Long-Term Health-Related Quality of Life Following Survival of Acute Respiratory Distress Syndrome and Extracorporeal Membrane Oxygenation Due to COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sites
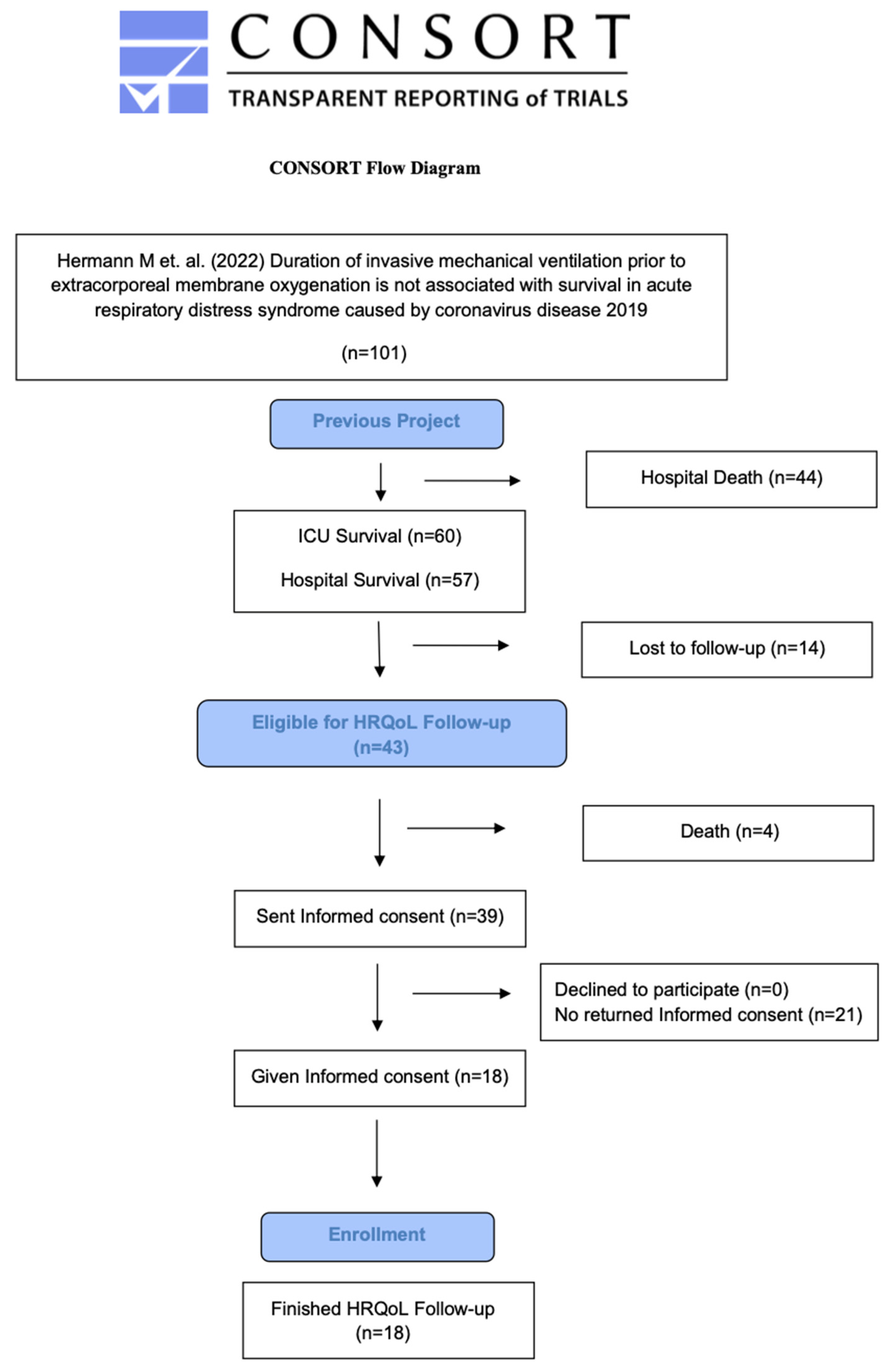
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Endpoints, Questionnaire and Interview
2.6. Data Collection
2.7. Statistical Methods
3. Results
3.1. Descriptive Data
3.2. HRQOL (SF-36 Scores)
3.3. HRQOL Outcomes Compared to a Non-COVID-19 ECMO Survivor Post-ICU Patients
3.3.1. Demographic Data
3.3.2. ICU-Related Data
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ARDS | Acute respiratory distress syndrome |
| BMI | Body mass index |
| COVID-19 | Coronavirus disease 2019 |
| ECMO | Extracorporeal membrane oxygenation |
| EQ-5D | EuroQOL-5D |
| HRQOL | Health-related quality of life |
| ICU | Intensive care unit |
| IMV | Invasive mechanical ventilation |
| IQR | Interquartile range |
| LOS | Length of stay |
| NMBA | Neuromuscular blocking agent |
| PCS | Post-acute COVID-19 syndrome |
| PICS | Post-intensive care syndrome |
| PTSD | Posttraumatic stress disorder |
| SAPS III | Simplified Acute Physiology Score III |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SD | Standard deviation |
| SF-36 | 36-Item Short-Form Health Survey |
References
- Bachner, D.; Rainer, L.; Zuba, M. Fact-Sheet Intensivpflege und COVID; Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz: Vienna, Austria, 2020. [Google Scholar]
- Müller, O.; Neuhann, F.; Razum, O. Epidemiology and control of COVID-19. Dtsch. Med. Wochenschr. 2020, 145, 670–674. [Google Scholar] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long covid symptoms after covid-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef] [PubMed]
- Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 189. [Google Scholar] [CrossRef]
- Nascimento, T.; do Valle Costa, L.; Ruiz, A.D.; Ledo, C.B.; Fernandes, V.P.L.; Cardoso, L.F.; Junior, J.M.V.; Saretta, R.; Kalil-Filho, R.; Drager, L.F. Vaccination status and long COVID symptoms in patients discharged from hospital. Sci. Rep. 2023, 13, 2481. [Google Scholar] [CrossRef]
- Singh, K.; Prabhakaran, D.; Nikhare, K.; Raspail, L.; Banerjee, A.; Narula, J.; Pineiro, D.; Perel, P.; Sliwa Hahnle, K. Vaccination status and long COVID symptoms among patients hospitalized with COVID-19: The WHF COVID-19 Long-term Study. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.3598. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving sepsis campaign: Guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19). Crit. Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef]
