Cytoimmunological Profile of Lower Airways in Post-COVID-19 Syndrome (PCS): Predictive Value of Bronchoalveolar Lavage
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- (1)
- Interstitial lesions with complete or partial resolution by 12 months after the first examination with predictive values of VC and DLCO greater than 80%; these patients were defined as PCS remission (n = 33).
- (2)
- Persistent interstitial lesions (defined as both non-fibrotic and fibrotic) observed at 12 months from baseline with associated ventilatory compromise (with VC and/or DLCO predicted values less than 80%); patients were defined as having PCS persistence (n = 25).
2.2. Bronchoalveolar Lavage (BAL)
2.3. BAL Lymphocyte Phenotyping and Flow Cytometry (FC)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA): BAL Cytokines/Chemokines
3. Statistics
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization. COVID-19 Epidemiological Update—24 December 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---24-december-2024 (accessed on 21 April 2025).
- Fernandez-de-Las-Peñas, C.; Notarte, K.I.; Macasaet, R.; Velasco, J.V.; Catahay, J.A.; Ver, A.T.; Chung, W.; Valera-Calero, J.A.; Navarro-Santana, M. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. 2024, 88, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Dorrian, D.; Linden, D.; Stanel, S.C.; Rivera-Ortega, P.; Chaudhuri, N. Pulmonary Sequelae of COVID-19: Focus on Interstitial Lung Disease. Cells 2023, 12, 2238. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. RECOVER Mechanistic Pathways Task Force. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86014. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Erratum in Nat. Rev. Microbiol. 2023, 21, 408. https://doi.org/10.1038/s41579-023-00896-0. [Google Scholar] [CrossRef]
- Sideratou, C.M.; Papaneophytou, C. Persisting Shadows: Unraveling the Impact of Long COVID-19 on Respiratory, Cardiovascular, and Nervous Systems. Infect. Dis. Rep. 2023, 15, 806–830. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Welson, N.N. Pathophysiology of Post-COVID syndromes: A new perspective. Virol. J. 2022, 19, 158. [Google Scholar] [CrossRef]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2021, 27, 328–337. [Google Scholar] [CrossRef]
