Abstract
Background/Objectives: Laryngeal cancer (LC), predominantly squamous cell carcinoma (SCC), represents a considerable health burden worldwide. Tumour subsite heterogeneity (supraglottic, glottic, subglottic) influences clinical behavior and outcomes. This review synthesizes current knowledge on epidemiology, risk factors, diagnostics, histological variants, biomarkers, treatment modalities, and survival. Results: This narrative review synthesizes current literature on the epidemiology, risk factors, diagnosis, histological variants, biomarkers, and prognosis of LC. The review highlights the critical influence of tumour sites (supraglottic, glottic, subglottic) on metastatic patterns and survival. Key risk factors of LC include tobacco and alcohol use, human papillomavirus (HPV) infection, and occupational exposures. The diagnostic process encompasses clinical examination, endoscopy, biopsy, and imaging. Several biomarkers that aid in diagnosis, treatment plan determination, and prognosis prediction have been established. These biomarkers include long noncoding RNAs, cell cycle regulators, apoptosis regulators, oncogenes, tumour suppressor genes, growth factor pathway components, angiogenic factors, structural proteins, sex hormone receptors, and immunological markers. Current treatment modalities range from organ-preserving surgery and radiotherapy to combined chemoradiotherapy and total laryngectomy. Finally, survival data are presented and stratified by stage and subsite. Conclusions: The review underscores the need for a multidisciplinary approach to LC management, integrating clinical, pathological, and molecular information to optimize patient outcomes.
1. Introduction
Cancers of the head and neck collectively represent the seventh most common type of cancer worldwide, encompassing a diverse array of tumours that originate within the upper aerodigestive tract [1]. According to global cancer statistics for 2022, laryngeal cancer (LC) is among the most prevalent cancers, with 188,960 new cases and 103,216 deaths reported [2]. The vast majority of these—between 85% and 95%—are classified as squamous cell carcinomas (SCC) [3]. The epidemiology of LC reveals a substantial burden. In 2022, global incidence rates reached over 165,598 cases in men and over 23,362 in women, with associated deaths exceeding 90,256 and 12,960, respectively [2].
Geographically, Cuba (7.8 per 100,000) and Montenegro (7.0 per 100,000) report the highest incidence rates, while Eswatini (0.18 per 100,000) and Cameroon (0.31 per 100,000) have the lowest. Asia is the most prevalent continent for LC, followed by Europe, whilst the epidemiologic burden in Africa remains low [4]. Within Europe, a high-prevalence region, incidence varies considerably; Spain reports rates above 12 per 100,000, while the UK’s rates are below 5 per 100,000 [5]. In Romania, oral malignant tumors are prevalent, with approximately 2388 new cases annually [6].
Regarding LC mortality, Cuba (3.9 per 100,000) and Montenegro (3.5 per 100,000) also register high mortality rates, in contrast to Iceland (0.08 per 100,000) and Martinique (0.11 per 100,000), which have the lowest [4]. These variations are linked to socioeconomic factors, with higher incidence in areas with lower average incomes and education [7]. Regarding gender differences, in 2021, the age-standardized DALY rate of LC in males was approximately 7.13 times higher than that in females, with males experiencing 282.12 cases per 100,000 individuals, compared to 39.59 cases per 100,000 individuals in females [8]. Higher Human Development Index (HDI) regions tend to have higher incidence but lower mortality, reflecting disparities in healthcare access and quality, while lower Sociodemographic Index (SDI) regions experience higher mortality rates [9,10]. Key etiological factors include tobacco smoking [11,12], alcohol consumption [13,14,15], high-risk human papillomavirus (HPV) infection [16], and occupational exposures such as asbestos [17,18].
Characteristic clinical manifestations often prompt the diagnosis of LC. These symptoms, which can vary significantly depending on the tumour’s location and size, commonly include a persistent lump or non-healing sore, ongoing throat pain, difficulty swallowing (dysphagia), and alterations in voice quality, such as hoarseness [19]. Despite significant advancements in instruments like flexible laryngoscopes, surgical techniques, and chemoradiation therapy, the mortality rate remains high; the 5-year survival rate is 64% because approximately two-thirds of patients are diagnosed with advanced cancer, leading to a poor prognosis [20]. Furthermore, even with improvements in treatment modalities, the American Cancer Society has reported a tendency for the 5-year survival rate for laryngeal cancer patients to decline [21].
Treatment options for LC include surgery, radiation therapy, and chemotherapy, used alone or in combination [22,23]. Recent advancements in immunotherapy and targeted therapy have significantly impacted laryngeal cancer treatment, offering hope for patients with recurrent or advanced disease. These therapies improve progression-free and overall survival rates, particularly in combination. The integration of immunotherapy with targeted therapies like anti-EGFR has shown promising results in tumor response, and is increasingly considered for managing recurrent locoregionally advanced squamous cell carcinoma of the head and neck, including laryngeal cancer [24]. A complex interplay of factors determines a patient’s prognosis in LC, broadly categorized as relating to the host, the tumour itself, or the treatment strategy [25]. Host factors encompass individual characteristics such as age, sex, nutritional status, general health and physical condition, coexisting medical conditions’ presence, and the immune response’s robustness. Tumour-related factors include the primary location of cancer, its TNM stage (which reflects the size, involvement of lymph nodes, and presence of distant metastasis), the microscopic grade (degree of abnormality), and whether any other primary cancers are present concurrently [25]. While current staging systems predominantly focus on tumour-related characteristics, a complete and accurate assessment of prognosis requires consideration of host and tumour factors. In addition, correctly determining the treatment plan is essential for accurately estimating the prognosis [26].
This review explore recent advancements in molecular biomarkers, histopathological subtypes, and innovative therapeutic approaches particularly immuno- and targeted therapies to enhance prognostic stratification and inform treatment decisions in laryngeal cancer. Our objectives are to assess emerging biomarkers for personalized risk evaluation, delineate the clinical and molecular diversity of LC subtypes, and propose an integrated management framework that optimizes both survival and functional outcomes.
2. Materials and Methods
To identify relevant literature, a comprehensive search was conducted using electronic databases such as PubMed/MEDLINE, Scopus, and Web of Science. The search strategy combined both Medical Subject Headings (MeSH) and free-text terms related to “laryngeal cancer”, “laryngeal carcinoma”, and “squamous cell carcinoma”, along with additional terms addressing specific aspects of the review (for example, “epidemiology”, “risk factors”, “diagnosis”, “histological variants”, “biomarkers”, “treatment”, “survival”, and “quality of life”). Searches were performed to capture all publications available from the inception of each database until March 2025. In order to ensure a comprehensive review, reference lists from the identified articles, including relevant review articles and original studies, were also examined.
Articles were considered eligible if they addressed key aspects of laryngeal cancer, particularly those relating to squamous cell carcinoma—and if they included information on epidemiology, risk factors, diagnostic methods, treatment strategies, prognostic biomarkers, survival outcomes, or quality-of-life measures. Only studies published in peer-reviewed journals and written in English were included. The review focused on original research articles, systematic reviews, meta-analyses, and established clinical guidelines. Articles such as isolated case reports or very small case series, studies focused solely on other head and neck cancers without specific details regarding LC, or non-peer-reviewed materials, abstracts, or conference proceedings with limited methodological detail were excluded from this review.
Because this review takes a narrative approach, the extracted data were synthesized qualitatively rather than quantitatively. Major themes were identified and then organized into coherent subsections that correspond to the different facets of LC. This approach facilitated an integrated discussion of the complex interplay between epidemiological trends, clinical parameters, molecular insights, treatment options, and patient outcomes.
3. Results
3.1. Risk Factors of LC
A combination of lifestyle and environmental factors significantly influences LC development (Figure 1).
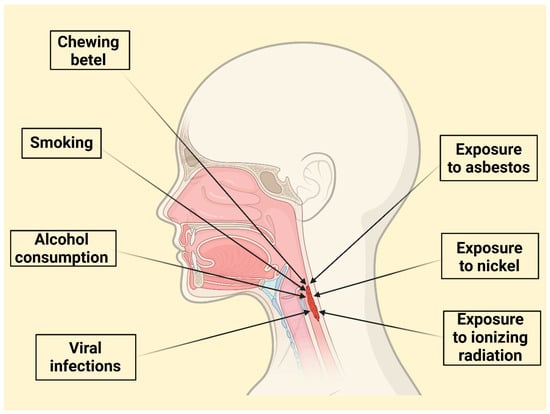
Figure 1.
Overview of Key Lifestyle, Viral, and Environmental Risk Factors in Laryngeal Cancer Development.
Undoubtedly, tobacco use is the most prominent risk factor [27]. The risk of LC increases substantially with both the duration and intensity of smoking, exhibiting a dose-response relationship. However, this relationship may not be perfectly linear, with a possible “saturation effect” at very high levels of consumption (more than 20 years and over 30 cigarettes per day) [11,12]. Smoking cessation reduces risk, but it remains elevated for up to 15 years after quitting [28]. The synergistic effect of tobacco’s carcinogens is amplified by human papillomavirus [16]. Cigarette smoking correlates with a sevenfold risk increase of LC [11]. Passive smoking also contributes to LC deaths [28]. Smokers have a higher likelihood of dying from LC [29]. Black smokers demonstrate a higher risk level than white smokers [30].
Excessive alcohol consumption is a significant independent risk factor for LC, exhibiting a clear dose–response relationship where both the amount and duration of intake proportionally elevate the risk [13,14,15]. Alcohol accounts for a substantial portion of laryngeal cancer-related mortality, especially in regions with high alcohol consumption levels [31]. In Europe, approximately 30% of individuals who died from LC were identified as alcoholics [31]. Moreover, the global contribution of alcohol to the incidence of LC may be on the rise [32].
While traditional risk factors like tobacco and alcohol remain significant, emerging concerns and socioeconomic disparities require further attention. Although initially marketed as a safer alternative, e-cigarettes have raised concerns due to the presence of potentially carcinogenic substances in some e-liquids [33]. A Korean study found formaldehyde and acetaldehyde in all 225 tested e-liquids [34]. Both of the compounds are classified as Group 1 carcinogens with links to head and neck cancers [35]. N′-nitrosonornicotine (NNN), a TSNA, has been shown to induce head and neck tumours in animal studies [36]. PAHs, like 1-hydroxypyrene (1-HOP) and benzopyrene, have demonstrated carcinogenic effects on the upper respiratory tract in animal models [36,37]. While in vivo studies on e-cigarettes and laryngeal mucosa are limited, one study in rats showed non-statistically significant hyperplasia and metaplasia after four weeks of exposure to e-cigarette aerosols [38].
Opium, classified as a human carcinogen by the IARC (International Agency for Research on Cancer), has shown preliminary links to increased head and neck squamous cell carcinoma (HNSCC) risk, including LC, although more research is needed to solidify this connection. The mechanism likely involves the carcinogenic alkaloids present in opium, which can induce DNA damage and mutations upon metabolic activation [39,40].
Beyond tobacco and alcohol, certain viral infections, notably high-risk strains of human papillomavirus (HPV), have been implicated in LC development [41].
Over the past few decades, HPV has been recognized as a key etiological agent, especially in oropharyngeal squamous cell carcinoma (OPSCC), where HPV-positive tumors form a distinct clinical and molecular subgroup compared with their HPV-negative counterparts [42]. However, the etiological role of HPV in LC appears to vary significantly by geographic region, with a lower prevalence in some areas, suggesting it is not always a primary cause [43,44,45]. Moreover, EBV has been suggested as a risk factor for LC. EBV’s genome and latent protein EBNA have been found in malignant laryngeal cells, suggesting a potential role as a risk factor or cofactor, though its presence in LSCC can be inconsistent [46]. EBV, like HPV, can produce oncoproteins that disrupt cell cycle control and promote uncontrolled cell growth, contributing to carcinogenesis [47].
Other identified risk factors include occupational exposure to asbestos [17,18] and possibly nickel or ionizing radiation [48]. Chewing betel, particularly in combination with tobacco and alcohol, substantially elevates risk, specifically in specific populations like Taiwan [49,50]. Helicobacter pylori infection has also been linked to an increased risk of LC [51], as has gastroesophageal reflux disease (GERD) and laryngopharyngeal reflux (LPR), likely due to chronic inflammation. Chronic inflammation leads to the release of reactive oxygen species (ROS) and inflammatory mediators, which can damage DNA, promote cell proliferation, and create a microenvironment conducive to tumour development [52,53,54]. Moreover, metabolic syndrome and its associated components (high blood glucose, increased waist circumference, elevated triglycerides, high blood pressure, and low HDL cholesterol) have also been identified as independent risk factors [55,56].
The oral and throat microbiome is emerging as a significant factor. Differences in bacterial composition, particularly elevated levels of genera like Fusobacterium, Prevotella, and Streptococcus, have been observed in LC patients compared to healthy individuals [57,58]. Fusobacterium, an invasive anaerobe, may contribute to chronic inflammation and carcinogenesis, and shifts in the oral microbiome could serve as an early marker of LC [58,59]. The microbiome’s influence extends to immune system modulation, metabolic regulation, and even cancer promotion, with interactions with alcohol and tobacco further contributing to LSCC development. Certain bacteria can produce carcinogenic metabolites, exacerbate inflammation, and suppress anti-tumor immune responses [60,61,62,63].
LC outcomes are significantly worsened by delays in diagnosis and treatment. Socioeconomic disparities such as lack of health insurance and limited healthcare access delaying cancer screening contribute to these delays and to the number of deaths from modifiable risk factors such as tobacco, alcohol and occupational exposures [64,65]. Men tend to have an increased risk of LC [66,67,68] and have a poor prognosis in comparison to women [69]. Preventive measures, particularly reducing tobacco and alcohol consumption and improving access to screening, are crucial for mitigating the burden of this disease [70,71]. Furthermore, in 2021, attributable deaths due to tobacco, occupational risks, and alcohol were 66.46%, 5.92%, and 12.4%, respectively. While deaths attributable to these factors have generally decreased since 1990, deaths due to occupational risks have increased in females. The proportion of LC deaths attributable to tobacco was highest in high-middle sociodemographic index (SDI) regions (76.15%), while high SDI regions had the highest proportions attributable to occupational risks (11.56%) and alcohol (19.31%) [72]. Country-specific data reveals that Armenia (middle SDI) had the highest proportion of LC deaths attributable to tobacco (83.86%), while the UK (high SDI) had the highest proportion linked to occupational risks (20.11%), and Czechia (high SDI) had the highest proportion attributable to alcohol (27.35%). These disparities highlight the complex interplay between individual behaviours, environmental exposures, and access to preventative care and treatment, underscoring the need for targeted interventions [72].
3.2. Histological Subtypes of LC
Laryngeal squamous cell carcinoma (LSCC), the most prevalent malignancy of the larynx, demonstrates a spectrum of differentiation, broadly categorized as keratinizing or non-keratinizing. Keratinizing SCC, typically encompassing well-differentiated and moderately differentiated forms, exhibits significant keratin production, often forming characteristic keratin pearls and demonstrating abundant intracellular keratin. In contrast, non-keratinizing SCC, usually associated with poorly differentiated tumours, lacks this well-developed keratinization; in these cases, immunohistochemical markers like CK5/6, p63, p40, and EMA are crucial to confirm the epithelial origin [73]. Beyond this broad distinction, laryngeal SCC encompasses several distinct variants, each with unique morphological features, clinical behaviours, and prognostic implications, as summarized in Table 1.

Table 1.
Histopathological and Prognostic Features of Laryngeal SCC Variants.
In contrast, papillary/exophytic SCC (PSCC/ESCC) demonstrates a predominantly exophytic or papillary growth pattern with frank cytomorphologic malignancy [74]. Verrucous carcinoma (VC) is a well-differentiated, slow-growing form characterized by a pushing border of infiltration, abundant keratinization, and a lack of cytologic atypia [75]. Spindle cell (sarcomatoid) SCC (SCSCC) is a biphasic tumour with both epithelial and spindle cell components, often presenting as a polypoid mass [76,77]. Basaloid SCC (BSCC) is a high-grade variant with a prominent basaloid component and often aggressive behaviour [78]. Adenosquamous carcinoma (ASC) is characterized by an admixture of SCC and adenocarcinoma components [79,80,81]. Distinguishing between keratinizing and non-keratinizing SCC and accurately classifying these variants is essential for guiding treatment decisions and predicting patient outcomes. Careful histological evaluation, often supplemented by immunohistochemistry, is paramount.