- Shekar, K.; Badulak, J.; Peek, G.; Boeken, U.; Dalton, H.J.; Arora, L.; Zakhary, B.; Ramanathan, K.; Starr, J.; Akkanti, B.; et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: A consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020, 66, 707–721. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, G.; Fisher, D.; Brodie, D. Preparing for the most critically Ill patients with COVID-19: The potential role of extracorporeal membrane oxygenation. JAMA 2020, 323, 1245–1246. [Google Scholar] [CrossRef]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; MacLaren, G.; Brodie, D.; Shekar, K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir. Med. 2020, 8, 518–526. [Google Scholar] [CrossRef]
- Jackson, J.C.; Pandharipande, P.P.; Girard, T.D.; Brummel, N.E.; Thompson, J.L.; Hughes, C.G.; Pun, B.T.; E Vasilevskis, E.; Morandi, A.; Shintani, A.K.; et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: A longitudinal cohort study. Lancet Respir. Med. 2014, 2, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.F.; Thomsen, T.; Overgaard, D.; Bestle, M.H.; Christensen, D.; Egerod, I. Impact of follow-up consultations for ICU survivors on post-ICU syndrome: A systematic review and meta-analysis. Intensive Care Med. 2015, 41, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, J.; Jeong, Y.J. Risk factors for post–intensive care syndrome: A systematic review and meta-analysis. Aust. Crit. Care 2020, 33, 287–294. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-a systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef]
- Vrettou, C.S.; Mantziou, V.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Post-intensive care syndrome in survivors from critical illness including COVID-19 patients: A narrative review. Life 2022, 12, 107. [Google Scholar] [CrossRef]
- Ambler, M.; Rhoads, S.; Peterson, R.; Jin, Y.; Armstrong, P.; Collier, P.; Cruse, M.H.; Csikesz, N.; Hua, M.; Engelberg, R.A.; et al. One year later: Family members of patients with COVID-19 experience persistent symptoms of posttraumatic stress disorder. Ann. Am. Thorac. Soc. 2023, 20, 713–720. [Google Scholar] [CrossRef]
- Fuke, R.; Hifumi, T.; Kondo, Y.; Hatakeyama, J.; Takei, T.; Yamakawa, K.; Inoue, S.; Nishida, O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: A systematic review and meta-analysis. BMJ Open 2018, 8, e019998. [Google Scholar] [CrossRef]
- Bullinger, M.; Kirchberger, I. SF-36. Fragebogen zum Gesundheitszustand; Hogrefe: Göttingen, Germany, 1998. [Google Scholar]
- Szende, A.; Oppe, M.; Devlin, N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli Against COVID-Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Nakanishi, N.; Amaya, F.; Fujinami, Y.; Hatakeyama, J.; Hifumi, T.; Iida, Y.; Kawakami, D.; Kawai, Y.; Kondo, Y.; et al. Post-intensive care syndrome: Recent advances and future directions. Acute Med. Surg. 2024, 11, e929. [Google Scholar] [CrossRef] [PubMed]