- Myall, K.J.; Mukherjee, B.; Castanheira, A.M.; Lam, J.L.; Benedetti, G.; Mak, S.M.; Preston, R.; Thillai, M.; Dewar, A.; Molyneaux, P.L.; et al. Persistent Post-COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann. Am. Thorac. Soc. 2021, 18, 799–806. [Google Scholar] [CrossRef]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.E.; Goldberg, S.A.; Forman, C.A.; Munter, S.E.; Hoh, R.; Tai, V.; et al. Markers of Immune Activation and Inflammation in Individuals with Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 224, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Gagiannis, D.; Hackenbroch, C.; Bloch, W.; Zech, F.; Kirchhoff, F.; Djudjaj, S.; von Stillfried, S.; Bülow, R.; Boor, P.; Steinestel, K. Clinical, Imaging, and Histopathological Features of Pulmonary Sequelae after Mild COVID-19. Am. J. Respir. Crit. Care Med. 2023, 208, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Le Bert, N.; Tan, A.T. SARS-CoV-2-specific T cells in the changing landscape of the COVID-19 pandemic. Immunity 2022, 55, 1764–1778. [Google Scholar] [CrossRef]
- Kopiński, P.; Wypasek, E.; Senderek, T.; Wędrowska, E.; Wandtke, T.; Przybylski, G. Different expression of immune checkpoint markers on bronchoalveolar lavage CD4+ cells: A comparison between hypersensitivity pneumonitis and sarcoidosis. Pol. Arch. Intern. Med. 2021, 131, 16084. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, F.; Fotovat, L. Post acute COVID-19 syndrome (PACS)—Long COVID. Bioinformation 2022, 18, 908–911. [Google Scholar] [CrossRef]
- Shafqat, A.; Omer, M.H.; Albalkhi, I.; Alabdul Razzak, G.; Abdulkader, H.; Abdul Rab, S.; Sabbah, B.N.; Alkattan, K.; Yaqinuddin, A. Neutrophil extracellular traps and long COVID. Front. Immunol. 2023, 14, 1254310. [Google Scholar] [CrossRef]
- Tomassetti, S.; Ciani, L.; Luzzi, V.; Gori, L.; Trigiani, M.; Giuntoli, L.; Lavorini, F.; Poletti, V.; Ravaglia, C.; Torrego, A.; et al. Utility of bronchoalveolar lavage for COVID-19: A perspective from the Dragon consortium. Front. Med. 2024, 11, 1259570. [Google Scholar] [CrossRef]
- Lee, J.H.; Yim, J.-J.; Park, J. Pulmonary Function and Chest Computed Tomography Abnormalities 6–12 Months after Recovery from COVID-19: A Systematic Review and Meta-Analysis. Respir. Res. 2022, 23, 233. [Google Scholar] [CrossRef]