3.3. Diagnosis
Accurate diagnosis of LC relies on a thorough patient history, a comprehensive physical examination, and appropriate diagnostic procedures. The patient’s history should include detailed inquiries about risk factors like tobacco and alcohol use, current medications, and any coexisting medical conditions that might influence treatment decisions [82]. The clinical presentation of LC varies considerably depending on the tumour’s location and size. Glottic tumours often present early with hoarseness, while supraglottic tumours may manifest later with symptoms such as pain, persistent hoarseness, or dysphagia (difficulty swallowing) [82]. The diagnostic modalities of LC are summarized in Table 2. Direct visualization of the larynx is essential, typically achieved through indirect laryngoscopy, flexible fiberoptic laryngoscopy, or video stroboscopy. Videostroboscopy, in particular, demonstrates high sensitivity (96.8%) and specificity (92.8%) in predicting the invasiveness of laryngeal lesions [83]. Tissue confirmation through biopsy of the primary tumour or fine-needle aspiration of suspicious lymph nodes is crucial for definitive diagnosis. Imaging studies play a vital role in staging cancer. Computed tomography (CT) is valuable for assessing bone involvement, while positron emission tomography combined with CT (PET/CT) helps detect recurrences, local and nodal spread, and distant metastases [84]. Magnetic resonance imaging (MRI) offers superior sensitivity (80%) and specificity (92.9%) compared to CT (60% sensitivity, 85.7% specificity) in evaluating cartilage and soft tissue invasion [85]. Narrow-band imaging (NBI) has also emerged as a highly sensitive (97%) and specific (92.5%) technique for identifying both LC and its precursor lesions [86].

Table 2.
Diagnostic Modalities in Laryngeal Cancer: Techniques, Strengths, and Limitations.
Traditional diagnostic approaches for LC, such as imaging and tissue biopsy, are fundamental but can be limited in detecting early-stage disease. Over the last decade, there has been increasing interest in using liquid biopsies to detect cancer-specific biomarkers in patients’ body fluids [87,88]. Liquid biopsy has been reported to play roles in early malignancy detection in diverse tumor types [89,90]. As a rapid and noninvasive approach, liquid biopsies have emerged as an exciting investigational avenue to obtain information on cancer diagnosis, treatment response, and progression [91,92]. Liquid biopsies are minimally invasive tests analyzing biomarkers in fluids like blood and saliva. These biomarkers, including circulating tumour cells (CTCs), circulating tumour DNA (ctDNA), exosomes carrying microRNAs (miRNAs), and even oral microbiome constituents, offer potential for earlier detection, refined prognosis, and real-time monitoring of treatment response in HNSCC, and specifically LC [93,94].
Since LC is frequently diagnosed at later stages, methods for earlier identification are critical. Research has demonstrated that ctDNA, fragments of DNA released by tumour cells, are detectable in the plasma and saliva of patients with LSCC, with levels correlating to the disease stage [95]. Epigenetic modifications, such as aberrant DNA methylation, also hold diagnostic promise. Studies have identified specific gene methylation patterns in plasma or serum associated with the early detection of respiratory cancers, including LSCC [96,97,98,99]. Furthermore, analysis of the oral microbiome, specifically detecting certain microbiota in mouthwash, has emerged as a novel liquid biopsy approach for LSCC [58].
Moreover, liquid biopsies provide valuable prognostic information for LC patients and enable monitoring of treatment response. Analyzing circulating tumour cells (CTCs), circulating tumour DNA (ctDNA), and microRNAs (miRNAs) is key. Higher preoperative CTC counts [100,101,102] and ctDNA hypermethylation [98] correlate with worse outcomes in LSCC. Changes in these markers and specific miRNAs [103,104,105,106] are linked to treatment and survival. Furthermore, liquid biopsies allow for minimally invasive, repeated sampling to dynamically monitor treatment efficacy and detect recurrence. Changes in CTC and ctDNA levels indicate treatment response or resistance [100,101,107].
Traditional diagnostic methods, relying on clinical examination, endoscopy, and histopathological analysis of biopsies, can be time-consuming and subject to inter-observer variability [108]. AI, particularly through machine learning (ML) and deep learning (DL) techniques, offers the potential to improve diagnostic accuracy and efficiency. In LC, various imaging modalities are used to train AI algorithms. Studies have utilized histological whole-slide images (WSI) [109] and endoscopic/clinical imaging [110]. For example, stimulated Raman scattering histology integrated with DL algorithms has shown an accuracy of 90% in diagnosing laryngeal SCC [111]. Other approaches have used traditional ML techniques to classify laryngeal tissues as normal or malignant based on textural information from narrow-band endoscopic images, achieving high recall rates [110]. These AI-driven diagnostic tools have the potential to assist clinicians in earlier and more accurate detection of LC, potentially leading to improved patient outcomes.
In addition, AI models, particularly deep neural networks (DNNs), can analyze diverse clinical data to provide more personalized risk assessments. A study by Choi et al., (2023) demonstrated that a DNN model incorporating various clinical factors significantly outperformed models using only the TNM stage for predicting survival in LSCC patients [111]. This aligns with previous findings highlighting the importance of factors like age, performance status, and lifestyle choices [112,113]. Unlike traditional regression models [114,115], AI can better handle the complex, non-linear relationships between these factors and survival outcomes, leading to more accurate and individualized predictions that could improve treatment strategies.
3.4. Molecular Biomarkers in Laryngeal Squamous Cell Carcinoma
Biomarkers, measurable indicators of biological states, are increasingly vital in understanding LSCC. They can refine diagnosis, predict prognosis, and guide treatment. Research has explored a wide range of molecules as potential biomarkers in LSCC, categorized by their biological roles: long noncoding RNAs (lncRNAs), cell cycle regulators, apoptosis regulators, oncogenes and tumour suppressors, growth factor pathway components, angiogenic factors, structural proteins, sex hormone signalling components, and immunological markers [116]. Table 3 summarize the important biomarkers and their function in laryngeal cell cancer.

Table 3.
Molecular Biomarkers in Laryngeal SCC: Biological Roles and Clinical Implications.
Alterations that enhance the function of the epidermal growth factor receptor (EGFR) axis and defects that diminish the function of the transforming growth factor-β receptor (TGF-βR) axis collectively promote unregulated cellular proliferation, reduced apoptosis, and increased cell survival. In the context of EGFR, overexpression or activating mutations result in the receptor being persistently active, thereby continuously stimulating the RAS–RAF–MEK–ERK and PI3K–AKT signaling pathways. This activation leads to the upregulation of pro-proliferative markers such as Cyclin D1 and Ki-67, as well as anti-apoptotic proteins like Bcl-2, while concurrently downregulating cell-cycle inhibitors such as p27. Conversely, the loss of TGF-βR signaling, due to receptor mutations or disruptions in the SMAD pathway, impairs its normal growth-inhibitory functions, resulting in decreased levels of p53 and p27. This loss effectively removes critical regulatory mechanisms that inhibit cell-cycle progression and apoptosis. These opposing molecular events converge within the nucleus, driving DNA replication, promoting survival under stress conditions, and ultimately contributing to the development and aggressiveness of laryngeal cancer (Figure 2).
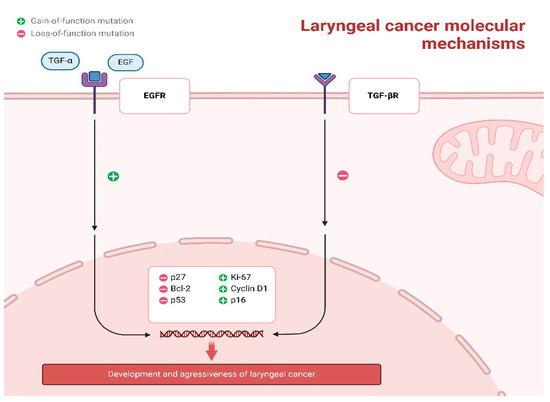
Figure 2.
Core Molecular Pathways Driving Laryngeal Squamous Cell Carcinoma Pathogenesis.
Biomarkers in laryngeal squamous cell carcinoma (LSCC) encompass a wide range of molecules that modulate key cellular processes and may impact tumor behavior and clinical outcomes.
Long noncoding RNAs (lncRNAs) are RNA molecules that do not code for proteins but regulate gene expression and various cellular functions. Their dysregulation is linked to cancer development, including LSCC, where many lncRNAs are upregulated in tumor tissues. This overexpression often promotes tumor progression, enhances metastasis, reduces radiosensitivity, and worsens overall survival, partly by activating pathways such as Wnt, Sox-2, and TGF-β1 [145,146,147,157]. Owing to their stability in body fluids and ease of detection by non-invasive methods, lncRNAs are considered promising biomarker candidates [158].
The cell cycle, which is frequently disrupted in cancer, offers several potential biomarkers. Ki-67, a nuclear protein expressed during all active phases of the cell cycle but absent in resting cells, serves as a direct marker of proliferation. In LSCC, elevated Ki-67 levels are associated with poorly differentiated tumors, advanced TNM stages, increased recurrence risk, and shorter disease-free survival [133,134,135,136]. However, its relationship with radiotherapy response is complex; while high levels might imply increased radiosensitivity in some studies [159,160], other reports suggest that low Ki-67 can be linked to better local control in early-stage tumors, possibly due to factors like DNA repair capacity and hypoxia [161,162]. Cyclin D1, which drives the G1 to S phase transition by complexing with CDKs 4 and 6, is commonly overexpressed in LSCC and correlates with lymph node metastasis and poorer survival; its overexpression may also occur alongside TP53 mutations [131,139,140]. In contrast, the cyclin-dependent inhibitor p27 is frequently underexpressed in LSCC, with low levels linked to tumor recurrence and poorer outcomes [140,163]. An inverse relationship between cyclin D1 and p27 is evident, with patients displaying cyclin D1 positivity alongside p27 negativity often faring worse [116]. In addition, p16—a CDKI closely related to the HPV 16 status frequently seen in head and neck cancers—is typically downregulated in tumors with high cyclin D1, and its presence tends to indicate better treatment response and survival [164].
Deregulation of apoptotic pathways is another hallmark of LSCC. Bcl-2, an intracellular membrane protein that inhibits apoptosis by modulating cytochrome C release and interacting with pro-apoptotic proteins, shows conflicting associations. While some studies report no correlation between Bcl-2 expression and clinical outcomes [117,118,119,120,121], other investigations have linked its overexpression to lymph node metastasis, advanced stage, poor differentiation, increased recurrence, and radioresistance [165]. A meta-analysis by Silva et al., (2023) even suggests that Bcl-2 overexpression may be associated with poorer lymph node metastasis, overall survival (OS), and disease-free survival (DFS), although these findings should be interpreted with caution because of study variability and potential bias [166].
Tumor suppressor and oncogenes also play crucial roles in LSCC. The tumor suppressor p53, which activates cell cycle arrest, DNA repair, senescence, or apoptosis in response to stress, is mutated in approximately 60–80% of LSCC cases [167,168,169]. These mutations are frequently linked to more aggressive disease features; however, consensus on the prognostic role of p53 remains elusive due to heterogeneous and sometimes conflicting study results [170,171,172,173].
Growth factor signaling contributes further to LSCC progression. Epidermal growth factor receptor (EGFR), a transmembrane receptor tyrosine kinase that binds ligands such as EGF and TGF-α, triggers intracellular pathways leading to increased cell proliferation, angiogenesis, and survival. EGFR overexpression in LSCC is associated with progression to malignancy, a higher risk of metastasis, and reduced survival, particularly when found together with high levels of cyclin D1 and Ki-67 [173,174,175,176,177]. Similarly, alterations in the TGF-β pathway—most notably the loss of TGF-β receptor II expression in lesions progressing to invasive carcinoma—reduce the growth-suppressive effects of TGF-β and promote tumor advancement [169,170,171,172,173,174,175,176,177,178].
Angiogenesis, a process vital for tumor growth and metastasis, is reflected by several markers in LSCC. Vascular endothelial growth factor (VEGF) increases vascular permeability and stimulates endothelial cell proliferation and migration, and its upregulation in LSCC correlates with dysplasia progression, local recurrence, metastasis, and shorter disease-free survival, though some studies find no significant association [126,150,179,180,181,182]. Additional angiogenic markers such as angiogenin and CD105 (endoglin) are also implicated, with their elevated expression correlating with recurrence, advanced disease, and poorer clinical outcomes [126,148,149,150,183,184].
Structural proteins that maintain cell adhesion and tissue integrity are also significant. E-cadherin, a transmembrane glycoprotein essential for cell-cell adhesion, is often downregulated in LSCC, correlating with poorer tumor differentiation, increased risk of nodal metastasis, and advanced tumor stage; reduced expression may also be linked to shorter disease-free survival [125,126,127,185]. The cell surface glycoprotein CD44, a known marker of cancer stem cells, is similarly associated with higher tumor grade and poorer 5-year survival [122,123,124]. Overexpression of focal adhesion kinase (FAK) and cortactin—an actin-binding protein involved in cell motility and invasion—has been linked to aggressive behavior and recurrence in LSCC [126,151,152,153,154].
The role of sex hormone signaling in LSCC remains debated. Estrogen receptors (ERα and ERβ, including variants such as ERα66 and ERα36) display variable expression patterns; while early-stage LSCC tumors may show increased levels compared to normal tissue, advanced stages often exhibit a shift in receptor subtype expression that correlates with a more unfavorable prognosis [155,186,187]. Likewise, progesterone receptor (PR) expression is higher in poorly differentiated LSCC and those with nodal metastasis, whereas androgen receptor (AR) expression is typically reduced in invasive tumors. Elevated prolactin receptor (PRLR) expression has also been noted in LSCC and correlates with poorer survival outcomes [155,156].
Immunological biomarkers reflect the critical role of the host immune response in modulating tumor growth. Tumor-infiltrating lymphocytes (TILs), particularly cytotoxic CD8+ T cells, are associated with improved survival in LSCC—even in cases of tobacco-related disease [188,189,190]. LSCC continues to have a poor prognosis, with a 5-year survival rate of 50 to 60% and suboptimal functional outcomes. The expression of PD-L1 and the tumor microenvironment markers (CD4, CD8, CD68, and CD163) were examined in LSCC using immunohistochemistry. PD-L1 expression demonstrated a statistically significant positive correlation with all the tumor microenvironment cells studied. Higher expressions of CD68 and CD163 were significantly associated with worse clinical outcomes in LSCC patients [191]. To determine which LSCC patients might benefit from immunomodulation therapies, it is crucial to understand the relationship between PD-L1 expression, immune cell distribution, and prognosis. Conversely, the expression of programmed death-ligand 1 (PD-L1) on tumor and immune cells, which aids tumors in evading immune attacks, has been linked to clinical outcomes and may make tumors amenable to immune checkpoint inhibitor therapy [140,192,193,194,195].
Emerging biomarkers in LSCC continue to broaden our understanding of tumor biology. Among these, heat shock proteins (HSPs) have diverse roles: HSP27 and HSP70 are linked to advanced tumor stage, chemoresistance (particularly to cisplatin), and radiotherapy resistance [196,197,198,199,200,201] whereas HSP47 appears to function as a tumor suppressor, with higher expression correlating with better prognosis and longer overall survival [202]. Metallothioneins (MTs), especially the MT1 and MT2 isoforms, are found at higher levels in malignant laryngeal lesions than in benign or dysplastic ones, and genetic variations in MT2A may increase LSCC risk, making them potential early biomarkers [203,204,205,206]. Components of the oxidative stress response, such as nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1), are also implicated; increased nuclear Nrf2 expression correlates with cisplatin resistance, while HO-1 may counteract cisplatin-induced apoptosis, although its prognostic role in LSCC is still under investigation [207,208,209,210,211,212]. Cyclooxygenase-2 (COX-2) is another emerging marker; its elevated expression in LSCC is linked to enhanced angiogenesis, inflammatory signaling, and resistance to radiotherapy, and it is associated with poorer clinical outcomes [213,214,215,216,217,218,219]. Lastly, several microRNAs (miRNAs)—such as Hs_miR-21_5p, Hs_miR-218_3p, and Hs_miR-210_3p, which are expressed exclusively in malignant laryngeal lesions—are implicated in regulating tumor cell migration, invasion, and treatment resistance, further supporting their utility as precise biomarkers [220,221,222,223,224,225,226,227].
