Database. Laryngoscope 2021, 131, E1139–E1146. [Google Scholar] [CrossRef]
- Nahavandipour, A.; Jakobsen, K.K.; Grønhøj, C.; Hebbelstrup Jensen, D.; Kim Schmidt Karnov, K.; Klitmøller Agander, T.; Specht, L.; von Buchwald, C. Incidence and Survival of Laryngeal Cancer in Denmark: A Nation-Wide Study from 1980 to 2014. Acta Oncol. 2019, 58, 977–982. [Google Scholar] [CrossRef]
- Petrakos, I.; Kontzoglou, K.; Nikolopoulos, T.P.; Papadopoulos, O.; Kostakis, A. Glottic and Supraglottic Laryngeal Cancer: Epidemiology, Treatment Patterns and Survival in 164 Patients. J. Buon 2012, 17, 700–705. [Google Scholar]
- Yang, F.; He, L.; Rao, Y.; Feng, Y.; Wang, J. Survival Analysis of Patients with Subglottic Squamous Cell Carcinoma Based on the SEER Database. Braz. J. Otorhinolaryngol. 2023, 88, S70–S80. [Google Scholar] [CrossRef]
- MacNeil, S.D.; Patel, K.; Liu, K.; Shariff, S.; Yoo, J.; Nichols, A.; Fung, K.; Garg, A.X. Survival of Patients with Subglottic Squamous Cell Carcinoma. Curr. Oncol. 2018, 25, e569. [Google Scholar] [CrossRef]
- Juan, X.; Jiali, H.; Ziqi, L.; Liqing, Z.; Han, Z. Development and Validation of Nomogram Models for Predicting Postoperative Prognosis of Early-Stage Laryngeal Squamous Cell Carcinoma. Curr. Probl. Cancer 2024, 49, 101079. [Google Scholar] [CrossRef]
- Shi, X.; Hu, W.-P.; Ji, Q.-H. Development of Comprehensive Nomograms for Evaluating Overall and Cancer-Specific Survival of Laryngeal Squamous Cell Carcinoma Patients Treated with Neck Dissection. Oncotarget 2017, 8, 29722–29740. [Google Scholar] [CrossRef]
- Qasem, M.; Qasem, N.; Kinshuck, A.; Milinis, K. Long-Term Laryngeal Function and Quality of Life Following Treatment of Early Glottic Cancer: A Meta-Analysis. Otolaryngol. Head Neck Surg. 2025, 172, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.-J.; Hung, L.-T.; Wang, L.-W.; Yang, M.-H.; Chu, P.-Y. Oncologic Results and Quality of Life in Patients with T3 Glottic Cancer after Transoral Laser Microsurgery. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Wulff, N.B.; Højager, A.; Wessel, I.; Dalton, S.O.; Homøe, P. Health-related Quality of Life Following Total Laryngectomy: A Systematic Review. Laryngoscope 2021, 131, 820–831. [Google Scholar] [CrossRef] [PubMed]

| Variant | Gross Appearance | Microscopic Features & Keratinization | Immunohistochemistry/Special Studies | Prognosis |
|---|---|---|---|---|
| Conventional SCC | Variable appearance: may be ulcerated or smooth; endophytic, exophytic, or polypoid; colour from red to tan-white; firm texture. | Invasive nests of squamous cells with variable differentiation; abnormal keratinization (prominent in well-differentiated types); desmoplastic reaction; possible perineural/vascular invasion. | In poorly differentiated cases: CK5/6, p63, p40, and EMA positivity help confirm epithelial origin. | Stage-dependent survival rates (e.g., Glottic: 80–85%, Supraglottic: 65–75%, Subglottic: ~40%). |
| Verrucous Carcinoma | Warty, broad-based, and fungating mass; firm-to-hard texture; generally tan or white. | Well-demarcated pushing border with blunt, club-shaped rete ridges; minimal atypia and rare mitoses; abundant keratin forming “church-spire” structures. | Lacks evidence of transcriptionally active HPV. | Excellent outlook with 5-year survival rates of approximately 85–95% (stage is key). |
| Papillary/Exophytic SCC | Polypoid and bulky, projecting outward with papillary or fungiform features; texture may vary from soft to firm. | Dominantly exophytic/papillary growth pattern with malignant features; surface keratinization; possible koilocytic atypia. | Diagnosis is usually made on morphology; additional studies are not typically required. | Generally favorable outcome with roughly 85% 5-year survival, better than conventional SCC. |
| Spindle Cell (Sarcomatoid) SCC | Often presents as a polyp-like mass with areas of surface ulceration; firm, fibrous texture. | Biphasic pattern showing both conventional SCC and atypical spindle cell components; high cell density, pleomorphism, and increased mitoses; keratinization is often focal. | Approximately 70% of cases show AE1/AE3, EMA, p63, and/or p40 positivity; frequent p53 overexpression. | Generally excellent, sometimes even better than conventional SCC. |