- Kopiński, P.; Wandtke, T.; Dyczek, A.; Wędrowska, E.; Roży, A.; Senderek, T.; Przybylski, G.; Chorostowska-Wynimko, J. Increased levels of interleukin 27 in patients with early clinical stages of non-small cell lung cancer. Pol. Arch. Intern. Med. 2018, 128, 105–114. [Google Scholar] [CrossRef]
- Davidson, K.R.; Ha, D.M.; Schwarz, M.I.; Chan, E.D. Bronchoalveolar lavage as a diagnostic procedure: A review of known cellular and molecular findings in various lung diseases. J. Thorac. Dis. 2020, 12, 4991–5019. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Bankier, A.A.; MacMahon, H.; Colby, T.; Gevenois, P.A.; Goo, J.M.; Leung, A.N.C.; Lynch, D.A.; Schaefer-Prokop, C.M.; Tomiyama, N.; Travis, W.D.; et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2024, 310, e232558. [Google Scholar] [CrossRef]
- Mehta, P.; Rosas, I.O.; Singer, M. Understanding Post-COVID-19 Interstitial Lung Disease (ILD): A New Fibroinflammatory Disease Entity. Intensive Care Med. 2022, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Tonkin, J.; Devaraj, A.; Philip, K.E.J.; Orton, C.M.; Desai, S.R.; Shah, P.L. CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge. Radiology 2022, 303, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-Month, 6-Month, 9-Month, and 12-Month Respiratory Outcomes in Patients Following COVID-19-Related Hospitalisation: A Prospective Study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Ruiu, P.; Paolucci, T.; Santilli, V.; Bernetti, A. Rehabilitation setting during and after COVID-19: An overview on recommendations. J. Rehabil. Med. 2021, 53, jrm00141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cojocaru, E.; Cojocaru, T.; Pînzariu, G.M.; Vasiliu, I.; Armașu, I.; Cojocaru, C. Perspectives on Post-COVID-19 Pulmonary Fibrosis Treatment. J. Pers. Med. 2023, 14, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceban, F.; Kulzhabayeva, D.; Rodrigues, N.B.; Di Vincenzo, J.D.; Gill, H.; Subramaniapillai, M.; Lui, L.M.W.; Cao, B.; Mansur, R.B.; Ho, R.C.; et al. COVID-19 vaccination for the prevention and treatment of long COVID: A systematic review and meta-analysis. Brain Behav. Immun. 2023, 111, 211–229, Erratum in Brain Behav. Immun. 2024, 115, 758. https://doi.org/10.1016/j.bbi.2023.09.020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šiklová, M.; Krauzová, E.; Svobodová, B.; Kračmerová, J.; Štěpán, M.; Koc, M.; Štich, V.; Rossmeislová, L. Circulating Monocyte and Lymphocyte Populations in Healthy First-Degree Relatives of Type 2 Diabetic Patients at Fasting and during Short-Term Hyperinsulinemia. Mediat. Inflamm. 