3.5. Metastatic Patterns of LC
LC metastasis predominantly follows a lymphatic route, with the specific pattern of spread heavily influenced by the primary tumour’s location within the larynx. The supraglottis, possessing a rich lymphatic network and a midline position, exhibits a high likelihood of bilateral lymph node involvement. Lymphatic drainage from the supraglottis flows through the thyrohyoid membrane into the jugular chain, making the jugular lymph nodes the primary recipients of metastases. Specifically, the sub-digastric (level II), mid-jugular (level III), and lower jugular (level IV) nodes are most frequently affected. In contrast, posterior cervical nodes (level V) are rarely involved, and submandibular (level IB) and submental (level IA) nodes are seldom involved. Approximately 55% of patients with supraglottic carcinoma present with clinically involved lymph nodes at diagnosis, with 16% showing bilateral involvement.
Furthermore, up to half of clinically node-negative patients may harbour occult nodal metastases. The risk of lymph node involvement increases with tumour size and grade, reaching approximately 40% for T1 and T2 tumours and 60% for T3 and T4 lesions [228,229,230]. In contrast, the subglottis has a less developed lymphatic network, resulting in a lower incidence of lymph node metastasis (20% to 50%). Subglottic lymphatic channels drain anteriorly through the cricothyroid membrane to the middle and lower jugular or Delphian nodes and posterolaterally to the paratracheal nodes. The true vocal cords are almost devoid of lymphatics, leading to a very low incidence of nodal metastasis in early-stage (T1) glottic cancers, approaching zero. This incidence increases with stage: 2% for T2, 15–20% for T3, and 20–30% for T4 lesions. Occult nodal involvement is found in approximately 16% of clinically node-negative T3 and T4 glottic cancers [229,230]. Glottic cancer spread typically involves extension into the supraglottis or subglottis, leveraging their more prosperous lymphatic supply. Distant metastasis is relatively uncommon in LC, occurring in 10–20% of patients, predominantly those with supraglottic and subglottic primaries, although autopsy studies reveal a higher rate of subclinical metastases. The lung is the most frequent site of distant metastasis, followed by bone (20% of patients with distant disease) and liver (10% clinically). Brain metastases are rare [229,230,231]. Because of the high risk of second primary cancers in these patients, tissue confirmation of suspected metastases is essential. Factors increasing the risk of distant metastasis include lymph node involvement, low neck metastases, advanced stage, and extranodal extension (ENE), with ENE potentially increasing the risk tenfold [19,232].
3.6. Recent Trials Further Investigating Biomarkers in LC
Several clinical trials are investigating the role of biomarkers in LSCC treatment and prognosis. One study focusing on locally advanced LSCC treated with radiotherapy found that high pre-treatment expression of the hypoxia marker HIF-1α, along with the presence of regional lymph node metastases, were independent predictors of locoregional recurrence [233]. Another trial examining advanced LC patients primarily treated with surgery found that while high tumour tissue expression of CD4+ and CD8+ T cells initially appeared to improve survival, only higher serum levels of the cytokine IL-8 were a significant negative predictor of disease-specific survival in multivariate analysis [234]. A third study examined inflammatory biomarkers in LSCC patients undergoing definitive radiotherapy, finding that pre-treatment C-reactive protein (CRP) levels were a significant predictor of progression-free survival, while neutrophil-to-lymphocyte ratio (NLR) and ECOG performance status predicted overall survival. Additionally, this study identified that monocyte-to-lymphocyte ratio (MLR), pan-immune inflammatory value (PIV), and CRP levels were significantly higher in patients with lymphatic metastasis [235].
3.7. Treatment
The treatment of LC is a multifaceted endeavor, with approaches tailored to the cancer’s stage, location, and the patient’s overall condition. For early-stage (T1-T2, N0, M0) LCs, treatments often aim to preserve the larynx. These can involve laryngeal-sparing surgeries, which selectively remove cancerous tissue, or definitive radiotherapy (RT), employing high-energy beams to eradicate cancer cells [236]. Table 4 summarizes the treatment modalities of LC.

Table 4.
Treatment Modalities for LC.
Currently, the standard treatment for locally advanced LC, when aiming for larynx preservation, is concurrent chemoradiation therapy (CRT). This approach combines high-dose cisplatin chemotherapy with radiation therapy, delivered simultaneously. The effectiveness of this concurrent approach was established by the RTOG 91-11 trial, which demonstrated superior larynx preservation rates compared to induction chemotherapy followed by radiation or radiation alone [237]. Intensity-modulated radiation therapy (IMRT) is a widely used technique that allows for highly conformal treatment plans, minimizing radiation dose to sensitive structures like the spinal cord, oesophagus, and salivary glands, thereby reducing toxicities [238].
Beyond the standard CRT approach, several other therapies and considerations are relevant in the treatment of LC. For early-stage disease, definitive radiotherapy alone or laryngeal-sparing surgery may be sufficient [236].
Alternative systemic therapies may be considered for patients who are not candidates for cisplatin. Although cetuximab, an EGFR inhibitor, can enhance radiation’s effects, it has shown inferior overall survival compared to cisplatin in certain head and neck cancers [239]. Thus, for platinum-ineligible cases, carboplatin and fluorouracil, or cetuximab alone, might be considered based on improvements in survival compared to radiation alone in clinical trials.
Immunotherapy, specifically with checkpoint inhibitors like pembrolizumab and nivolumab, is currently approved for recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) [240]. Pembrolizumab and nivolumab received FDA approval in 2016 [240,241]. In the pivotal CheckMate 141 Asian subset, nivolumab conferred a median overall survival of 12.1 months vs. 6.2 months with investigator’s choice therapy, and the estimated 2-year OS rates were 22.7% vs. 0%, respectively; notably, patients who developed any treatment-related adverse events particularly skin-related disorders experienced superior survival, suggesting that early immune-related toxicity may predict benefit [242]. Multiple clinical trials are actively investigating the role of these agents in combination with radiation therapy for locally advanced LC, exploring their potential as alternative radiosensitizers [243,244]. For example, the multinational, Phase III, double-blind, placebo-controlled JAVELIN Head and Neck 100 trial (NCT02952586) is assessing whether adding the PD-L1 inhibitor avelumab to standard cisplatin-based chemoradiotherapy improves progression-free and overall survival compared with chemoradiotherapy plus placebo in patients with high-risk, nonmetastatic, locoregionally advanced HNSCC [245].
Similarly, the Phase III, double-blind, placebo-controlled KEYNOTE-412 trial (NCT03040999) evaluated the addition of pembrolizumab (200 mg q3w) to standard cisplatin-based chemoradiotherapy (70 Gy in 35 fractions) in 804 patients with newly diagnosed, high-risk, unresected, locoregionally advanced HNSCC. Pembrolizumab was given one dose before CRT, two doses during CRT, and up to 14 maintenance doses thereafter. After a median follow-up of 47.7 months, median event-free survival was not reached in the pembrolizumab arm versus 46.6 months in the placebo arm (hazard ratio 0.83 [95% CI 0.68–1.03]; log-rank p = 0.043, above the prespecified threshold of p ≤ 0.024), and median overall survival was not reached in either arm. Grade ≥3 adverse events occurred in 92% of pembrolizumab-treated versus 88% of placebo-treated patients, with similar safety profiles and no new signals [246]. This trial highlights the challenge of integrating PD-1 inhibitors into definitive CRT for locally advanced HNSCC and underscores the need for biomarker-driven strategies.
Pembrolizumab, a highly selective humanized monoclonal antibody, is designed to disrupt the PD-1 immune checkpoint pathway. It works by binding to the PD-1 receptor on T cells, preventing it from interacting with its ligands, PD-L1 and PD-L2, which are often expressed on tumour cells and other cells in the tumour microenvironment. This blockade releases the “brake” on the immune system, allowing T cells to become activated and attack cancer cells. In the context of recurrent or metastatic head and neck squamous cell carcinoma (HNSCC), including LSCC, the Keynote 040 study investigated the effectiveness of pembrolizumab against standard treatments. In this trial, 247 participants were randomly assigned to receive either pembrolizumab or one of the following standard-of-care therapies: methotrexate (40–60 mg/m2), docetaxel (30–40 mg/m2), or cetuximab (250 mg/m2 weekly after an initial 400 mg/m2 loading dose). The median overall survival for patients treated with pembrolizumab was 8.4 months, compared to 6.9 months for those receiving standard therapy. This difference represented a statistically significant improvement in survival for the pembrolizumab group, with a hazard ratio (HR) of 0.80 (95% CI 0.65–0.98; p = 0.0161) [247]. The benefit of pembrolizumab was even more pronounced in patients whose tumours had a high proportion of cells expressing PD-L1, as indicated by a tumour proportion score (TPS) of 50% or greater. In this subgroup, the median overall survival was 11.6 months with pembrolizumab versus 6.6 months with standard care, demonstrating a more substantial and statistically significant improvement (HR: 0.53, 95% CI 0.35–0.81; p = 0.014) [247].
CTLA-4 inhibitors, such as ipilimumab and tremelimumab, block the CTLA-4 immune checkpoint on T cells preventing its engagement with CD 80/86—and thereby sustain T-cell activation to enhance antitumor immunity. In CheckMate 651 (NCT02741570), 947 patients with previously untreated R/M SCCHN were randomized to first-line nivolumab + ipilimumab versus the EXTREME regimen. Neither the overall population (median OS 13.9 vs. 13.5 months; HR 0.95; p = 0.4951) nor the PD-L1 CPS ≥ 20 subgroup (17.6 vs 14.6 months; HR 0.78; p = 0.0469) met the primary OS endpoint, although nivolumab + ipilimumab had fewer grade 3–4 treatment-related adverse events (28.2% vs. 70.7%) [248]. In the phase III KESTREL trial (NCT02551159), neither tumor PD-L1 expression (TC ≥ 50%/IC ≥ 25% or TC ≥ 25%) nor a low neutrophil-to-lymphocyte ratio (≤7) enriched for improved outcomes with first-line durvalumab (D) or durvalumab + tremelimumab (D + T) versus EXTREME. However, in patients whose blood tumor mutational burden was ≥16 mut/Mb, D + T achieved a median OS hazard ratio of 0.69 (95% CI 0.39–1.25) and a complete response rate of 8.6% versus 4.3% with EXTREME, suggesting bTMB may help identify those most likely to benefit from checkpoint blockade [249].
In the phase III KESTREL trial (NCT02551159), first-line durvalumab monotherapy or durvalumab plus tremelimumab was compared with EXTREME in recurrent/metastatic HNSCC, and archival tumors or blood were assayed for PD-L1, blood tumor mutational burden (bTMB), and neutrophil-to-lymphocyte ratio (NLR). PD-L1 expression (TC ≥ 50%/IC ≥ 25% or TC ≥ 25%) and NLR ≤ 7 failed to enrich for either overall survival or response rates, whereas in the bTMB ≥ 16 mut/Mb subgroup, durvalumab plus tremelimumab achieved an OS hazard ratio of 0.69 (95% CI 0.39–1.25) versus EXTREME and doubled the complete response rate (8.6% vs. 4.3%), suggesting high bTMB may identify patients most likely to benefit from PD-L1/CTLA-4 blockade.
In the phase III CheckMate 651 trial, first-line nivolumab plus ipilimumab did not improve overall survival compared with the EXTREME regimen in patients with recurrent or metastatic SCCHN, yielding a median OS of 13.9 versus 13.5 months in the intent-to-treat population (HR 0.95, p = 0.495) and 17.6 versus 14.6 months in those with PD-L1 CPS ≥ 20 (HR 0.78, p = 0.047), despite a substantially lower rate of grade 3–4 treatment-related adverse events (28.2% vs. 70.7%). Among responders, the duration of response was markedly longer with nivolumab/ipilimumab (32.6 vs. 7.0 months), but progression-free survival and response rates were similar between arms.
Proton therapy represents another evolving radiation modality. It offers the potential for a more favourable therapeutic window compared to photons due to the Bragg Peak phenomenon, where the majority of the radiation dose is deposited at a specific depth, minimizing the dose beyond the target. However, access to proton therapy is limited, and prospective, multi-institutional data comparing it to IMRT are still emerging [250]. Stereotactic body radiation therapy (SBRT), which delivers very high doses of radiation in a few fractions, is being investigated for early-stage glottic cancers but is not yet established for laryngeal preservation and should only be used within a clinical trial [251].
While many treatments focus on larynx preservation, total laryngectomy (TL) remains a crucial option, particularly for patients with extensive tumour invasion, significant pre-existing swallowing dysfunction, or those who are not candidates for or have failed organ preservation approaches. Total laryngectomy involves the complete removal of the larynx (voice box), resulting in permanent loss of natural voice production. This procedure necessitates the creation of a permanent tracheostoma, an opening in the neck, for breathing. While a significant intervention, TL can be the most effective treatment for achieving complete tumour removal in certain cases. After a total laryngectomy, patients require relearning for tasks like speaking [252].
In addition, a tracheostomy can secure the airway in patients with significant tumour obstruction, allowing them to undergo CRT instead of requiring a total laryngectomy [253].
Minimally invasive surgical techniques have significantly advanced, offering patients less invasive options with potentially improved functional outcomes. Two prominent techniques are transoral laser microsurgery (TLM) and transoral robotic surgery (TORS).
TLM employs a CO2 laser to precisely resect laryngeal tumours through the mouth, avoiding external incisions. Unlike open partial laryngectomy, TLM focuses on removing the tumour with margins while preserving normal structures to optimize function. The European Laryngological Society (ELS) has a classification system for cordectomies, with six types based on resection depth and area [254].
TLM’s primary indication is early-stage vocal fold cancers (Tis, T1, and T2), with consideration for some advanced cases [255]. Limitations include tumours with reduced vocal fold mobility from cricoarytenoid joint fixation/infiltration (though usable with muscle infiltration without joint fixation), cricoid cartilage resection, and the necessity to preserve at least one cricoarytenoid unit [256,257]. TLM has also been applied to supraglottic and hypopharyngeal tumours, demonstrating comparable oncological results and better functional outcomes (less tracheotomy, faster swallowing, shorter hospital stays) than open surgeries [258,259].
TLM is a gold standard for early vocal fold cancers due to high disease-free survival, local/regional control, and larynx preservation rates, sometimes exceeding those of radiotherapy. It offers better postoperative and quality-of-life outcomes than open surgery or radiotherapy and is more cost-effective [260,261,262].
TORS uses the da Vinci system, providing 3D visualization, enhanced instrument manoeuvrability, tremor reduction, and improved movement. TORS received FDA approval in 2009 for specific laryngeal, oropharyngeal, and oral carcinomas and benign tumours. TORS is commonly used for cT1-T2 and some selected cT3-T4a oropharyngeal and supraglottic cancers with adequate visualization and instrument access. TORS supraglottic laryngectomy shows similar oncological results to open procedures, with good functional outcomes (rapid oral diet resumption, low permanent tracheotomy rates) [263,264].
TORS’s role in glottic cancer is less defined. Studies are controversial, with the current robotic instrument length not ideally suited. Research indicates that TORS for glottic cancer has exposure issues, higher costs, increased feeding tube and tracheotomy needs, and more complications than TLM [265,266,267].
3.8. Survival
The prognosis for patients with LC is strongly correlated with the disease stage at initial diagnosis and treatment. Other factors that play a role in survival are the patient’s general health and smoking cessation. In the United States, the overall 5-year survival rate for LC is 61% [268]. Survival rates decline significantly with the advancing stage. For diseases confined to the larynx (stages I and II), the 5-year survival rate is 78%. However, regional lymph node metastases (stage III) reduce the 5-year survival to 46%, and distant metastases lower it to 34% [269]. Survival outcomes also vary by laryngeal subsite. Glottic cancer generally has the most favourable prognosis, followed by supraglottic cancer, with subglottic tumours having the least favourable outlook [113]. Specifically, the overall 5-year survival for glottic SCC is 77%, reaching 84% for localized disease (stages I and II) but decreasing to 52% with nodal involvement (stage III) and 45% with distant spread [270]. For supraglottic SCC, the overall 5-year survival is 45%, with rates of 61% for localized disease, 46% for stage III, and 30% for metastatic disease [271,272]. Subglottic SCC has an overall 5-year survival rate of 49%, 59% for stages I and II, 38% for stage III, and 44% for metastatic disease [273,274].