| Basaloid SCC | Firm to hard mass, frequently with central necrosis. | Deep invasive lobules of basaloid cells with a high nuclear-to-cytoplasmic ratio; palisading at the periphery; abrupt squamous differentiation with focal keratinization; comedonecrosis is common; may show hyaline stroma. | Consistent epithelial marker expression (e.g., pan-cytokeratin, p63, p40) and p53 overexpression; typically negative for neuroendocrine markers. | Tends to have a worse overall prognosis compared to conventional SCC. |
| Adenosquamous Carcinoma | Typically presents as a submucosal, indurated mass. | Infiltrative growth with two distinct components: a conventional squamous carcinoma element and an adenocarcinoma (glandular) component; abrupt or focal keratinization (keratin pearls may be seen). | Glandular areas are positive for mucin, supporting a dual differentiation profile. | When matched for stage, outcomes are similar to conventional SCC, though the lesion may behave more aggressively. |
| Method | Description | Advantages | Limitations |
|---|---|---|---|
| Clinical Examination | Patient history, physical exam (neck palpation, etc.) | The essential first step is to identify symptoms and risk factors. | Cannot visualize the larynx directly; subjective. |
| Laryngoscopy | Indirect, flexible fiberoptic, video stroboscopy | Direct visualization of the larynx; videostroboscopy assesses vocal fold vibration. | It can be uncomfortable and may require local anaesthesia and inter-observer variability. |
| Biopsy | A tissue sample from the primary tumour or lymph nodes (fine-needle aspiration) | The gold standard for definitive diagnosis allows for histological analysis. | Invasive; potential for complications (bleeding, infection); sampling error. |
| Imaging | CT, MRI, PET/CT | CT: assesses bone involvement; MRI: superior for soft tissue and cartilage; PET/CT: detects recurrences and metastases. | CT: radiation exposure; MRI: cost, claustrophobia; PET/CT: cost, availability, radiation exposure. |
| Narrow-band Imaging (NBI) | Uses specific wavelengths of light to enhance visualization of mucosal changes | High sensitivity and specificity for identifying LC and precursor lesions. | It requires specialized equipment; it may not detect deep invasion. |
| Liquid Biopsy | Analysis of biomarkers in blood/saliva (CTCs, ctDNA, exosomes, microbiome) | Minimally invasive; potential for early detection, prognosis, and monitoring treatment response. | Biomarker validation is ongoing; standardization is needed; it may not replace tissue biopsy. |
| AI-Assisted Diagnosis | Machine learning/deep learning applied to imaging (histology, endoscopy) | Potential to improve diagnostic accuracy and efficiency; personalized risk assessment. | It requires large datasets for training, the “black box” nature of some algorithms, and ethical considerations. |
| Biomarker (Symbol) | Primary Function | LC-Specific Application | Clinical Impact |
|---|---|---|---|
| BCL2 | Suppresses apoptosis | IHC to identify tumors with high anti-apoptotic tone | Overexpression correlates with radio-/chemo-resistance and poorer OS and DFS [117,118,119,120,121] |
| CD44 | Regulates cell–cell/ECM interactions; CSC marker | CSC enrichment assays; marker for minimal residual disease | High CD44+ fraction associates with increased recurrence and metastasis [122,123,124] |
| E-cadherin (CDH1) | Mediates calcium-dependent cell–cell adhesion | IHC loss as indicator of EMT | Reduced expression predicts invasion, nodal spread, and worse prognosis [125,126,127,128] |
| p16 (CDKN2A) | Inhibits CDK4/6; surrogate of HPV-driven oncogenesis | p16 IHC to stratify HPV+ vs. HPV− tumors | p16 positivity correlates with better OS in HPV-associated LC [129] |
| p27 (CDKN1B) | Restrains cyclin-CDK activity | IHC gauge of intact growth-inhibitory signaling | Low p27 levels associate with aggressive phenotype and reduced DFS [130] |
| Cyclin D1 (CCND1) | Drives G1–S cell-cycle transition | FISH/IHC for gene amplification and overexpression | Overexpression predicts lymph node metastasis and shorter DFS [131] |
| EGFR | RTK regulating proliferation, survival, angiogenesis | IHC/FISH to select patients for EGFR-targeted therapies (cetuximab, TKIs) | High EGFR expression correlates with poor prognosis; targetable with cetuximab [132] |
| Ki-67 (MKI67) | Marks actively cycling cells | Ki-67 index quantification in biopsy specimens | Elevated Ki-67 (>30–40%) predicts high tumor grade and reduced survival [133,134,135,136] |