2019, 2019, 1491083. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Fesu, D.; Polivka, L.; Barczi, E.; Foldesi, M.; Horvath, G.; Hidvegi, E.; Bohacs, A.; Muller, V. Post-COVID interstitial lung disease in symptomatic patients after COVID-19 disease. Inflammopharmacology 2023, 31, 565–571. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; Carmona, P.; Santisteve, S.; Monge, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Pinilla, L.; Carratalá, A.; Zuil, M.; et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest 2021, 160, 187–198. [Google Scholar] [CrossRef]
- Hallek, M.; Adorjan, K.; Behrends, U.; Ertl, G.; Suttorp, N.; Lehmann, C. Post-COVID Syndrome. Dtsch. Arztebl. Int. 2023, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.C.; Kemp, K.; Hayton, P.; Mudawi, D.; Wang, R.; Greaves, M.; Yioe, V.; Rivera-Ortega, P.; Avram, C.; Chaudhuri, N. Pulmonary Sequelae at 4 Months After COVID-19 Infection: A Single-Centre Experience of a COVID Follow-Up Service. Adv. Ther. 2021, 38, 4505–4519. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, R.J.; Bailey, J.I.; Sznajder, J.I.; Sala, M.A. Northwestern Comprehensive COVID Center Consortium. Recovering from a pandemic: Pulmonary fibrosis after SARS-CoV-2 infection. Eur. Respir. Rev. 2021, 30, 210194. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef]
- Gelarden, I.; Nguyen, J.; Gao, J.; Chen, Q.; Morales-Nebreda, L.; Wunderink, R.; Li, L.; Chmiel, J.S.; Hrisinko, M.; Marszalek, L.; et al. Comprehensive evaluation of bronchoalveolar lavage from patients with severe COVID-19 and correlation with clinical outcomes. Hum. Pathol. 2021, 113, 92–103. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Baron, A.; Hachem, M.; Tran Van Nhieu, J.; Botterel, F.; Fourati, S.; Carteaux, G.; De Prost, N.; Maitre, B.; Mekontso-Dessap, A.; Schlemmer, F. Bronchoalveolar Lavage in Patients with COVID-19 with Invasive Mechanical Ventilation for Acute Respiratory Distress Syndrome. Ann. Am. Thorac. Soc. 2021, 18, 723–726. [Google Scholar] [CrossRef]