The accurate prediction of prognosis in LC is crucial for tailoring treatment strategies. While the TNM staging system provides a foundation, it does not fully capture the heterogeneity of the disease, particularly regarding the impact of lymph node involvement. Therefore, integrating both clinical factors and biomarkers into comprehensive prognostic models is an area of active investigation. Recent efforts have focused on developing nomograms which is a statistical tools that combine multiple variables to provide individualized risk assessments. For instance, Juan et al., (2024) [275] developed and validated nomograms specifically for early-stage LSCC to predict postoperative recurrence-free survival (RFS) and overall survival (OS). Their models incorporated readily available preoperative blood markers (platelet counts, fibrinogen, platelet-to-lymphocyte ratio, systemic immune-inflammation index, and hemoglobin) alongside clinicopathological characteristics (tumor diameter and differentiation degree). These nomograms demonstrated superior predictive accuracy compared to T staging alone [275].
Similarly, Shi et al., (2017) [276] developed nomograms for LSCC patients undergoing neck dissection, incorporating the lymph node ratio (LNR)—a factor reflecting both the extent of nodal disease and the thoroughness of the dissection—along with a comprehensive set of clinical and pathological factors, including T stage, N stage, tumor size and demographics. Their models, validated both internally and externally, also showed improved predictive performance for both OS and cancer-specific survival (CSS) compared to models without LNR and to traditional TNM staging [276]. These studies highlight that readily available clinical parameters and biomarkers can significantly enhance prognostication in LSCC, there is a need for further investigations into developing new scoring systems. Further research that include novel biomarkers discussed in the review may improve current scoring system and therefore, be used by clinicians to decide the optimal personalized treatment.
Quality-of-life (QoL) outcomes are paramount in the treatment of LC, particularly when comparing organ-preserving approaches versus TL. For early-stage (T1-T2) glottic cancers, both TLM and RT aim for larynx preservation, but their impact on voice and QoL can differ. A meta-analysis by Qasem et al., (2024) [277] found no significant difference between TLM and RT in overall voice-related QoL measures, including the Voice Handicap Index-30 (VHI-30). However, TLM was associated with significantly better outcomes in specific acoustic parameters like jitter and shimmer, suggesting potentially improved voice stability [277]. In more advanced T3 glottic cancers, TLM can still be an option, and Chien et al., (2020) [278] showed that carefully selected T3 glottic SCC patients could achieve satisfactory QoL and larynx preservation rates after CO2 TLM. Even after total laryngectomy, where the larynx is removed, voice rehabilitation is crucial for QoL [278]. However, Wulff et al., (2020) [279], in a systematic review, found that individuals who underwent TL generally reported worse health-related quality of life (HRQoL) compared to a male normative reference population, though the reported symptom burden was often mild. The heterogeneity and generally low quality of existing studies highlight a need for more rigorous research in this area [279]. These studies, overall, emphasize a need for individualized assessment when choosing a treatment and obtaining a high level of QoL. Table 5 shows a summary of survival data provided within this section.

Table 5.
Survival Rates and Prognostic Factors in LC.
4. Conclusions
LC, a complex and heterogeneous disease, presents significant diagnosis, treatment, and prognostication challenges. This review has highlighted the crucial role of accurate staging in guiding treatment decisions, incorporating both clinical and pathological findings. The distinct histological variants of LSCC, each with unique characteristics and behaviors, necessitate careful pathological evaluation. The expanding knowledge of biomarkers offers the potential for improved risk stratification and personalized treatment approaches, although further validation is needed for many of these markers. While treatment strategies have evolved, with a growing emphasis on organ preservation, the advanced-stage disease continues to pose a significant challenge.
Recognizing that no single specialist can address every nuance of LC care, it is essential to integrate multidisciplinary team (MDT) decision-making throughout the patient journey. Regular MDT reviews, which bring together head and neck surgeons, radiation and medical oncologists, radiologists, pathologists, and allied health professionals, ensure that emerging biomarkers and novel therapies are interpreted within a comprehensive clinical context.
Future research in LC must address several critical gaps to advance the field. A primary focus should be the rigorous validation of promising biomarkers, moving beyond exploratory studies to establish their clinical utility in diverse patient populations and treatment settings. This includes standardizing assays and determining optimal cut-off values for clinical decision-making. Simultaneously, the development of novel targeted therapies is crucial, particularly those addressing specific molecular alterations driving tumor growth and resistance. Emphasis should also be on personalized medicine. This means creating strategies that are individualized and focus on the combination of specific tumoral factors and host factors. This personalized approach necessitates integrating comprehensive genomic and proteomic profiling with detailed clinical data to tailor treatment selection and predict response to therapies. Finally, optimizing treatment strategies, including refining radiation techniques, exploring immunotherapy combinations, and developing strategies to overcome resistance, is essential to improve patient outcomes and quality of life.
Author Contributions
Conceptualization, A.-R.H. and E.R.B.; methodology, A.-R.H.; software, D.P.; validation, G.I., D.H. and L.M.; formal analysis, G.I.; investigation, D.H. and N.C.B.; resources, R.G.; data curation, D.P.; writing—original draft preparation, A.-R.H.; writing—review and editing, A.C.M. and N.C.B.; visualization, M.C.; supervision, A.C.M.; project administration, E.R.B.; funding acquisition, E.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to acknowledge “Victor Babes” University of Medicine and Pharmacy for their support in covering the costs of publication for this research paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ciolofan, M.S.; Vlăescu, A.N.; Mogoantă, C.-A.; Ioniță, E.; Ioniță, I.; Căpitănescu, A.-N.; Mitroi, M.-R.; Anghelina, F. Clinical, Histological and Immunohistochemical Evaluation of Larynx Cancer. Curr. Health Sci. J. 2017, 43, 367–375. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F.; et al. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/14-larynx-fact-sheet.pdf (accessed on 14 March 2025).
- Zuo, J.-J.; Tao, Z.-Z.; Chen, C.; Hu, Z.-W.; Xu, Y.-X.; Zheng, A.-Y.; Guo, Y. Characteristics of Cigarette Smoking without Alcohol Consumption and Laryngeal Cancer: Overall and Time-Risk Relation. A Meta-Analysis of Observational Studies. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.M.; Watz, C.G.; Motofelea, A.C.; Chiriac, S.; Poenaru, M.; Dehelean, C.A.; Borza, C.; Ionita, I. Challenges of Pharyngeal Cancer Screening in Lower-Income Countries during Economic and Social Transitions: A Population-Based Analysis. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2226–2237. [Google Scholar] [CrossRef]
- Tang, J.A.; Lango, M.N. Diverging Incidence Trends for Larynx and Tonsil Cancer in Low Socioeconomic Regions of the US. Oral. Oncol. 2019, 91, 65–68. [Google Scholar] [CrossRef]
- Liao, L.; Wang, H.; Cui, W.; Zhang, Q.; He, X.; Wang, L.; Xiong, Y.; Jiang, L.; Xie, Y. Global, Regional and National Burden and Trends of Larynx Cancer among Adults Aged 55 and Older from 1990 to 2021: Results from the Global Burden of Disease Study 2021. BMC Public. Health 2025, 25, 906. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Zhang, Q.-W.; Wen, K.; Wang, C.; Ji, X.; Zhang, L. Temporal Trends in Incidence and Mortality Rates of Laryngeal Cancer at the Global, Regional and National Levels, 1990–2017. BMJ Open 2021, 11, e050387. [Google Scholar] [CrossRef]
- Balogun, O.; Rodin, D.; Ngwa, W.; Grover, S.; Longo, J. Challenges and Prospects for Providing Radiation Oncology Services in Africa. Semin. Radiat. Oncol. 2017, 27, 184–188. [Google Scholar] [CrossRef]
- Chang, C.-P.; Chang, S.-C.; Chuang, S.-C.; Berthiller, J.; Ferro, G.; Matsuo, K.; Wünsch-Filho, V.; Toporcov, T.N.; de Carvalho, M.B.; La Vecchia, C. Age at Start of Using Tobacco on the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium (INHANCE). Cancer Epidemiol. 2019, 63, 101615. [Google Scholar] [CrossRef]
- Di Credico, G.; Edefonti, V.; Polesel, J.; Pauli, F.; Torelli, N.; Serraino, D.; Negri, E.; Luce, D.; Stucker, I.; Matsuo, K. Joint Effects of Intensity and Duration of Cigarette Smoking on the Risk of Head and Neck Cancer: A Bivariate Spline Model Approach. Oral. Oncol. 2019, 94, 47–57. [Google Scholar] [CrossRef] [PubMed]
- LoConte, N.K.; Brewster, A.M.; Kaur, J.S.; Merrill, J.K.; Alberg, A.J. Alcohol and Cancer: A Statement of the American Society of Clinical Oncology. J. Clin. Oncol. 2018, 36, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Berdzuli, N.; Ferreira-Borges, C.; Gual, A.; Rehm, J. Alcohol Control Policy in Europe: Overview and Exemplary Countries. Int. J. Environ. Res. Public Health 2020, 17, 8162. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E. Alcohol Consumption and Site-Specific Cancer Risk: A Comprehensive Dose–Response Meta-Analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- He, J.; Meng, H.; Yao, G.; Fu, Y.; Geng, J. New Research Progress of HPV and Larynx Cancer. Progress. Mod. Biomed. 2017, 17, 5989–5992. [Google Scholar]
- Peng, W.; Mi, J.; Jiang, Y. Asbestos Exposure and Laryngeal Cancer Mortality. Laryngoscope 2016, 126, 1169–1174. [Google Scholar] [CrossRef]
- Hall, A.L.; Kromhout, H.; Schüz, J.; Peters, S.; Portengen, L.; Vermeulen, R.; Agudo, A.; Ahrens, W.; Boffetta, P.; Brennan, P. Laryngeal Cancer Risks in Workers Exposed to Lung Carcinogens: Exposure–Effect Analyses Using a Quantitative Job Exposure Matrix. Epidemiology 2020, 31, 145–154. [Google Scholar] [CrossRef]
- Haddad, G.; Sataloff, R.T.; Hamdan, A.-L. Laryngeal Metastatic Lesions: A Literature Review. J. Voice 2024, 38, 1458–1464. [Google Scholar] [CrossRef]
- Santos, A.; Santos, I.C.; dos Reis, P.F.; Rodrigues, V.D.; Peres, W.A.F. Impact of Nutritional Status on Survival in Head and Neck Cancer Patients After Total Laryngectomy. Nutr. Cancer 2022, 74, 1252–1260. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Hoffmann, T.K. Systemic therapy strategies for head-neck carcinomas: Current status. Laryngo Rhino Otol. 2012, 91 (Suppl. 1), S23–S143. [Google Scholar] [CrossRef]
- Baird, B.J.; Sung, C.K.; Beadle, B.M.; Divi, V. Treatment of Early-Stage Laryngeal Cancer: A Comparison of Treatment Options. Oral. Oncol. 2018, 87, 8–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Fu, Q.; Han, Z.; Wang, D.; Umar Shinge, S.A.; Muluh, T.A.; Lu, X. Combined Immunotherapy and Targeted Therapies for Cancer Treatment: Recent Advances and Future Perspectives. Curr. Cancer Drug Targets 2023, 23, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.R.; Ferlito, A.; Devaney, K.O.; Mäkitie, A.A.; Rinaldo, A. Prognostic Factors in Laryngeal Squamous Cell Carcinoma. Laryngoscope Investig. Otolaryngol. 2020, 5, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Sauer, A.G.; Siegel, R.L.; Miller, K.D.; Islami, F.; Fedewa, S.A.; Jacobs, E.J.; Jemal, A. State-Level Cancer Mortality Attributable to Cigarette Smoking in the United States. JAMA Intern. Med. 2016, 176, 1792–1798. [Google Scholar] [CrossRef]
- Permitasari, A.L.; Satibi, S.; Kristina, S.A. National Burden of Cancers Attributable to Secondhand Smoking in Indonesia. Asian Pac. J. Cancer Prev. 2018, 19, 1951–1955. [Google Scholar]
- Allegra, E.; Bianco, M.R.; Ralli, M.; Greco, A.; Angeletti, D.; De Vincentiis, M. Role of Clinical-Demographic Data in Survival Rates of Advanced Laryngeal Cancer. Medicina 2021, 57, 267. [Google Scholar] [CrossRef]
- Voltzke, K.J.; Lee, Y.-C.A.; Zhang, Z.-F.; Zevallos, J.P.; Yu, G.-P.; Winn, D.M.; Vaughan, T.L.; Sturgis, E.M.; Smith, E.; Schwartz, S.M. Racial Differences in the Relationship between Tobacco, Alcohol, and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the INHANCE Consortium. Cancer Causes Control 2018, 29, 619–630. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global Alcohol Exposure between 1990 and 2017 and Forecasts until 2030: A Modelling Study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef]
- Grevers, X.; Ruan, Y.; Poirier, A.E.; Walter, S.D.; Villeneuve, P.J.; Friedenreich, C.M.; Brenner, D.R.; Team, C.S. Estimates of the Current and Future Burden of Cancer Attributable to Alcohol Consumption in Canada. Prev. Med. 2019, 122, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Szukalska, M.; Szyfter, K.; Florek, E.; Rodrigo, J.P.; Rinaldo, A.; Mäkitie, A.A.; Strojan, P.; Takes, R.P.; Suárez, C.; Saba, N.F.; et al. Electronic Cigarettes and Head and Neck Cancer Risk-Current State of Art. Cancers 2020, 12, 3274. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-H.; Shin, H.-S. Measurement of Aldehydes in Replacement Liquids of Electronic Cigarettes by Headspace Gas Chromatography-Mass Spectrometry. Bull. Korean Chem. Soc. 2013, 34, 2691–2696. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking; IARC: Lyon, France, 2004; ISBN 9283212835. [Google Scholar]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-Related Carcinogenesis in Head and Neck Cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef]
- Khariwala, S.S.; Carmella, S.G.; Stepanov, I.; Fernandes, P.; Lassig, A.A.; Yueh, B.; Hatsukami, D.; Hecht, S.S. Elevated Levels of 1-hydroxypyrene and N′-nitrosonornicotine in Smokers with Head and Neck Cancer: A Matched Control Study. Head Neck 2013, 35, 1096–1100. [Google Scholar] [CrossRef]