| PCNA | Cofactor for DNA polymerase δ during replication | IHC metric of S-phase fraction and proliferative activity | High PCNA levels associate with rapid growth and adverse outcome [137] |
| SPP1 (Osteopontin) | Adhesion molecule and cytokine | Serum/plasma ELISA and tissue IHC | Elevated SPP1 correlates with metastasis, chemoresistance, and shorter OS [138] |
| p53 (TP53) | Orchestrates DNA-damage response, apoptosis, senescence | Mutational analysis by NGS/IHC for risk stratification | TP53 mutation denotes higher recurrence risk and poor prognosis [131,139,140] |
| VEGFA | Stimulates angiogenesis and vascular permeability [141] | IHC/ELISA to assess angiogenic index | High VEGFA expression correlates with microvessel density and reduced OS [142,143,144] |
| TGF-βR | Mediates TGF-β growth-inhibitory signaling | IHC/genomic profiling for receptor or SMAD pathway defects | Loss correlates with invasion, EMT, and poor survival [145,146,147] |
| Endoglin (CD105) | Accessory TGF-β receptor controlling angiogenesis | IHC microvessel density marker | High CD105+ microvessel count predicts aggressive behavior and reduced OS [126,148,149,150] |
| FAK (PTK2) | Integrates integrin and growth-factor signals to regulate motility | IHC and activity assays; under investigation as therapeutic target | Overexpression linked to metastasis, diminished DFS, and poorer OS [151,152,153,154] |
| Hormone receptors (ER, PR, AR, PRLR) | Ligand-activated TFs mediating hormone-driven growth and differentiation | IHC detection to explore endocrine manipulation | AR+ tumors may be less aggressive; PRLR elevation correlates with poorer survival [155,156] |
| Treatment Modality | Description | Indications | Advantages | Disadvantages |
|---|---|---|---|---|
| Surgery | ||||
| Laryngeal Sparing Surgery | Selective removal of cancerous tissue. | Early-stage (T1, T2, N0, M0) | It preserves the larynx and potentially improves voice and swallowing function. | It may not be suitable for advanced disease due to the risk of recurrence. |
| Transoral Laser Microsurgery (TLM) | CO2 laser resection through the mouth. | Early-stage vocal fold cancers (Tis, T1, T2); selected advanced cases. | Minimally invasive; good oncological outcomes; better postoperative and QoL outcomes than open surgery or radiotherapy; cost-effective. | Limited by tumour extent and location; requires specialized equipment and expertise. |
| Transoral Robotic Surgery (TORS) | Robotic-assisted surgery through the mouth. | Selected supraglottic cancers (cT1,T2, some cT3,T4a); oropharyngeal cancers. | Minimally invasive; improved visualization and manoeuvrability. | Role in glottic cancer less defined; potential for exposure issues, higher costs, and complications compared to TLM. |
| Total Laryngectomy (TL) | Complete removal of the larynx. | Extensive tumour invasion; significant pre-existing swallowing dysfunction; failed organ preservation. | Definitive tumour removal. | Permanent loss of natural voice requires tracheostomy, which has a significant impact on QoL. |
| Radiation Therapy (RT) | ||||
| Definitive RT | High-energy beams to eradicate cancer cells. | Early-stage disease; alternative to surgery. | Preserves larynx. | Potential for side effects (mucositis, xerostomia, dysphagia); risk of recurrence. |
| Intensity-Modulated RT (IMRT) | Conformal radiation delivery, minimizing dose to surrounding tissues. | Locally advanced disease (often combined with chemotherapy). | Reduced toxicities compared to conventional RT. | It requires specialized equipment and expertise; there is potential for late effects. |
| Proton Therapy | Radiation using protons, with the potential for reduced dose to surrounding tissues. | Investigational; potential for locally advanced disease. | It may reduce side effects compared to IMRT. | Limited availability, cost, and lack of robust comparative data. |
| Stereotactic Body RT (SBRT) | High doses of radiation in a few fractions. | Investigational; potential for early-stage glottic cancers (clinical trial only). | Short treatment course. | Not established for laryngeal preservation; potential for severe toxicities. |
| Chemotherapy | ||||
| Concurrent Chemoradiotherapy (CRT) | Cisplatin chemotherapy combined with radiation therapy. | Locally advanced disease (standard of care for larynx preservation). | Superior larynx preservation rates compared to induction chemotherapy or radiation alone. | Significant toxicities (mucositis, nephrotoxicity, myelosuppression). |