- Voiriot, G.; Dorgham, K.; Bachelot, G.; Fajac, A.; Morand-Joubert, L.; Parizot, C.; Gerotziafas, G.; Farabos, D.; Trugnan, G.; Eguether, T.; et al. Identification of bronchoalveolar and blood immune-inflammatory biomarker signature associated with poor 28-day outcome in critically ill COVID-19 patients. Sci. Rep. 2022, 12, 9502. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020, 92, 1733–1734. [Google Scholar] [CrossRef]
- Cheon, I.S.; Son, Y.M.; Sun, J. Tissue-resident memory T cells and lung immunopathology. Immunol. Rev. 2023, 316, 63–83. [Google Scholar] [CrossRef]
- Vijayakumar, B.; Boustani, K.; Ogger, P.P.; Papadaki, A.; Tonkin, J.; Orton, C.M.; Ghai, P.; Suveizdyte, K.; Hewitt, R.J.; Desai, S.R.; et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity 2022, 55, 542–556.e5. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Kitaichi, M.; Itoh, H.; Nishimura, K.; Izumi, T.; Colby, T.V. Idiopathic nonspecific interstitial pneumonia/fibrosis: Comparison with idiopathic pulmonary fibrosis and BOOP. Eur. Respir. J. 1998, 12, 1010–1019, Erratum in Eur. Respir. J. 1999, 13, 711. https://doi.org/10.1183/09031936.98.12051010. [Google Scholar] [CrossRef]
- Chiappelli, F.; Fotovat, L. Virus interference in COVID-19. Bioinformation 2022, 18, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.J.; Yu, W.C.; Peng, G.R.; Ye, C.H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.C.; Yu, S.H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar] [CrossRef]
- Opsteen, S.; Files, J.K.; Fram, T.; Erdmann, N. The role of immune activation and antigen persistence in acute and long COVID. J. Investig. Med. 2023, 71, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef]
- Resende, G.G.; da Cruz Lage, R.; Lobê, S.Q.; Medeiros, A.F.; Costa ESilva, A.D.; Nogueira Sá, A.T.; Oliveira, A.J.A.; Sousa, D.; Guimarães, H.C.; Gomes, I.C.; et al. Blockade of interleukin seventeen (IL-17A) with secukinumab in hospitalized COVID-19 patients—The BISHOP study. Infect. Dis. 2022, 54, 591–599. [Google Scholar] [CrossRef]
- Hirata, T.; Osuga, Y.; Hamasaki, K.; Yoshino, O.; Ito, M.; Hasegawa, A.; Takemura, Y.; Hirota, Y.; Nose, E.; Morimoto, C.; et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology 2008, 149, 1260–1267. [Google Scholar] [CrossRef]
- MacDonald, L.; Alivernini, S.; Tolusso, B.; Elmesmari, A.; Somma, D.; Perniola, S.; Paglionico, A.; Petricca, L.; Bosello, S.L.; Carfì, A.; et al. COVID-19 and RA share an SPP1 myeloid pathway that drives PD-L1+ neutrophils and CD14+ monocytes. JCI Insight 2021, 6, e147413. [Google Scholar] [CrossRef] [PubMed]