- Salturk, Z.; Çakır, Ç.; Sünnetçi, G.; Atar, Y.; Kumral, T.L.; Yıldırım, G.; Berkiten, G.; Uyar, Y. Effects of Electronic Nicotine Delivery System on Larynx: Experimental Study. J. Voice 2015, 29, 560–563. [Google Scholar] [CrossRef]
- Sheikh, M.; Shakeri, R.; Poustchi, H.; Pourshams, A.; Etemadi, A.; Islami, F.; Khoshnia, M.; Gharavi, A.; Roshandel, G.; Khademi, H. Opium Use and Subsequent Incidence of Cancer: Results from the Golestan Cohort Study. Lancet Glob. Health 2020, 8, e649–e660. [Google Scholar] [CrossRef]
- Mohebbi, E.; Hadji, M.; Rashidian, H.; Rezaianzadeh, A.; Marzban, M.; Haghdoost, A.A.; Naghibzadeh Tahami, A.; Moradi, A.; Gholipour, M.; Najafi, F. Opium Use and the Risk of Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2021, 148, 1066–1076. [Google Scholar] [CrossRef]
- Tsimplaki, E.; Argyri, E.; Sakellaridis, A.; Kyrodimos, E.; Xesfyngi, D.; Panotopoulou, E. Oropharyngeal and Laryngeal but Not Oral Cancers Are Strongly Associated with High-risk Human Papillomavirus in 172 Greek Patients. J. Med. Virol. 2017, 89, 170–176. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Nahet, A.; Boublenza, L.; Hassaine, H.; Masdoua, N.; Pretet, J.-L.; Belglaiaa, E.; Mougin, C. HPV DNA Genotyping: A Study of Anogenital, Head and Neck and Skin Cancers in a Population from West Algerian. HPV Detection in Different Cancers from an Algerian Population. Bull. Cancer 2016, 103, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kariche, N.; Hortal, M.T.; Benyahia, S.; Alemany, L.; Moulaï, N.; Clavero, O.; Muñoz, M.; Ouahioune, W.; Djennaoui, D.; Touil-Boukoffa, C. Comparative Assessment of HPV, Alcohol and Tobacco Etiological Fractions in Algerian Patients with Laryngeal Squamous Cell Carcinoma. Infect. Agents Cancer 2018, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chuang, H.-C.; Lin, Y.-T.; Huang, C.-C.; Chien, C.-Y. Clinical Impact of Human Papillomavirus in Laryngeal Squamous Cell Carcinoma: A Retrospective Study. PeerJ 2017, 5, e3395. [Google Scholar] [CrossRef]
- De Lima, M.A.P.; Silva, Á.D.L.; do Nascimento Filho, A.C.S.; Cordeiro, T.L.; Bezerra, J.P.d.S.; Rocha, M.A.B.; Pinheiro, S.d.F.L.; Pinheiro, R.F.F., Jr.; Gadelha, M.d.S.V.; da Silva, C.G.L. Epstein-Barr Virus-Associated Carcinoma of the Larynx: A Systematic Review with Meta-Analysis. Pathogens 2021, 10, 1429. [Google Scholar] [CrossRef]
- Vazquez-Guillen, J.M.; Palacios-Saucedo, G.C.; Alanis-Valdez, A.Y.; Huerta-Escobedo, A.; Zavala-Pompa, A.; Rivera-Morales, L.G.; Martinez-Torres, A.C.; Gonzalez-Villasana, V.; Serna-Hernandez, J.C.; Hernandez-Martinez, S.J. p16INK4a and pRb Expression in Laryngeal Squamous Cell Carcinoma with and without Infection by EBV or Different Genotypes of HPV: A Retrospective Study. Infect. Agents Cancer 2023, 18, 43. [Google Scholar] [CrossRef]
- Igissin, N.; Zatonskikh, V.; Telmanova, Z.; Tulebaev, R.; Moore, M. Laryngeal Cancer: Epidemiology, Etiology, and Prevention: A Narrative Review. Iran. J. Public Health 2023, 52, 2248–2259. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Yen, C.-J.; Hsiao, J.-R.; Ou, C.-Y.; Huang, J.-S.; Wong, T.-Y.; Tsai, S.-T.; Huang, C.-C.; Lee, W.-T.; Chen, K.-C. A Comprehensive Analysis on the Association between Tobacco-Free Betel Quid and Risk of Head and Neck Cancer in Taiwanese Men. PLoS ONE 2016, 11, e0164937. [Google Scholar] [CrossRef]
- Chang, C.; Siwakoti, B.; Sapkota, A.; Gautam, D.K.; Lee, Y.A.; Monroe, M.; Hashibe, M. Tobacco Smoking, Chewing Habits, Alcohol Drinking and the Risk of Head and Neck Cancer in Nepal. Int. J. Cancer 2020, 147, 866–875. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Yang, Y.; Zhou, L.; Tao, L. Association between Helicobacter Pylori Infection and Carcinoma of the Larynx or Pharynx. Head Neck 2016, 38, E2291–E2296. [Google Scholar] [CrossRef]
- Eells, A.C.; Mackintosh, C.; Marks, L.; Marino, M.J. Gastroesophageal Reflux Disease and Head and Neck Cancers: A Systematic Review and Meta-Analysis. Am. J. Otolaryngol. 2020, 41, 102653. [Google Scholar] [CrossRef]
- Parsel, S.M.; Wu, E.L.; Riley, C.A.; McCoul, E.D. Gastroesophageal and Laryngopharyngeal Reflux Associated with Laryngeal Malignancy: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Sereg-Bahar, M.; Jerin, A.; Hocevar-Boltezar, I. Higher Levels of Total Pepsin and Bile Acids in the Saliva as a Possible Risk Factor for Early Laryngeal Cancer. Radiol. Oncol. 2015, 49, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Han, K.; Joo, Y.-H. Metabolic Syndrome and Incidence of Laryngeal Cancer: A Nationwide Cohort Study. Sci. Rep. 2019, 9, 667. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kim, G.-J.; Han, K.; Joo, Y.-H. Changes in Metabolic Syndrome Status and Risk of Laryngeal Cancer: A Nationwide Cohort Study. PLoS ONE 2021, 16, e0252872. [Google Scholar] [CrossRef]
- Gong, H.-L.; Shi, Y.; Zhou, L.; Wu, C.-P.; Cao, P.-Y.; Tao, L.; Xu, C.; Hou, D.-S.; Wang, Y.-Z. The Composition of Microbiome in Larynx and the Throat Biodiversity between Laryngeal Squamous Cell Carcinoma Patients and Control Population. PLoS ONE 2013, 8, e66476. [Google Scholar] [CrossRef]
- Yu, S.; Chen, J.; Zhao, Y.; Yan, F.; Fan, Y.; Xia, X.; Shan, G.; Zhang, P.; Chen, X. Oral-Microbiome-Derived Signatures Enable Non-Invasive Diagnosis of Laryngeal Cancers. J. Transl. Med. 2023, 21, 438. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S.; et al. Composition and Function of Oral Microbiota between Gingival Squamous Cell Carcinoma and Periodontitis. Oral. Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Kelly, D.; Mulder, I.E. Microbiome and Immunological Interactions. Nutr. Rev. 2012, 70, S18–S30. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed]
- McMaughan, D.J.; Oloruntoba, O.; Smith, M.L. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front. Public Health 2020, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Fullmer, T.; Wilde, D.C.; Shi, J.W.; Wagner, T.; Skinner, H.; Eicher, S.A.; Sandulache, V.C.; Hernandez, D.J. Demographic and Tumor Characteristic Impact on Laryngeal Cancer Outcomes in a Minority Underserved Patient Population. Otolaryngol. Head Neck Surg. 2020, 162, 888–896. [Google Scholar] [CrossRef]
- Lu, Y.; Li, P.; Luo, G.; Liu, D.; Zou, H. Cancer Attributable to Human Papillomavirus Infection in China: Burden and Trends. Cancer 2020, 126, 3719–3732. [Google Scholar] [CrossRef]
- Buttmann-Schweiger, N.; Deleré, Y.; Klug, S.J.; Kraywinkel, K. Cancer Incidence in Germany Attributable to Human Papillomavirus in 2013. BMC Cancer 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Senkomago, V. Human Papillomavirus–Attributable Cancers—United States, 2012–2016. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef]
- Wang, N.; Lv, H.; Huang, M. Impact of Gender on Survival in Patients with Laryngeal Squamous Cell Carcinoma: A Propensity Score Matching Analysis. Int. J. Clin. Exp. Pathol. 2020, 13, 573–581. [Google Scholar]
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and Neck Cancer Prevention: From Primary Prevention to Impact of Clinicians on Reducing Burden. Ann. Oncol. 2019, 30, 744–756. [Google Scholar] [CrossRef]
- Andersson, T.M.L.; Engholm, G.; Pukkala, E.; Stenbeck, M.; Tryggvadottir, L.; Storm, H.; Weiderpass, E. Avoidable Cancers in the Nordic Countries—The Impact of Alcohol Consumption. Eur. J. Cancer 2018, 103, 299–307. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Zhu, Q.; Zhou, E.; Zhang, J.; Song, F.; Xu, C.; Shen, Y.; Zou, J.; Zhu, H.; et al. Global Trends and Risk Factors of Laryngeal Cancer: A Systematic Analysis for the Global Burden of Disease Study (1990–2021). BMC Cancer 2025, 25, 296. [Google Scholar] [CrossRef]
- Liberale, C.; Soloperto, D.; Marchioni, A.; Monzani, D.; Sacchetto, L. Updates on Larynx Cancer: Risk Factors and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 12913. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Husain, Q.; Kam, D.; Dubal, P.M.; Baredes, S.; Eloy, J.A. Laryngeal Papillary Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2015, 153, 54–59. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S.D. Non-Squamous Laryngeal Cancer. Otolaryngol. Clin. N. Am. 2023, 56, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Dubal, P.M.; Marchiano, E.; Kam, D.; Dutta, R.; Kalyoussef, E.; Baredes, S.; Eloy, J.A. Laryngeal Spindle Cell Carcinoma: A Population-Based Analysis of Incidence and Survival. Laryngoscope 2015, 125, 2709–2714. [Google Scholar] [CrossRef]
- Gerry, D.; Fritsch, V.A.; Lentsch, E.J. Spindle Cell Carcinoma of the Upper Aerodigestive Tract. Ann. Otol. Rhinol. Laryngol. 2014, 123, 576–583. [Google Scholar] [CrossRef]
- Fritsch, V.A.; Lentsch, E.J. Basaloid Squamous Cell Carcinoma of the Larynx: Analysis of 145 Cases with Comparison to Conventional Squamous Cell Carcinoma. Head Neck 2013, 36, 164–170. [Google Scholar] [CrossRef]
- Kass, J.I.; Lee, S.C.; Abberbock, S.; Seethala, R.R.; Duvvuri, U. Adenosquamous Carcinoma of the Head and Neck: Molecular Analysis Using CRTC-MAML FISH and Survival Comparison with Paired Conventional Squamous Cell Carcinoma. Laryngoscope 2015, 125, E371–E376. [Google Scholar] [CrossRef]
- Kusafuka, K.; Muramatsu, K.; Iida, Y.; Mori, K.; Miki, T.; Suda, T.; Fuke, T.; Kamijo, T.; Onitsuka, T.; Nakajima, T. MUC Expression in Adenosquamous Carcinoma of the Head and Neck Regions of Japanese Patients: Immunohistochemical Analysis. Pathol. Int. 2014, 64, 104–114. [Google Scholar] [CrossRef]
- Schick, U.; Pusztaszeri, M.; Betz, M.; Ghadjar, P.; Demiroz, C.; Kaanders, J.H.A.M.; Ozsahin, M. Adenosquamous Carcinoma of the Head and Neck: Report of 20 Cases and Review of the Literature. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 116, 313–320. [Google Scholar] [CrossRef]
- Jones, T.M.; De, M.; Foran, B.; Harrington, K.; Mortimore, S. Laryngeal Cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S75–S82. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Fawaz, S.A.; Sabri, S.M.; Sweed, A.; Rabie, H. Sensitivity and Specificity of Stroboscopy in Preoperative Differentiation of Dysplasia from Early Invasive Glottic Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Master, Z.; Kannan, S.; Agarwal, J.P.; Ghsoh-Laskar, S.; Rangarajan, V.; Murthy, V.; Budrukkar, A. Diagnostic Performance of Post-Treatment FDG PET or FDG PET/CT Imaging in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Sharma, N.; Raghavan, A. Role of Computed Tomography, Magnetic Resonance Imaging, and Endoscopy in Pretherapeutic Evaluation of Laryngeal Tumors. Astrocyte 2016, 2, 172. Available online: https://go.gale.com/ps/i.do?id=GALE%7CA466753779&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=23490977&p=AONE&sw=w&userGroupName=anon%7Ec07fdcb8&aty=open-web-entry (accessed on 14 March 2025). [CrossRef]
- De Vito, A.; Meccariello, G.; Vicini, C. Narrow Band Imaging as Screening Test for Early Detection of Laryngeal Cancer: A Prospective Study. Clin. Otolaryngol. 2016, 42, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, Plasma and Saliva Biomarkers for Head and Neck Cancer. Expert. Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined Circulating Tumor DNA and Protein Biomarker-Based Liquid Biopsy for the Earlier Detection of Pancreatic Cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Correction to: Liquid Biopsy and Tumor Heterogeneity in Metastatic Solid Tumors: The Potentiality of Blood Samples. J. Exp. Clin. Cancer Res. 2020, 39, 120. [Google Scholar] [CrossRef]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The Systemic Inflammation-Based Neutrophil–Lymphocyte Ratio: Experience in Patients with Cancer. Crit. Rev. Oncol./Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Iacob, R.; Mandea, M.; Iacob, S.; Pietrosanu, C.; Paul, D.; Hainarosie, R.; Gheorghe, C. Liquid Biopsy in Squamous Cell Carcinoma of the Esophagus and of the Head and Neck. Front. Med. 2022, 9, 827297. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Y.; Feng, L.-F.; Meng, X.; Chen, R.; Xu, W.-H.; Hou, J.; Xu, T.; Zhang, L. Liquid Biopsy in Head and Neck Squamous Cell Carcinoma: Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes. Expert. Rev. Mol. Diagn. 2020, 20, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Spruce, R.; Beggs, A.; Sharma, N.; Kong, A.; Martin, T.; Parmar, S.; Praveen, P.; Nankivell, P.; Mehanna, H. Circulating Tumor DNA as a Biomarker and Liquid Biopsy in Head and Neck Squamous Cell Carcinoma. Head Neck 2018, 40, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Vrba, L.; Oshiro, M.M.; Kim, S.S.; Garland, L.L.; Placencia, C.; Mahadevan, D.; Nelson, M.A.; Futscher, B.W. DNA Methylation Biomarkers Discovered in Silico Detect Cancer in Liquid Biopsies from Non-Small Cell Lung Cancer Patients. Epigenetics 2020, 15, 419–430. [Google Scholar] [CrossRef]
- Ooki, A.; Maleki, Z.; Tsay, J.-C.J.; Goparaju, C.; Brait, M.; Turaga, N.; Nam, H.-S.; Rom, W.N.; Pass, H.I.; Sidransky, D. A Panel of Novel Detection and Prognostic Methylated DNA Markers in Primary Non–Small Cell Lung Cancer and Serum DNA. Clin. Cancer Res. 2017, 23, 7141–7152. [Google Scholar] [CrossRef]
- Schröck, A.; Leisse, A.; de Vos, L.; Gevensleben, H.; Dröge, F.; Franzen, A.; Wachendörfer, M.; Schröck, F.; Ellinger, J.; Teschke, M. Free-Circulating Methylated DNA in Blood for Diagnosis, Staging, Prognosis, and Monitoring of Head and Neck Squamous Cell Carcinoma Patients: An Observational Prospective Cohort Study. Clin. Chem. 2017, 63, 1288–1296. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M.; Esteller, M.; Wu, L.; Nawroz-Danish, H.; Yoo, G.H.; Koch, W.M.; Jen, J.; Herman, J.G.; Sidransky, D. Gene Promoter Hypermethylation in Tumors and Serum of Head and Neck Cancer Patients. Cancer Res. 2000, 60, 892–895. [Google Scholar]
- Kawada, T.; Takahashi, H.; Sakakura, K.; Ida, S.; Mito, I.; Toyoda, M.; Chikamatsu, K. Circulating Tumor Cells in Patients with Head and Neck Squamous Cell Carcinoma: Feasibility of Detection and Quantitation. Head Neck 2017, 39, 2180–2186. [Google Scholar] [CrossRef]