| Alternative Systemic Therapies | Carboplatin/fluorouracil, cetuximab (for platinum-ineligible patients). | Patients not candidates for cisplatin. | It may be better tolerated than cisplatin. | Cetuximab may have inferior overall survival compared to cisplatin in certain cases. |
| Immunotherapy | Pembrolizumab, Nivolumab (checkpoint inhibitors) | Investigational; potential for locally advanced disease. approved for recurrent or metastatic disease. | It may reduce side effects compared to IMRT. | Limited availability; cost; lack of robust comparative data. |
| Tracheostomy | Creating a hole in the neck to breathe | In cases of significant tumor obstruction | Secures the airway in patients and allows patients to get CRT. | Patient will have to relearn to speak |
| Factor | Subcategory/Details | Impact on Survival |
|---|---|---|
| Stage (TNM) | I & II (localized) | Showed the best prognosis. 5-year survival: 78% [269]. |
| III (regional lymph node involvement) | Showed an intermediate prognosis. 5-year survival: 46% [269]. | |
| IV (distant metastases) | Showed the poorest prognosis. 5-year survival: 34% [269]. | |
| Laryngeal Subsite | Glottic | Generally best prognosis; overall 5-year survival: 77%; localized: 84%; nodal involvement: 52%; distant spread: 45% [270]. |
| Supraglottic | Overall 5-year survival: 45%; localized: 61%; stage III: 46%; metastatic: 30% [270]. | |
| Subglottic | Worst prognosis; overall 5-year survival: 49%; stages I & II: 59%; stage III: 38%; metastatic: 44% [270]. | |
| Lymph Node Status | N0 (no nodal involvement) | Better prognosis. |
| N+ (nodal involvement) | Significantly worse prognosis; higher risk of recurrence and distant metastasis. | |
| Extranodal Extension (ENE) | Significantly increases risk of distant metastasis (potentially tenfold). | |
| Histological Grade | Well-differentiated | Generally better prognosis. |
| Poorly differentiated | Generally worse prognosis; higher risk of recurrence and metastasis. | |
| Patient Factors | Age, general health, performance status, comorbidities, smoking | Poorer general health, older age, and continued smoking associated with worse outcomes. |
| Biomarkers | See Table 3 (multiple biomarkers with varying prognostic value) | Can provide additional prognostic information beyond TNM staging; potential for personalized risk assessment. |
| Prognostic Models | Nomograms incorporating clinical factors and biomarkers | Can improve prognostication in LSCC. |
| QoL | TLM, RT, and TL | Overall, voice-related QoL measures are not significantly different between TLM and RT, TLM patients demonstrated better outcomes in specific acoustic measures. Carefully selected T3 glottic SCC patients achieve satisfactory QoL and larynx preservation rates after CO2 TLM. Individuals who underwent TL reported worse HRQoL compared to a male normative reference population. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hut, A.-R.; Boia, E.R.; Para, D.; Iovanescu, G.; Horhat, D.; Mikša, L.; Chiriac, M.; Galant, R.; Motofelea, A.C.; Balica, N.C. Laryngeal Cancer in the Modern Era: Evolving Trends in Diagnosis, Treatment, and Survival Outcomes. J. Clin. Med. 2025, 14, 3367. https://doi.org/10.3390/jcm14103367
Hut A-R, Boia ER, Para D, Iovanescu G, Horhat D, Mikša L, Chiriac M, Galant R, Motofelea AC, Balica NC. Laryngeal Cancer in the Modern Era: Evolving Trends in Diagnosis, Treatment, and Survival Outcomes. Journal of Clinical Medicine. 2025; 14(10):3367. https://doi.org/10.3390/jcm14103367
Chicago/Turabian StyleHut, Alexandru-Romulus, Eugen Radu Boia, Diana Para, Gheorghe Iovanescu, Delia Horhat, Loredan Mikša, Maria Chiriac, Raphaël Galant, Alexandru Catalin Motofelea, and Nicolae Constantin Balica. 2025. "Laryngeal Cancer in the Modern Era: Evolving Trends in Diagnosis, Treatment, and Survival Outcomes" Journal of Clinical Medicine 14, no. 10: 3367. https://doi.org/10.3390/jcm14103367
APA StyleHut, A.-R., Boia, E. R., Para, D., Iovanescu, G., Horhat, D., Mikša, L., Chiriac, M., Galant, R., Motofelea, A. C., & Balica, N. C. (2025). Laryngeal Cancer in the Modern Era: Evolving Trends in Diagnosis, Treatment, and Survival Outcomes. Journal of Clinical Medicine, 14(10), 3367. https://doi.org/10.3390/jcm14103367