- Oatis, D.; Herman, H.; Balta, C.; Ciceu, A.; Simon-Repolski, E.; Mihu, A.G.; Lepre, C.C.; Russo, M.; Trotta, M.C.; Gravina, A.G.; et al. Dynamic shifts in lung cytokine patterns in post-COVID-19 interstitial lung disease patients: A pilot study. Ther. Adv. Chronic Dis. 2024, 15, 20406223241236257. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Getz, M.; Macklin, P.; Versypt, A.N.F. An agent-based modeling approach for lung fibrosis in response to COVID-19. PLoS Comput. Biol. 2023, 19, e1011741. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, M.; Szepanowski, F.; Mausberg, A.K.; Asan, L.; Uslar, E.; Zwanziger, D.; Volbracht, L.; Stettner, M.; Kleinschnitz, C. Cytokines (IL1β, IL6, TNFα) and serum cortisol levels may not constitute reliable biomarkers to identify individuals with post-acute sequelae of COVID-19. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241229567. [Google Scholar] [CrossRef]
- Yang, O.O. The immunopathogenesis of SARS-CoV-2 infection: Overview of lessons learned in the first 5 years. J. Immunol. 2025, vkaf033. [Google Scholar] [CrossRef]
- Forsslund, H.; Mikko, M.; Karimi, R.; Grunewald, J.; Wheelock, Å.M.; Wahlström, J.; Sköld, C.M. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014, 145, 711–722. [Google Scholar] [CrossRef]
- Hoser, G.; Kawiak, J.; Domagała-Kulawik, J.; Kopiński, P.; Droszcz, W. Flow cytometric evaluation of lymphocyte subpopulations in BALF of healthy smokers and nonsmokers. Folia Histochem. Cytobiol. 1999, 37, 25–30. [Google Scholar]
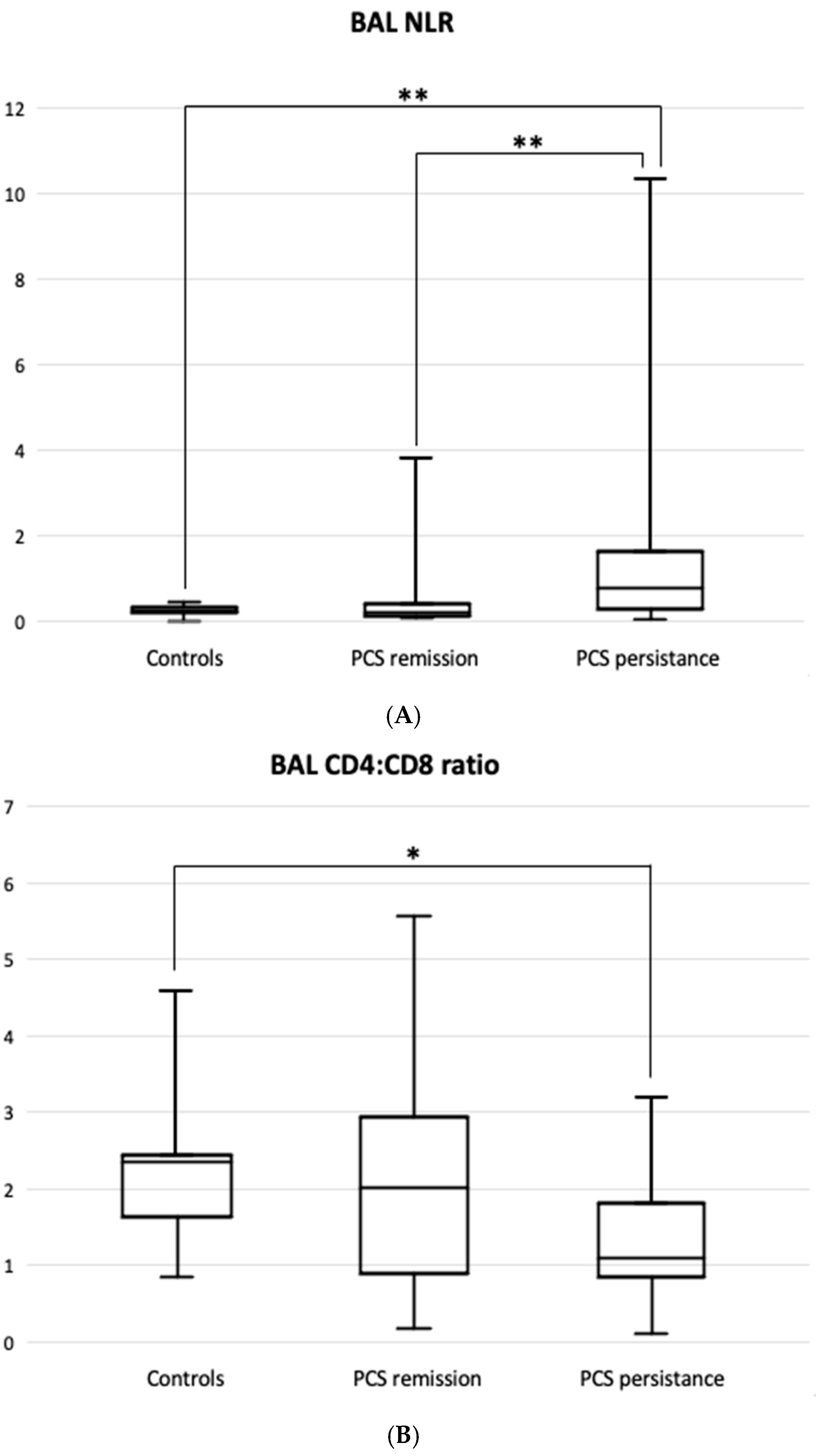
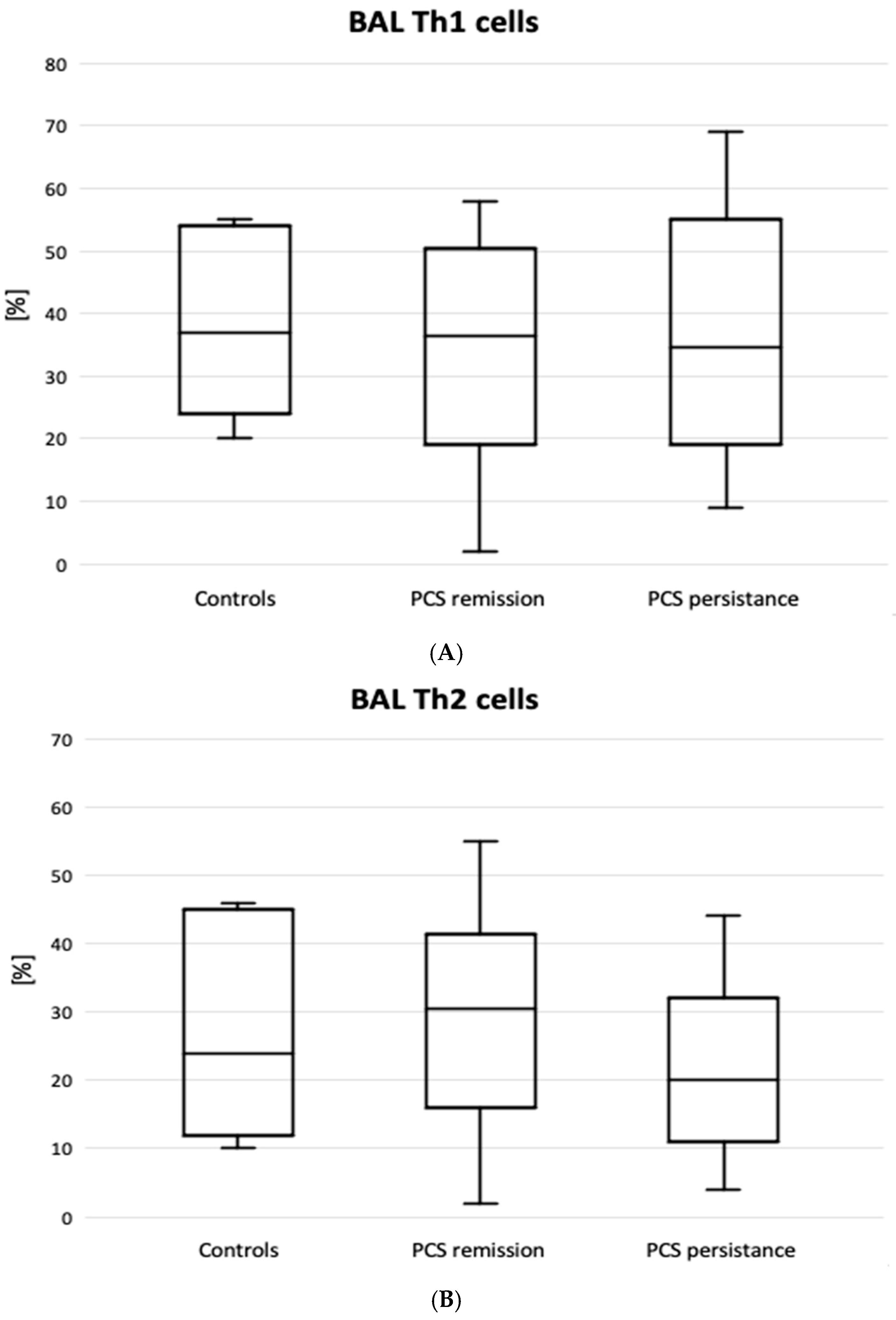
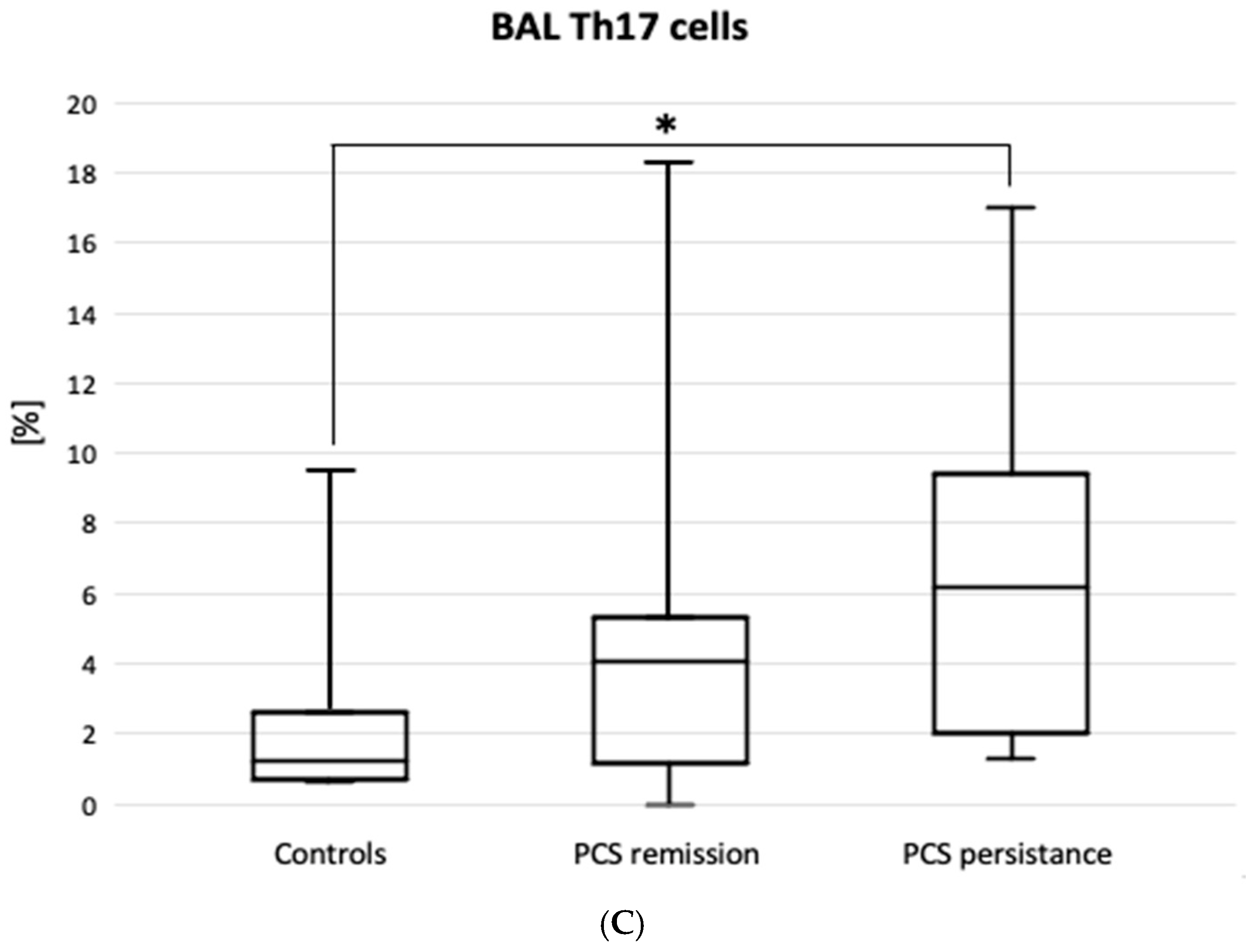
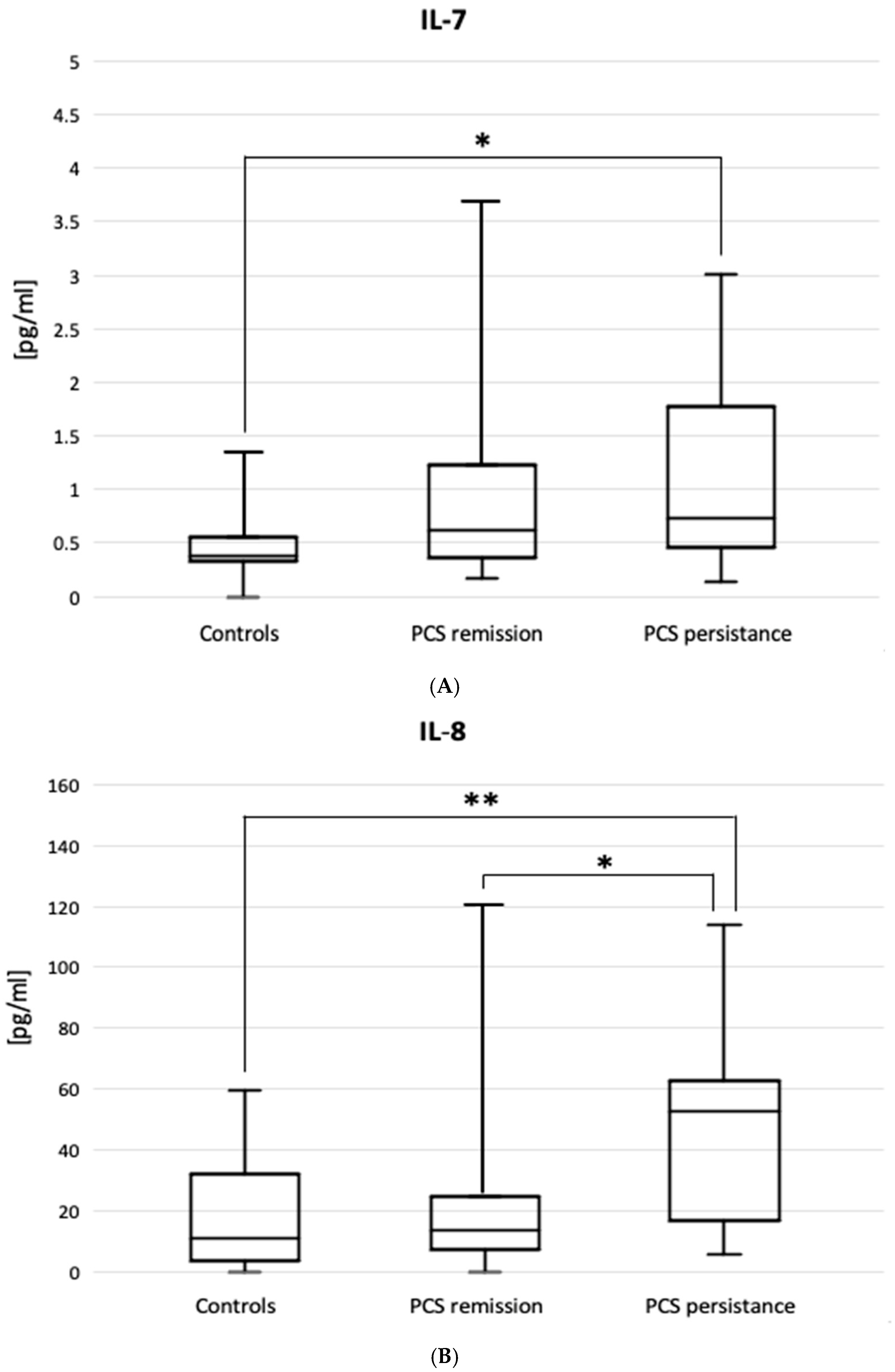

| Variable | Patients with PCS Remission (n = 33) | Patients with PCS Persistence (n = 25) | Total PCS Patients (n = 58) | Controls (n = 11) |
|---|---|---|---|---|
| Age, years | 55.1 ± 12.4 | 59.1 ± 11.1 | 57.7 ± 11.9 | 49.1 ± 16.4 |
| Sex (M) | 15 (45.5) | 19 (76.0) * | 34 (58.6) | 6 (54.5) |
| Acute COVID-19 disease form | ||||
| Mild | 7 (21.2) | 5 (20.0) | 12 (20.7) | 7 (63.6) |
| Moderate | 16 (48.5) | 13 (52.0) | 29 (50.0) | 1 (9.1) |
| Severe | 7 (21.2) | 4 (16.0) | 11 (19.0) | 1 (9.1) |