- Nichols, A.C.; Lowes, L.E.; Szeto, C.C.T.; Basmaji, J.; Dhaliwal, S.; Chapeskie, C.; Todorovic, B.; Read, N.; Venkatesan, V.; Hammond, A.; et al. Detection of Circulating Tumor Cells in Advanced Head and Neck Cancer Using the Cellsearch System. Head Neck 2011, 34, 1440–1444. [Google Scholar] [CrossRef]
- Rizzo, M.I.; Ralli, M.; Nicolazzo, C.; Gradilone, A.; Carletti, R.; Di Gioia, C.; De Vincentiis, M.; Greco, A. Detection of Circulating Tumor Cells in Patients with Laryngeal Cancer Using ScreenCell: Comparative Pre- and Post-Operative Analysis and Association with Prognosis. Oncol. Lett. 2020, 19, 4183–4188. [Google Scholar] [CrossRef]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. miRNA-130a Significantly Improves Accuracy of SGA Nutritional Assessment Tool in Prediction of Malnutrition and Cachexia in Radiotherapy-Treated Head and Neck Cancer Patients. Cancers 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-C.; Song, L.-Q.; Xu, W.-W.; Qi, J.-J.; Wang, X.-Y.; Su, Y. Serum miR-632 Is a Potential Marker for the Diagnosis and Prognosis in Laryngeal Squamous Cell Carcinoma. Acta Oto-Laryngol. 2020, 140, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wang, X.; Yang, D.; Shi, W.J. The Expression of MicroRNA-155 in Plasma and Tissue Is Matched in Human Laryngeal Squamous Cell Carcinoma. Yonsei Med. J. 2016, 57, 298–305. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Lu, J.; Sun, Y.; Xiao, H.; Liu, M.; Tian, L. Combined Detection of Serum Exosomal miR-21 and HOTAIR as Diagnostic and Prognostic Biomarkers for Laryngeal Squamous Cell Carcinoma. Med. Oncol. 2014, 31, 148. [Google Scholar] [CrossRef]
- Cabanero, M.; Tsao, M.S. Circulating Tumour DNA in EGFR-Mutant Non-Small-Cell Lung Cancer. Curr. Oncol. 2018, 25, 38–44. [Google Scholar] [CrossRef]
- Mahmood, H.; Shaban, M.; Rajpoot, N.; Khurram, S.A. Artificial Intelligence-Based Methods in Head and Neck Cancer Diagnosis: An Overview. Br. J. Cancer 2021, 124, 1934–1940. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Zheng, B.; Su, L.; Chen, Y.; Ma, S.; Hu, Q.; Zou, X.; Yao, L.; Yang, Y.; et al. Rapid Histology of Laryngeal Squamous Cell Carcinoma with Deep-Learning Based Stimulated Raman Scattering Microscopy. Theranostics 2019, 9, 2541–2554. [Google Scholar] [CrossRef]
- Moccia, S.; De Momi, E.; Guarnaschelli, M.; Savazzi, M.; Laborai, A.; Guastini, L.; Peretti, G.; Mattos, L.S. Confident Texture-Based Laryngeal Tissue Classification for Early Stage Diagnosis Support. J. Med. Imaging (Bellingham Wash.) 2017, 4, 34502. [Google Scholar] [CrossRef]
- Choi, N.; Kim, J.; Yi, H.; Kim, H.; Kim, T.H.; Chung, M.J.; Ji, M.; Kim, Z.; Son, Y.-I. The Use of Artificial Intelligence Models to Predict Survival in Patients with Laryngeal Squamous Cell Carcinoma. Sci. Rep. 2023, 13, 9734. [Google Scholar] [CrossRef]
- Brandstorp-Boesen, J.; Sørum Falk, R.; Boysen, M.; Brøndbo, K. Impact of Stage, Management and Recurrence on Survival Rates in Laryngeal Cancer. PLoS ONE 2017, 12, e0179371. [Google Scholar] [CrossRef]
- Mulcahy, C.F.; Mohamed, A.S.R.; Kanwar, A.; Hutcheson, K.A.; Ghosh, A.; Vock, D.; Weber, R.S.; Lai, S.Y.; Gunn, G.B. Age-adjusted Comorbidity and Survival in Locally Advanced Laryngeal Cancer. Head Neck 2018, 40, 2060–2069. [Google Scholar] [PubMed]
- Egelmeer, A.G.T.M.; Velazquez, E.R.; de Jong, J.M.A.; Oberije, C.; Geussens, Y.; Nuyts, S.; Kremer, B.; Rietveld, D.; René Leemans, C.; de Jong, M.C.; et al. Development and Validation of a Nomogram for Prediction of Survival and Local Control in Laryngeal Carcinoma Patients Treated with Radiotherapy Alone: A Cohort Study Based on 994 Patients. Radiother. Oncol. 2011, 100, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Datema, F.R.; Ferrier, M.B.; Vergouwe, Y.; Moya, A.; Molenaar, J.; Piccirillo, J.F.; Baatenburg de Jong, R.J. Update and External Validation of a Head and Neck Cancer Prognostic Model. Head Neck 2012, 35, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, M.; Bisogno, A.; Scarpa, A.; D’Urso, A.; Marra, P.; Colacurcio, V.; De Luca, P.; Ralli, M.; Cassandro, E.; Cassandro, C. Biomarkers of Laryngeal Squamous Cell Carcinoma: A Review. Ann. Diagn. Pathol. 2021, 54, 151787. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Rubini, C.; Magliulo, G.; Re, M. Prognostic Value of Bcl-2 Expression in Squamous Cell Carcinoma of the Larynx: A Systematic Review. Int. J. Biol. Markers 2015, 30, 155–160. [Google Scholar] [CrossRef]
- Rashad, U.M.; Hussein, M.R.; Algizawy, S.M. Alterations of P53 and Bcl-2 Protein Expression in the Recurrent Laryngeal and Pharyngeal Squamous Cell Carcinoma. Am. J. Otolaryngol. 2011, 32, 210–214. [Google Scholar] [CrossRef]
- Yildirim, S.; Cermik, H.; Işitmangil, T.; Baloglu, H.; Gungor, A.; Pekkafali, Z. Significance of P53 and Bcl-2 Immunoexpression in the Prognosis of Laryngeal Squamous Cell Carcinoma. J. Int. Med. Res. 2002, 30, 597–600. [Google Scholar] [CrossRef]
- Ozdek, A.; Sarac, S.; Akyol, M.U.; Sungur, A.; Yilmaz, T. C-Myc and Bcl-2 Expression In Supraglottic Squamous Cell Carcinoma of the Larynx. Otolaryngol. Head Neck Surg. 2004, 131, 77–83. [Google Scholar] [CrossRef]
- Jakstas, T.; Bartnykaite, A.; Padervinskis, E.; Vegiene, A.; Juozaityte, E.; Uloza, V.; Ugenskiene, R. The Association of TP53, BCL2, BAX and NOXA SNPs and Laryngeal Squamous Cell Carcinoma Development. Int. J. Mol. Sci. 2024, 25, 11849. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 Expression in Head and Neck Cancer: A Systemic Review and Meta-Analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef]
- Trapasso, S.; Allegra, E. Role of CD44 as a Marker of Cancer Stem Cells in Head and Neck Cancer. Biol. Targets Ther. 2012, 6, 379–383. [Google Scholar] [CrossRef][Green Version]
- Joshua, B.; Kaplan, M.J.; Doweck, I.; Pai, R.; Weissman, I.L.; Prince, M.E.; Ailles, L.E. Frequency of Cells Expressing CD44, a Head and Neck Cancer Stem Cell Marker: Correlation with Tumor Aggressiveness. Head Neck 2011, 34, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Gioacchini, F.M.; Scarpa, A.; Cassandro, C.; Tulli, M.; Cassandro, E. The Prognostic Significance of E-Cadherin Expression in Laryngeal Squamous-Cell Carcinoma: A Systematic Review. Acta Otorhinolaryngol. Ital. 2018, 38, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Yang, X.; Li, S.; Liu, X.; Yang, Q.; Li, Y.; Ye, J. Reduced E-Cadherin Expression Is Associated with Lymph Node Metastases in Laryngeal Squamous Cell Carcinoma. Auris Nasus Larynx 2012, 39, 186–192. [Google Scholar] [CrossRef]
- Akdeniz, O.; Akduman, D.; Haksever, M.; Ozkarakas, H.; Müezzinoglu, B. Relationships between Clinical Behavior of Laryngeal Squamous Cell Carcinomas and Expression of VEGF, MMP-9 and E-Cadherin. Asian Pac. J. Cancer Prev. 2013, 14, 5301–5310. [Google Scholar] [CrossRef]
- Cappellesso, R.; Marioni, G.; Crescenzi, M.; Giacomelli, L.; Guzzardo, V.; Mussato, A.; Staffieri, A.; Martini, A.; Blandamura, S.; Fassina, A. The Prognostic Role of the Epithelial-Mesenchymal Transition Markers E-Cadherin and Slug in Laryngeal Squamous Cell Carcinoma. Histopathology 2015, 67, 491–500. [Google Scholar] [CrossRef]
- CDKN2A Gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/cdkn2a/ (accessed on 8 May 2025).
- Nowosad, A.; Besson, A. CDKN1B/P27 Regulates Autophagy via the Control of Ragulator and MTOR Activity in Amino Acid-Deprived Cells. Autophagy 2020, 16, 2297–2298. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Kaleci, S.; Magliulo, G.; Presutti, L.; Re, M. The Prognostic Value of Cyclin D1 Expression in Head and Neck Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2014, 273, 801–809. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Lin, J.; Hu, Y.; Luo, G.; Luo, Z.; Cheng, C.; Wang, P. Clinicopathologic and Prognostic Significance of Human Epidermal Growth Factor Receptor in Patients with Gastric Cancer: An Updated Meta-Analysis. Oncotarget 2017, 8, 17202–17215. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Magliulo, G.; Rubini, C.; Presutti, L.; Re, M. The Clinical Relevance of Ki-67 Expression in Laryngeal Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2014, 272, 1569–1576. [Google Scholar] [CrossRef]
- Mirza, S.; Jeannon, J.P.; Soames, J.; Wilson, J.A. Is Ki67 a Marker for the Transformation of Laryngeal Dysplasia to Carcinoma? Acta Oto-Laryngol. 2006, 126, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Acikalin, M.F.; Öner, Ü.; Tel, N.; Paşaoğlu, Ö.; Çakli, H.; Çolak, E. Prognostic Significance of Ki-67 Expression for Patients with Laryngeal Squamous Cell Carcinoma Primarily Treated by Total Laryngectomy. Eur. Arch. Oto-Rhino-Laryngol. 2003, 261, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Zizzi, A.; Ferrante, L.; Stramazzotti, D.; Goteri, G.; Gioacchini, F.M.; Olivieri, F.; Magliulo, G.; Rubini, C. P63 and Ki-67 Immunostainings in Laryngeal Squamous Cell Carcinoma Are Related to Survival. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. In The Enzymes; Academic Press: Cambridge, MA, USA, 2016; Volume 39, pp. 231–254. [Google Scholar]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The Role of Osteopontin in the Progression of Solid Organ Tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Bradford, C.R.; Kumar, B.; Bellile, E.; Lee, J.; Taylor, J.; D’Silva, N.; Cordell, K.; Kleer, C.; Kupfer, R.; Kumar, P.; et al. Biomarkers in Advanced Larynx Cancer. Laryngoscope 2014, 124, 179–187. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Teknos, T.N.; Cox, C.; Yoo, S.; Chepeha, D.B.; Wolf, G.T.; Bradford, C.R.; Carey, T.E.; Fisher, S.G. Elevated Serum Vascular Endothelial Growth Factor and Decreased Survival in Advanced Laryngeal Carcinoma. Head Neck 2002, 24, 1004–1011. [Google Scholar] [CrossRef]
- Parikh, R.R.; Yang, Q.; Haffty, B.G. Prognostic Significance of Vascular Endothelial Growth Factor Protein Levels in T1-2 N0 Laryngeal Cancer Treated with Primary Radiation Therapy. Cancer 2007, 109, 566–573. [Google Scholar] [CrossRef]
- Neuchrist, C.; Quint, C.; Pammer, A.; Burian, M. Vascular Endothelial Growth Factor (VEGF) and Microvessel Density in Squamous Cell Carcinomas of the Larynx: An Immunohistochemical Study. Acta Otolaryngol. 1999, 119, 732–738. [Google Scholar] [CrossRef]
- Cossu, A.M.; Mosca, L.; Zappavigna, S.; Misso, G.; Bocchetti, M.; De Micco, F.; Quagliuolo, L.; Porcelli, M.; Caraglia, M.; Boccellino, M. Long Non-Coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. Int. J. Mol. Sci. 2019, 20, 3444. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.M.; Johnson, A.M. Exploring the Mechanisms behind Long Noncoding RNAs and Cancer. Non-Coding RNA Res. 2018, 3, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, K.K.; Zhang, J.; Xiao, B.; Huang, Z.; Ju, C.; Sun, J.; Zhang, F.; Lv, X.-B.; Huang, G. The Decade of Exosomal Long RNA Species: An Emerging Cancer Antagonist. Mol. Cancer 2018, 17, 75. [Google Scholar] [CrossRef]
- Zvrko, E.; Mikic, A.; Vuckovic, L. CD105 Expression as a Measure of Microvessel Density in Supraglottic Laryngeal Squamous Cell Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1971–1976. [Google Scholar] [CrossRef]
- Zvrko, E.; Mikic, A.; Vuckovic, L. Clinicopathologic Significance of CD105-Assessed Microvessel Density in Glottic Laryngeal Squamous Cell Carcinoma. Auris Nasus Larynx 2010, 37, 77–83. [Google Scholar] [CrossRef]
- Marioni, G.; Blandamura, S.; Loreggian, L.; Koussis, H.; Lionello, M.; Giacomelli, L.; Fasanaro, E.; Lovato, A.; Staffieri, A. Laryngeal Carcinoma Prognosis after Postoperative Radiotherapy Correlates with CD105 Expression, but Not with Angiogenin or EGFR Expression. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1779–1787. [Google Scholar] [CrossRef]
- Villaronga, M.Á.; Hermida-Prado, F.; Granda-Díaz, R.; Menéndez, S.T.; Álvarez-Teijeiro, S.; Quer, M.; Vilaseca, I.; Allonca, E.; Garzón-Arango, M.; Sanz-Moreno, V.; et al. Immunohistochemical Expression of Cortactin and Focal Adhesion Kinase Predicts Recurrence Risk and Laryngeal Cancer Risk Beyond Histologic Grading. Cancer Epidemiol. Biomark. Prev. 2018, 27, 805–813. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Álvarez-Alija, G.; Menéndez, S.T.; Mancebo, G.; Allonca, E.; García-Carracedo, D.; Fresno, M.F.; Suárez, C.; García-Pedrero, J.M. Cortactin and Focal Adhesion Kinase as Predictors of Cancer Risk in Patients with Laryngeal Premalignancy. Cancer Prev. Res. 2011, 4, 1333–1341. [Google Scholar] [CrossRef]
- Marioni, G.; Lionello, M.; Marchese-Ragona, R.; Fasanaro, E.; Valentini, E.; Zanoletti, E.; Stritoni, P.; Ramacciotti, G.; Guzzardo, V.; Giacomelli, L.; et al. Cortactin and Phosphorylated Cortactin Tyr421 and Tyr466 Expression in Supraglottic Laryngeal Carcinomas and Lymph Node Metastases. Int. J. Biol. Markers 2017, 33, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gibcus, J.H.; Mastik, M.F.; Menkema, L.; de Bock, G.H.; Kluin, P.M.; Schuuring, E.; van der Wal, J.E. Cortactin Expression Predicts Poor Survival in Laryngeal Carcinoma. Br. J. Cancer 2008, 98, 950–955. [Google Scholar] [CrossRef]
- Fei, M.; Zhang, J.; Zhou, J.; Xu, Y.; Wang, J. Sex-Related Hormone Receptor in Laryngeal Squamous Cell Carcinoma: Correlation with Androgen Estrogen-α and Prolactin Receptor Expression and Influence of Prognosis. Acta Oto-Laryngol. 2017, 138, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kalyi, V.V.; Goncharuk-Khomyn, M.Y. Analysis of the Role and Impact of Sex Hormones in Diagnostics and Assessment of Clinical Progress of Laryngeal Cancer. Morphologia 2016, 10, 41–45. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, Evolution, and Mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Kretz, M. The More the Merrier-Complexity in Long Non-Coding RNA Loci. Front. Endocrinol. 2017, 8, 90. [Google Scholar] [CrossRef]
- Ahmed, W.A.; Suzuki, K.; Imaeda, Y.; Horibe, Y. Ki-67, P53 and Epidermal Growth Factor Receptor Expression in Early Glottic Cancer Involving the Anterior Commissure Treated with Radiotherapy. Auris Nasus Larynx 2008, 35, 213–219. [Google Scholar] [CrossRef]
- Aras, S.; Ozkanli, S.; Erdem, E.; Gokalp, S.; Erdogan, C.E. Investigation of Low and High Dose Rate X-Ray Effects on Histopathological Changes and Prognostic Importance of Ki-67 in Laryngeal Cancer Radiotherapy. Appl. Radiat. Isot. 2023, 197, 110823. [Google Scholar] [CrossRef]
- Sakata, K.; Oouchi, A.; Nagakura, H.; Akiba, H.; Tamakawa, M.; Koito, K.; Hareyama, M.; Asakura, K.; Satoh, M.; Ohtani, S. Accelerated Radiotherapy for T1, 2 Glottic Carcinoma: Analysis of Results with KI-67 Index. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 81–88. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hanjing, S.; Zhang, B.-H.; Luo, X.-Y. Expression of P16 and Ki67 in Laryngeal Squamous Cell Carcinoma and Their Clinical Significance. Front. Oncol. 2024, 14, 1430830. [Google Scholar] [CrossRef]
- DE Almeida, M.R.; Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Somoza-Martín, J.M.; García-García, A. P27(Kip1) Expression as a Prognostic Marker for Squamous Cell Carcinoma of the Head and Neck. Oncol. Lett. 2015, 10, 2675–2682. [Google Scholar] [CrossRef]
- Allegra, E.; Bianco, M.R.; Mignogna, C.; Caltabiano, R.; Grasso, M.; Puzzo, L. Role of P16 Expression in the Prognosis of Patients with Laryngeal Cancer: A Single Retrospective Analysis. Cancer Control 2021, 28, 10732748211033544. [Google Scholar] [CrossRef]
- Lee, L.-A.; Fang, T.-J.; Li, H.-Y.; Chuang, H.-H.; Kang, C.-J.; Chang, K.-P.; Liao, C.-T.; Chen, T.-C.; Huang, C.-G.; Yen, T.-C. Effects of Epstein-Barr Virus Infection on the Risk and Prognosis of Primary Laryngeal Squamous Cell Carcinoma: A Hospital-Based Case-Control Study in Taiwan. Cancers 2021, 13, 1741. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.V.e.; Caponio, V.C.A.; Camolesi, G.C.V.; Padín-Iruegas, M.E.; Lorenzo-Pouso, A.I.; Lima, K.C.; Vieira, S.L.S.; Chamorro-Petronacci, C.M.; Suaréz-Peñaranda, J.M.; Pérez-Sayáns, M. Correlation of Bcl-2 Expression with Prognosis and Survival in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol./Hematol. 2023, 187, 104021. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.K.; Zhen, G.; Agrawal, N. The Role of Tumor DNA as a Diagnostic Tool for Head and Neck Squamous Cell Carcinoma. Semin. Cancer Biol. 2019, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, A.; Mathe, E.; Kato, S.; Ishioka, C.; Tavtigian, S.V.; Hainaut, P.; Olivier, M. Impact of Mutant P53 Functional Properties on TP53 Mutation Patterns and Tumor Phenotype: Lessons from Recent Developments in the IARC TP53 Database. Human. Mutat. 2007, 28, 622–629. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The Molecular Biology of Head and Neck Cancer. Nat. Rev. Cancer 2010, 11, 9–22. [Google Scholar] [CrossRef]
- Poeta, M.L.; Manola, J.; Goldwasser, M.A.; Forastiere, A.; Benoit, N.; Califano, J.A.; Ridge, J.A.; Goodwin, J.; Kenady, D.; Saunders, J.; et al. TP53 Mutations and Survival in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2007, 357, 2552–2561. [Google Scholar] [CrossRef]
- Ioachim, E. Expression Patterns of Cyclins D1, E and Cyclin-Dependent Kinase Inhibitors P21waf1/Cip1, P27kip1 in Colorectal Carcinoma: Correlation with Other Cell Cycle Regulators (pRb, P53 and Ki-67 and PCNA) and Clinicopathological Features. Int. J. Clin. Pract. 2008, 62, 1736–1743. [Google Scholar] [CrossRef]
- Basyuni, S.; Nugent, G.; Ferro, A.; Barker, E.; Reddin, I.; Jones, O.; Lechner, M.; O’Leary, B.; Jones, T.; Masterson, L.; et al. Value of P53 Sequencing in the Prognostication of Head and Neck Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 20776. [Google Scholar] [CrossRef]
- Tan, A.; Eskiizmir, G.; Kamiloglu, U.; Sarioglu, S. P53 and PTEN Expression Evaluation with Molecular Evident Recent Criteria in Laryngeal Carcinoma. Medicine 2023, 102, e33676. [Google Scholar] [CrossRef]
- Chong, C.R.; Jänne, P.A. The Quest to Overcome Resistance to EGFR-Targeted Therapies in Cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef]
- Lionello, M.; Staffieri, A.; Marioni, G. Potential Prognostic and Therapeutic Role for Angiogenesis Markers in Laryngeal Carcinoma. Acta Oto-Laryngol. 2012, 132, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kontić, M.; Čolović, Z.; Paladin, I.; Gabelica, M.; Barić, A.; Pešutić-Pisac, V. Association between EGFR Expression and Clinical Outcome of Laryngeal HPV Squamous Cell Carcinoma. Acta Oto-Laryngol. 2019, 139, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Marijić, B.; Braut, T.; Babarović, E.; Krstulja, M.; Maržić, D.; Avirović, M.; Kujundžić, M.; Hadžisejdić, I. Nuclear EGFR Expression Is Associated with Poor Survival in Laryngeal Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.; Strzalka, B.; Kowalczyk, M.; Jurzak, M.; Mazurek, U.; Gierek, T.; Paluch, J.; Markowski, J.; Swiatkowska, L.; Weglarz, L. Transforming Growth Factor Beta Isoforms (TGF-Beta1, TGF-Beta2, TGF-Beta3) Messenger RNA Expression in Laryngeal Cancer. Am. J. Otolaryngol. 2008, 29, 233–237. [Google Scholar] [CrossRef]
- Mosch, B.; Reissenweber, B.; Neuber, C.; Pietzsch, J. Eph Receptors and Ephrin Ligands: Important Players in Angiogenesis and Tumor Angiogenesis. J. Oncol. 2010, 2010, 135285. [Google Scholar] [CrossRef]
- Shigyo, H.; Nonaka, S.; Katada, A.; Bandoh, N.; Ogino, T.; Katayama, A.; Takahara, M.; Hayashi, T.; Harabuchi, Y. Inducible Nitric Oxide Synthase Expression in Various Laryngeal Lesions in Relation to Carcinogenesis, Angiogenesis, and Patients’ Prognosis. Acta Oto-Laryngol. 2007, 127, 970–979. [Google Scholar] [CrossRef]
- Rueda, A.; Cazorla, O.; Pérez, L.; Álvarez, M.; Redondo, M.; Gallego, E.; Sáez, M.; Medina, J.A.; Solano, J.; Matilla, A. Vascular Endothelial Growth Factor and Vascular Endothelial Growth Factor Receptor-2 Tumor Expression in Patients with Advanced Laryngeal Cancer after Induction Chemotherapy for Organ Preservation. Head Neck 2010, 33, 808–816. [Google Scholar] [CrossRef]
- Bonhin, R.G.; Rocha, V.B.C.; Carvalho, G.M.d.; Guimarães, A.C.; Crespo, A.N.; Chone, C.T.; Amstalden, E.M.I. Correlation between Vascular Endothelial Growth Factor Expression and Presence of Lymph Node Metastasis in Advanced Squamous Cell Carcinoma of the Larynx. Braz. J. Otorhinolaryngol. 2015, 81, 58–62. [Google Scholar] [CrossRef]
- Yoshioka, N.; Wang, L.; Kishimoto, K.; Tsuji, T.; Hu, G. A Therapeutic Target for Prostate Cancer Based on Angiogenin-Stimulated Angiogenesis and Cancer Cell Proliferation. Proc. Natl. Acad. Sci. USA 2006, 103, 14519–14524. [Google Scholar] [CrossRef]
- Marioni, G.; Marino, F.; Blandamura, S.; D’Alessandro, E.; Giacomelli, L.; Guzzardo, V.; Lionello, M.; De Filippis, C.; Staffieri, A. Neoangiogenesis in Laryngeal Carcinoma: Angiogenin and CD105 Expression Is Related to Carcinoma Recurrence Rate and Disease-free Survival. Histopathology 2010, 57, 535–543. [Google Scholar] [CrossRef]
- Carico, E.; Radici, M.; Bucci, B.; Firrisi, L.; Fabiano, A.; Salerno, G.; Giovagnoli, M.R.; Vecchione, A. p16INK4/Ki-67 Dual-Staining Expression as a Prognostic Indicator in Laryngeal Cancer. J. Cancer Prev. Curr. Res. 2014, 1, 68–72. [Google Scholar] [CrossRef][Green Version]
- Verma, A.; Schwartz, N.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Estrogen Signaling and Estrogen Receptors as Prognostic Indicators in Laryngeal Cancer. Steroids 2019, 152, 108498. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Verma, A.; Muktipaty, C.; Bivens, C.; Schwartz, Z.; Boyan, B.D. Estradiol Receptor Profile and Estrogen Responsiveness in Laryngeal Cancer and Clinical Outcomes. Steroids 2019, 142, 34–42. [Google Scholar] [CrossRef]
- Ou, D.; Adam, J.; Garberis, I.; Blanchard, P.; Nguyen, F.; Levy, A.; Casiraghi, O.; Gorphe, P.; Breuskin, I.; Janot, F.; et al. Clinical Relevance of Tumor Infiltrating Lymphocytes, PD-L1 Expression and Correlation with HPV/P16 in Head and Neck Cancer Treated with Bio- or Chemo-Radiotherapy. Oncoimmunology 2017, 6, e1341030. [Google Scholar] [CrossRef]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Song, X.; Zeng, W.; Wang, S.; Chen, F.; Ding, H. The Prognostic Value of Systemic and Local Inflammation in Patients with Laryngeal Squamous Cell Carcinoma. OncoTargets Ther. 2016, 9, 7177–7185. [Google Scholar] [CrossRef]
- Tudor, F.; Marijić, B.; Babarović, E.; Hadžisejdić, I. Significance of PD-L1 and Tumor Microenvironment in Laryngeal Squamous Cell Cancer. Cancers 2024, 16, 2645. [Google Scholar] [CrossRef]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-Novo and Acquired Resistance to Immune Checkpoint Targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Ritprajak, P.; Azuma, M. Intrinsic and Extrinsic Control of Expression of the Immunoregulatory Molecule PD-L1 in Epithelial Cells and Squamous Cell Carcinoma. Oral. Oncol. 2015, 51, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; Fadous-Khalifé, M.C.; Tannous, R.; Fakhreddine, S.; Massoud, M.; Hadchity, J.; Aftimos, G.; Hadchity, E. Role of Krüppel-like Factor 4 and Heat Shock Protein 27 in Cancer of the Larynx. Mol. Clin. Oncol. 2017, 7, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Kaigorodova, E.V.; Zavyalova, M.V.; Bychkov, V.A.; Perelmuter, V.M.; Choynzonov, E.L. Functional State of the Hsp27 Chaperone as a Molecular Marker of an Unfavorable Course of Larynx Cancer. Cancer Biomark. 2016, 17, 145–153. [Google Scholar] [CrossRef]
- Lee, J.-H.; Sun, D.; Cho, K.-J.; Kim, M.-S.; Hong, M.-H.; Kim, I.-K.; Lee, J.-S.; Lee, J.-H. Overexpression of Human 27 kDa Heat Shock Protein in Laryngeal Cancer Cells Confers Chemoresistance Associated with Cell Growth Delay. J. Cancer Res. Clin. Oncol. 2006, 133, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; Conway de Macario, E.; et al. Quantitative Patterns of Hsps in Tubular Adenoma Compared with Normal and Tumor Tissues Reveal the Value of Hsp10 and Hsp60 in Early Diagnosis of Large Bowel Cancer. Cell Stress Chaperones 2016, 21, 927–933. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zhang, X.; Qiu, Y.; Huang, D.; Li, W.; Xiao, X.; Tian, Y. HSP70: A Promising Target for Laryngeal Carcinoma Radiaotherapy by Inhibiting Cleavage and Degradation of Nucleolin. J. Exp. Clin. Cancer Res. 2010, 29, 106. [Google Scholar] [CrossRef]
- Orasan, A.; Negru, M.-C.; Morgovan, A.I.; Fleser, R.C.; Sandu, D.; Sitaru, A.M.; Motofelea, A.-C.; Balica, N.C. Strategies to Mitigate Cisplatin-Induced Ototoxicity: A Literature Review of Protective Agents, Mechanisms, and Clinical Gaps. Audiol. Res. 2025, 15, 22. [Google Scholar] [CrossRef]
- Song, X.; Liao, Z.; Zhou, C.; Lin, R.; Lu, J.; Cai, L.; Tan, X.; Zeng, W.; Lu, X.; Zheng, W.; et al. HSP47 Is Associated with the Prognosis of Laryngeal Squamous Cell Carcinoma by Inhibiting Cell Viability and Invasion and Promoting Apoptosis. Oncol. Rep. 2017, 38, 2444–2452. [Google Scholar] [CrossRef][Green Version]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Ioachim, E.; Assimakopoulos, D.; Peschos, D.; Zissi, A.; Skevas, A.; Agnantis, N.J. Immunohistochemical Expression of Metallothionein in Benign Premalignant and Malignant Epithelium of the Larynx: Correlation with P53 and Proliferative Cell Nuclear Antigen. Pathol.—Res. Pract. 1999, 195, 809–814. [Google Scholar] [CrossRef]
- Nowinska, K.; Chmielewska, M.; Piotrowska, A.; Pula, B.; Pastuszewski, W.; Krecicki, T.; Podhorska-Okołow, M.; Zabel, M.; Dziegiel, P. Correlation between Levels of Expression of Minichromosome Maintenance Proteins, Ki-67 Proliferation Antigen and Metallothionein I/II in Laryngeal Squamous Cell Cancer. Int. J. Oncol. 2015, 48, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Krześlak, A.; Forma, E.; Olszewski, J.; Lewy-Trenda, I.; Osuch-Wójcikiewicz, E.; Bryś, M. Genetic Polymorphism of Metallothionein 2A and Risk of Laryngeal Cancer in a Polish Population. Med. Oncol. 2014, 31, 75. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; Celesia, A.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Giuliano, M. The Good and Bad of Nrf2: An Update in Cancer and New Perspectives in COVID-19. Int. J. Mol. Sci. 2021, 22, 7963. [Google Scholar] [CrossRef]
- Namani, A.; Matiur Rahaman, M.; Chen, M.; Tang, X. Gene-Expression Signature Regulated by the KEAP1-NRF2-CUL3 Axis Is Associated with a Poor Prognosis in Head and Neck Squamous Cell Cancer. BMC Cancer 2018, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, X.-R.; Ma, Y.-L.; Zhao, Z.-J.; Zhao, R.; Wang, Y.-Y. Nrf2 Promotes Esophageal Squamous Cell Carcinoma (ESCC) Resistance to Radiotherapy through the CaMKIIα-Associated Activation of Autophagy. Cell Biosci. 2020, 10, 90. [Google Scholar] [CrossRef]
- Sheth, S.; Farquhar, D.R.; Schrank, T.P.; Stepp, W.; Mazul, A.; Hayward, M.; Lenze, N.; Little, P.; Jo, H.; Major, M.B.; et al. Correlation of Alterations in the KEAP1/CUL3/NFE2L2 Pathway with Radiation Failure in Larynx Squamous Cell Carcinoma. Laryngoscope Investig. Otolaryngol. 2021, 6, 699–707. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Wang, S.; Zhu, J. Expression and Correlation of NRF2, KEAP1, NQO-1 and HO-1 in Advanced Squamous Cell Carcinoma of the Larynx and Their Association with Clinicopathologic Features. Mol. Med. Rep. 2016, 14, 5171–5179. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Bao, X.; Shi, J.; Liu, B.; Chen, Y.; Li, J. Nuclear Nrf2 Activity in Laryngeal Carcinoma Is Regulated by SENP3 After Cisplatin-Induced Reactive Oxygen Species Stress. J. Cancer 2019, 10, 3427–3434. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-Inflammatory Agents: An Update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Wei, Y.; Xu, H. Prognostic and Clinical Significance of Cyclooxygenase-2 Overexpression in Endometrial Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 1202. [Google Scholar] [CrossRef]
- Kourelis, K.; Vandoros, G.; Kourelis, T.; Papadas, T.; Goumas, P.; Sotiropoulou-Bonikou, G. Low COX2 in Tumor and Upregulation in Stroma Mark Laryngeal Squamous Cell Carcinoma Progression. Laryngoscope 2009, 119, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Luo, R.-Z.; Li, Y.; Cui, B.-K.; Song, M.; Yang, A.-K.; Chen, W.-K. High Expression Levels of COX-2 and P300 Are Associated with Unfavorable Survival in Laryngeal Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2013, 270, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Feng, J.; Luo, D.; Peng, L. Prognostic and Clinical Significance of COX-2 Overexpression in Laryngeal Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 854946. [Google Scholar] [CrossRef] [PubMed]
- Sackett, M.K.; Bairati, I.; Meyer, F.; Jobin, E.; Lussier, S.; Fortin, A.; Gélinas, M.; Nabid, A.; Brochet, F.; Têtu, B. Prognostic Significance of Cyclooxygenase-2 Overexpression in Glottic Cancer. Clin. Cancer Res. 2008, 14, 67–73. [Google Scholar] [CrossRef]