| Critical | 3 (9.1) | 3 (12.0) | 6 (10.3) | 0 |
| Weeks since the onset of acute COVID-19 # | 33 [20–44] | 25 [17–46] | 28 [18–43] | 38 [25–51] |
| Unvaccinated status | 4 (12.1) | 4 (16.0) | 8 (13.8) | 2 (18.2) |
| Comorbidities | ||||
| Arterial hypertension | 12 (36.3) | 12 (48.0) | 24 (41.4) | 3 (27.3) |
| Bronchial asthma | 4 (12.1) | 3 (12.0) | 7 (12.1) | 0 |
| Diabetes mellitus | 9 (27.2) | 9 (36.0) | 18 (31.0) | 0 |
| Obesity | 10 (30.3) | 6 (24.0) | 16 (27.5) | 1 (9.1) |
| Coronary artery disease | 11 (33.3) | 8 (32.0) | 19 (32.8) | 2 (18.2) |
| COPD | 1 (3.3) | 2 (8.0) | 3 (5.2) | 0 |
| More than one comorbidity | 11 (33.3) | 10 (40.0) | 21 (36.2) | 1 (9.1) |
| No comorbidity | 9 (27.2) | 5 (20.0) | 14 (24.1) | 10 (90.9) |
| Lung function tests | ||||
| VC, predicted value% | 100.9 ± 15.0 | 85.7 ± 15.0 | 97.6 ± 17.1 | 99.2 ± 14.5 |
| DLCO, predicted value% | 87.3 ± 12.7 | 58.7 ± 21.3 ** | 73.8 ± 24.6 * | 93.3 ± 10.9 |
| Laboratory investigations | ||||
| Blood Neutrophils, 103/µL | 4.3 [3.1–5.1] | 3.9 [3.3–6.5] | 4.2 [3.1–5.7] | 4.3 [3.1–5.1] |
| Blood Lymphocytes, 103/µL | 2.0 [1.6–2.4] | 1.9 [1.4–2.3] | 2.0 [1.5–2.4] | 2.0 [1.6–2.4] |
| Blood NLR | 2.0 [1.6–2.8] | 2.4 [1.7–3.9] | 2.1 [1.6–3.6] | 2.5 [1.9–4.0] |
| C-reactive protein, mg/L | 2.1 [0.9–4.9] | 3.8 [3.1–6.0] * | 3.1 [1.2–6.0] | 1.9 [0.6–3.9] |
| Variable | Patients with PCS Remission (n = 33) | Patients with PCS Persistence (n = 25) | Total PCS Patients (n = 58) | Controls (n = 11) |
|---|---|---|---|---|
| BAL fluid recovery% | 47 [43–60] | 46 [35–47] | 47 [41–60] | 47 [45–51.5] |
| Total cell count 103/mL | 270 [198–480] * | 320 [165–439] * | 315 [184–535] * | 118 [90–167] |
| Macrophages% | 78 [60–85] | 72 [49–82] * | 78 [49–85] | 84.5 [81–88] |
| Lymphocytes% | 17 [12–27] * | 13.5 [10–29] | 15.5 [10–27] | 12 [9.5–14] |
| Neutrophils% | 4.0 [1.9–5.9] | 7.9 [4.9–16] *** # | 5.1 [3.4–9.3] * | 2.4 [1.1–3.2] |
| Eosinophils% | 0.0 [0–1.1] | 0.9 [0.5–1.0] | 0.8 [0–1.3] | 0.0 [0–0.8] |
| Lymphocytes 103/mL | 47.5 [27–81.5] ** | 32 [16–89.5] * | 40.8 [20–89.5] * | 14.4 [12.7–21] |
| T cells, CD3+ 103/mL | 44.3 [26.5–78] ** | 21.8 [14.5–79.7] | 38.4 [17.2–79.7] * | 13.1 [10.7–17.5] |
| Th cells, CD4+ 103/mL | 25.1 [14.2–55.2] * | 11.7 [6.9–23.4] | 19.5 [9.1–53] * | 8.7 [6.7–12] |