- Klatka, J.; Grywalska, E.; Hymos, A.; Guz, M.; Polberg, K.; Roliński, J.; Stepulak, A. Cyclooxygenase-2 Inhibition Enhances Proliferation of NKT Cells Derived from Patients with Laryngeal Cancer. Anticancer Res. 2017, 37, 4059–4066. [Google Scholar] [CrossRef][Green Version]
- Kinoshita, T.; Hanazawa, T.; Nohata, N.; Kikkawa, N.; Enokida, H.; Yoshino, H.; Yamasaki, T.; Hidaka, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumor Suppressive microRNA-218 Inhibits Cancer Cell Migration and Invasion through Targeting Laminin-332 in Head and Neck Squamous Cell Carcinoma. Oncotarget 2012, 3, 1386–1400. [Google Scholar] [CrossRef]
- Bruzgielewicz, A.; Osuch-Wojcikiewicz, E.; Niemczyk, K.; Sieniawska-Buccella, O.; Siwak, M.; Walczak, A.; Nowak, A.; Majsterek, I. Altered Expression of miRNAs Is Related to Larynx Cancer TNM Stage and Patients’ Smoking Status. DNA Cell Biol. 2017, 36, 581–588. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.-P.; Yang, D.; Zhang, G.; Zhou, H.-F. Clinical Significance of miR-149 in the Survival of Patients with Laryngeal Squamous Cell Carcinoma. BioMed Res. Int. 2016, 2016, 8561251. [Google Scholar] [CrossRef]
- Karatas, O.F. Antiproliferative Potential of miR-33a in Laryngeal Cancer Hep-2 Cells via Targeting PIM1. Head Neck 2018, 40, 2455–2461. [Google Scholar] [CrossRef]
- Chen, H.; Cai, X.; Du, B.; Cai, J.; Luo, Z. MicroRNA-150-5p Inhibits the Proliferation and Invasion of Human Larynx Epidermiod Cancer Cells Though Regulating Peptidyl-Prolyl Cis/Trans Isomerase. Braz. J. Otorhinolaryngol. 2023, 89, 383–392. [Google Scholar] [CrossRef]
- Luo, M.; Sun, G.; Sun, J. MiR-196b Affects the Progression and Prognosis of Human LSCC through Targeting PCDH-17. Auris Nasus Larynx 2019, 46, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, H. MicroRNA-143-3p Suppresses Cell Growth and Invasion in Laryngeal Squamous Cell Carcinoma via Targeting the k-Ras/Raf/MEK/ERK Signaling Pathway. Int. J. Oncol. 2018, 54, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.; de Carvalho, A.C.; Horst, M.A.; Carvalho, A.L.; Scapulatempo-Neto, C.; Vettore, A.L. Expression of miR-296-5p as Predictive Marker for Radiotherapy Resistance in Early-Stage Laryngeal Carcinoma. J. Transl. Med. 2015, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Oztürkcan, S.; Katilmiş, H.; Ozdemir, I.; Tuna, B.; Güvenç, I.A.; Dündar, R. Occult Contralateral Nodal Metastases in Supraglottic Laryngeal Cancer Crossing the Midline. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 117–120. [Google Scholar] [CrossRef]
- Koroulakis, A.; Agarwal, M. Laryngeal Cancer. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Sharbel, D.D.; Abkemeier, M.; Groves, M.W.; Albergotti, W.G.; Byrd, J.K.; Reyes-Gelves, C. Occult Metastasis in Laryngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Ann. Otol. Rhinol. Laryngol. 2021, 130, 67–77. [Google Scholar] [CrossRef]
- Khan, U.; MacKay, C.; Rigby, M.; Trites, J.; Corsten, M.; Taylor, S.M. Management of Positive Resection Margins Following Transoral Laser Microsurgery for Glottic Cancer. Laryngoscope Investig. Otolaryngol. 2023, 8, 1579–1583. [Google Scholar] [CrossRef]
- Kjems, J.; Zukauskaite, R.; Johansen, J.; Eriksen, J.G.; Lassen, P.; Andersen, E.; Andersen, M.; Farhadi, M.; Overgaard, J.; Vogelius, I.R. Distant Metastases in Squamous Cell Carcinoma of the Pharynx and Larynx: A Population-Based DAHANCA Study. Acta Oncol. 2021, 60, 1472–1480. [Google Scholar] [CrossRef]
- Van den Bovenkamp, K.; van der Vegt, B.; Halmos, G.B.; Slagter-Menkema, L.; Langendijk, J.A.; van Dijk, B.A.C.; Schuuring, E.; van der Laan, B.F.A.M. The Relation between Hypoxia and Proliferation Biomarkers with Radiosensitivity in Locally Advanced Laryngeal Cancer. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 3801–3809. [Google Scholar] [CrossRef]
- Jotic, A.; Milovanovic, J.; Savic-Vujovic, K.; Radin, Z.; Medic, B.; Folic, M.; Pavlovic, B.; Vujovic, A.; Dundjerovic, D. Immune Cell and Biochemical Biomarkers in Advanced Laryngeal Cancer. Dose Response 2022, 20, 15593258221115537. [Google Scholar] [CrossRef]
- Koca, T.; Cetmi, D.A.; Aksoy, R.; Korcum, A.F. The Predictive Role of Inflammatory Biomarkers in Patients with Larynx Cancer Undergoing Definitive Radiotherapy. Technol. Cancer Res. Treat. 2024, 23, 15330338241280433. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients with Locally Advanced Larynx Cancer. JCO 2013, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Harris, J.; Garden, A.S.; Chao, C.K.S.; Straube, W.; Harari, P.M. Multi-Institutional Trial of Accelerated Hypofractionated Intensity-Modulated Radiation Therapy for Early-Stage Oropharyngeal Cancer (RTOG 00-22). Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus Cetuximab or Cisplatin in Human Papillomavirus-Positive Oropharyngeal Cancer (NRG Oncology RTOG 1016): A Randomised, Multicentre, Non-Inferiority Trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; Castro, G. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- NCI. FDA Approves Nivolumab for Head and Neck Cancer. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2016/fda-nivolumab-scchn (accessed on 4 May 2025).
- Yen, C.-J.; Kiyota, N.; Hanai, N.; Takahashi, S.; Yokota, T.; Iwae, S.; Shimizu, Y.; Hong, R.-L.; Goto, M.; Kang, J.-H.; et al. Two-Year Follow-up of a Randomized Phase III Clinical Trial of Nivolumab vs. the Investigator’s Choice of Therapy in the Asian Population for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (CheckMate 141). Head Neck 2020, 42, 2852–2862. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.H.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus Standard-of-Care Chemoradiotherapy versus Chemoradiotherapy Alone in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomised, Double-Blind, Placebo-Controlled, Multicentre, Phase 3 Trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, N.Y. JAVELIN Head and Neck 100: A Phase III Trial of Avelumab and Chemoradiation for Locally Advanced Head and Neck Cancer. Future Oncol. 2019, 15, 687–694. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Tao, Y.; Licitra, L.; Burtness, B.; Tahara, M.; Rischin, D.; Alves, G.; Lima, I.P.F.; Hughes, B.G.M.; Pointreau, Y.; et al. Pembrolizumab plus Concurrent Chemoradiotherapy versus Placebo plus Concurrent Chemoradiotherapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-412): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2024, 25, 572–587. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2023, 41, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Wildsmith, S.; Fayette, J.; Harrington, K.; Gillison, M.; Ahn, M.-J.; Takahashi, S.; Weiss, J.; Machiels, J.-P.; Baxi, S.; et al. Outcomes in Biomarker-Selected Subgroups from the KESTREL Study of Durvalumab and Tremelimumab in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Immunother. 2024, 73, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, A.; Cohen, M.A.; Sherman, E.J.; Lee, N.Y. Past, Present and Future of Proton Therapy for Head and Neck Cancer. Oral. Oncol. 2020, 110, 104879. [Google Scholar] [CrossRef]
- Kang, B.-H.; Yu, T.; Kim, J.H.; Park, J.M.; Kim, J.-I.; Chung, E.-J.; Kwon, S.K.; Kim, J.H.; Wu, H.G. Early Closure of a Phase 1 Clinical Trial for SABR in Early-Stage Glottic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 104–109. [Google Scholar] [CrossRef]
- Chotipanich, A. Total Laryngectomy: A Review of Surgical Techniques. Cureus 2021, 13, e18181. [Google Scholar] [CrossRef]
- Campbell, G.; Glazer, T.A.; Kimple, R.J.; Bruce, J.Y. Advances in Organ Preservation for Laryngeal Cancer. Curr. Treat. Options Oncol. 2022, 23, 594–608. [Google Scholar] [CrossRef]
- Remacle, M.; Van Haverbeke, C.; Eckel, H.; Bradley, P.; Chevalier, D.; Djukic, V.; de Vicentiis, M.; Friedrich, G.; Olofsson, J.; Peretti, G.; et al. Proposal for Revision of the European Laryngological Society Classification of Endoscopic Cordectomies. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 709. [Google Scholar] [CrossRef]
- Canis, M.; Ihler, F.; Martin, A.; Wolff, H.A.; Matthias, C.; Steiner, W. Organ Preservation in T4a Laryngeal Cancer: Is Transoral Laser Microsurgery an Option? Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2719–2727. [Google Scholar] [CrossRef]
- Peretti, G.; Piazza, C.; Mora, F.; Garofolo, S.; Guastini, L. Reasonable Limits for Transoral Laser Microsurgery in Laryngeal Cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 135–139. [Google Scholar] [CrossRef]
- Day, A.T.; Sinha, P.; Nussenbaum, B.; Kallogjeri, D.; Haughey, B.H. Management of Primary T1–T4 Glottic Squamous Cell Carcinoma by Transoral Laser Microsurgery. Laryngoscope 2016, 127, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.G.; Ihler, F.; Wolff, H.A.; Schneider, S.; Canis, M.; Steiner, W.; Welz, C. Transoral Laser Microsurgery for Treatment for Hypopharyngeal Cancer in 211 Patients. Head Neck 2017, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Martin, A.; Ihler, F.; Wolff, H.A.; Kron, M.; Matthias, C.; Steiner, W. Results of Transoral Laser Microsurgery for Supraglottic Carcinoma in 277 Patients. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Cornu, N.; Hans, S.; Sadoughi, B.; Badoual, C.; Brasnu, D. Early Glottic Cancer Involving the Anterior Commissure Treated by Transoral Laser Cordectomy. Laryngoscope 2015, 126, 1817–1822. [Google Scholar] [CrossRef]
- Breda, E.; Catarino, R.; Monteiro, E. Transoral Laser Microsurgery for Laryngeal Carcinoma: Survival Analysis in a Hospital-based Population. Head Neck 2014, 37, 1181–1186. [Google Scholar] [CrossRef]
- Vilaseca, I.; Aviles-Jurado, F.X.; Valduvieco, I.; Berenguer, J.; Grau, J.J.; Baste, N.; Muxí, Á.; Castillo, P.; Lehrer, E.; Jordana, M. Transoral Laser Microsurgery in Locally Advanced Laryngeal Cancer: Prognostic Impact of Anterior versus Posterior Compartments. Head Neck 2021, 43, 3832–3842. [Google Scholar] [CrossRef]
- Hans, S.; Chekkoury-Idrissi, Y.; Circiu, M.P.; Distinguin, L.; Crevier-Buchman, L.; Lechien, J.R. Surgical, Oncological, and Functional Outcomes of Transoral Robotic Supraglottic Laryngectomy. Laryngoscope 2020, 131, 1060–1065. [Google Scholar] [CrossRef]
- Lechien, J.R.; Fakhry, N.; Saussez, S.; Chiesa-Estomba, C.-M.; Chekkoury-Idrissi, Y.; Cammaroto, G.; Melkane, A.E.; Barillari, M.R.; Crevier-Buchman, L.; Ayad, T.; et al. Surgical, Clinical and Functional Outcomes of Transoral Robotic Surgery for Supraglottic Laryngeal Cancers: A Systematic Review. Oral. Oncol. 2020, 109, 104848. [Google Scholar] [CrossRef]
- Lechien, J.R.; Baudouin, R.; Circiu, M.P.; Chiesa-Estomba, C.M.; Crevier-Buchman, L.; Hans, S. Transoral Robotic Cordectomy for Glottic Carcinoma: A Rapid Review. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 5449–5456. [Google Scholar] [CrossRef]
- Remacle, M.; Prasad, V.M.N. Preliminary Experience in Transoral Laryngeal Surgery with a Flexible Robotic System for Benign Lesions of the Vocal Folds. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 761–765. [Google Scholar] [CrossRef]
- Hans, S.; Chebib, E.; Lisan, Q.; Chekkoury-Idrissi, Y.; Distinguin, L.; Circiu, M.P.; Crevier-Buchman, L.; Lechien, J.R. Oncological, Surgical and Functional Outcomes of Transoral Robotic Cordectomy for Early Glottic Carcinoma. J. Voice 2023, 37, 801.e3–801.e7. [Google Scholar] [CrossRef] [PubMed]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An Update on Larynx Cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Zhao, S.; Eskander, A.; Rygalski, C.; Brock, G.; Parikh, A.S.; Haring, C.T.; Swendseid, B.; Zhan, K.Y.; Bradford, C.R. Stage Migration and Survival Trends in Laryngeal Cancer. Ann. Surg. Oncol. 2021, 28, 7300–7309. [Google Scholar] [CrossRef]
- Patel, T.R.; Eggerstedt, M.; Toor, J.; Tajudeen, B.A.; Husain, I.; Stenson, K.; Al-Khudari, S. Occult Lymph Node Metastasis in Early-stage Glottic Cancer in the National Cancer Database. Laryngoscope 2021, 131, E1139–E1146. [Google Scholar] [CrossRef]
- Nahavandipour, A.; Jakobsen, K.K.; Grønhøj, C.; Hebbelstrup Jensen, D.; Kim Schmidt Karnov, K.; Klitmøller Agander, T.; Specht, L.; von Buchwald, C. Incidence and Survival of Laryngeal Cancer in Denmark: A Nation-Wide Study from 1980 to 2014. Acta Oncol. 2019, 58, 977–982. [Google Scholar] [CrossRef]
- Petrakos, I.; Kontzoglou, K.; Nikolopoulos, T.P.; Papadopoulos, O.; Kostakis, A. Glottic and Supraglottic Laryngeal Cancer: Epidemiology, Treatment Patterns and Survival in 164 Patients. J. Buon 2012, 17, 700–705. [Google Scholar]
- Yang, F.; He, L.; Rao, Y.; Feng, Y.; Wang, J. Survival Analysis of Patients with Subglottic Squamous Cell Carcinoma Based on the SEER Database. Braz. J. Otorhinolaryngol. 2023, 88, S70–S80. [Google Scholar] [CrossRef]
- MacNeil, S.D.; Patel, K.; Liu, K.; Shariff, S.; Yoo, J.; Nichols, A.; Fung, K.; Garg, A.X. Survival of Patients with Subglottic Squamous Cell Carcinoma. Curr. Oncol. 2018, 25, e569. [Google Scholar] [CrossRef]
- Juan, X.; Jiali, H.; Ziqi, L.; Liqing, Z.; Han, Z. Development and Validation of Nomogram Models for Predicting Postoperative Prognosis of Early-Stage Laryngeal Squamous Cell Carcinoma. Curr. Probl. Cancer 2024, 49, 101079. [Google Scholar] [CrossRef]
- Shi, X.; Hu, W.-P.; Ji, Q.-H. Development of Comprehensive Nomograms for Evaluating Overall and Cancer-Specific Survival of Laryngeal Squamous Cell Carcinoma Patients Treated with Neck Dissection. Oncotarget 2017, 8, 29722–29740. [Google Scholar] [CrossRef]
- Qasem, M.; Qasem, N.; Kinshuck, A.; Milinis, K. Long-Term Laryngeal Function and Quality of Life Following Treatment of Early Glottic Cancer: A Meta-Analysis. Otolaryngol. Head Neck Surg. 2025, 172, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.-J.; Hung, L.-T.; Wang, L.-W.; Yang, M.-H.; Chu, P.-Y. Oncologic Results and Quality of Life in Patients with T3 Glottic Cancer after Transoral Laser Microsurgery. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Wulff, N.B.; Højager, A.; Wessel, I.; Dalton, S.O.; Homøe, P. Health-related Quality of Life Following Total Laryngectomy: A Systematic Review. Laryngoscope 2021, 131, 820–831. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).