| Tc cells, CD8+ 103/mL | 20.0 [8.2–25.2] * | 10.7 [5.4–27.6] * | 16.9 [7.6–27.6] * | 5.1 [3.4–5.6] |
| NK cells, 103/mL | 1.5 [0.7–2.7] * | 1.6 [0.7–4.2] * | 1.6 [0.7–4.4] * | 0.4 [0.3–0.9] |
| Variable | Patients with PCS Remission (n = 33) | Patients with PCS Persistence (n = 25) | Total PCS Patients (n = 58) | Controls (n = 11) |
|---|---|---|---|---|
| CD4+PD1+% | 31.5 [5.7–68] | 31.5 [16–89] | 32 [5.7–89] | 26 [10–52] |
| CD4+PDL1+% | 8.1 [6.4–13] | 7.0 [4.2–6.9] | 7.2 [4.2–13] | 5.1 [0–7.7] |
| CD4+CTLA4+% | 8.0 [5.4–13.5] | 7.2 [5.9–8.0] | 7.5 [4.9–12] | 8.3 [6.9–10] |
| CD8+PD1+% | 35.5 [28–41] | 43.5 [34–60.5] * | 41 [28–49] | 24.5 [22–44] |
| CD8+PDL1+% | 3.9 [1.9–6.0] | 2 [1.0–3.3] | 2.4 [1.0–5.3] | 1.9 [1.5–2.9] |
| CD8+CTLA4+% | 7.9 [5.9–13] | 6.8 [5.0–7.8] | 7.1 [5.0–11] | 8.1 [5.8–10] |
| Activated T cells CD3+HLA-DR+% | 31 [24–36] | 28.5 [19–39] | 30.5 [20–36] * | 23.5 [15–36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolna-Michno, J.; Kopiński, P.; Przybylski, G.; Wypasek, E.; Szymańska, M.; Wędrowska, E.; Mikołajczyk, K.; Senderek, T.; Gagat, M. Cytoimmunological Profile of Lower Airways in Post-COVID-19 Syndrome (PCS): Predictive Value of Bronchoalveolar Lavage. J. Clin. Med. 2025, 14, 3361. https://doi.org/10.3390/jcm14103361
Dolna-Michno J, Kopiński P, Przybylski G, Wypasek E, Szymańska M, Wędrowska E, Mikołajczyk K, Senderek T, Gagat M. Cytoimmunological Profile of Lower Airways in Post-COVID-19 Syndrome (PCS): Predictive Value of Bronchoalveolar Lavage. Journal of Clinical Medicine. 2025; 14(10):3361. https://doi.org/10.3390/jcm14103361
Chicago/Turabian StyleDolna-Michno, Justyna, Piotr Kopiński, Grzegorz Przybylski, Ewa Wypasek, Magdalena Szymańska, Ewelina Wędrowska, Klaudia Mikołajczyk, Tomasz Senderek, and Maciej Gagat. 2025. "Cytoimmunological Profile of Lower Airways in Post-COVID-19 Syndrome (PCS): Predictive Value of Bronchoalveolar Lavage" Journal of Clinical Medicine 14, no. 10: 3361. https://doi.org/10.3390/jcm14103361
APA StyleDolna-Michno, J., Kopiński, P., Przybylski, G., Wypasek, E., Szymańska, M., Wędrowska, E., Mikołajczyk, K., Senderek, T., & Gagat, M. (2025). Cytoimmunological Profile of Lower Airways in Post-COVID-19 Syndrome (PCS): Predictive Value of Bronchoalveolar Lavage. Journal of Clinical Medicine, 14(10), 3361. https://doi.org/10.3390/jcm14103361