- Hiser, S.L.; Fatima, A.; Ali, M.; Needham, D.M. Post-intensive care syndrome (PICS): Recent updates. J. Intensive Care 2023, 11, 23. [Google Scholar] [CrossRef]
- Chommeloux, J.; Valentin, S.; Winiszewski, H.; Adda, M.; Pineton de Chambrun, M.; Moyon, Q.; Mathian, A.; Capellier, G.; Guervilly, C.; Levy, B.; et al. One-Year Mental and Physical Health Assessment in Survivors after Extracorporeal Membrane Oxygenation for COVID-19-related Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2023, 207, 150–159. [Google Scholar] [CrossRef]
- Hodgson, C.L.; Hayes, K.; Everard, T.; Nichol, A.; Davies, A.R.; Bailey, M.J.; Tuxen, D.V.; Cooper, D.J.; Pellegrino, V. Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit. Care 2012, 16, R202. [Google Scholar] [CrossRef]
- Knudson, K.A.; Funk, M.; Redeker, N.S.; Andrews, L.K.; Whittemore, R.; Mangi, A.A.; Sadler, L.S. An unbelievable ordeal: The experiences of adult survivors treated with extracorporeal membrane oxygenation. Aust. Crit. Care 2022, 35, 391–401. [Google Scholar] [CrossRef]
- Kurniawati, E.R.; Rutjens, V.G.H.; Vranken, N.P.A.; Delnoij, T.S.R.; Lorusso, R.; van der Horst, I.C.C.; Maessen, J.G.; Weerwind, P.W. Quality of life following adult veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review. Qual. Life Res. 2021, 30, 2123–2135. [Google Scholar] [CrossRef]
- Rilinger, J.; Krotzsch, K.; Bemtgen, X.; Jackel, M.; Zotzmann, V.; Lang, C.N.; Kaier, K.; Duerschmied, D.; Supady, A.; Bode, C.; et al. Long-term survival and health-related quality of life in patients with severe acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation support. Crit. Care 2021, 25, 410. [Google Scholar] [CrossRef]
- Stoll, C.; Haller, M.; Briegel, J.; Meier, M.; Manert, W.; Hummel, T.; Heyduck, M.; Lenhart, A.; Polasek, J.; Bullinger, M.; et al. Health-related quality of life. Long-term survival in patients with ARDS following extracorporeal membrane oxygenation (ECMO). Anaesthesist 1998, 47, 24–29. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Hermann, M.; Laxar, D.; Krall, C.; Hafner, C.; Herzog, O.; Kimberger, O.; Koenig, S.; Kraft, F.; Maleczek, M.; Markstaller, K.; et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann. Intensive Care 2022, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.P.; Metnitz, P.G.H.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.-R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef]
- Turgeon, J.; Venkatamaran, V.; Englesakis, M.; Fan, E. Long-term outcomes of patients supported with extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Intensive Care Med. 2024, 50, 350–370. [Google Scholar] [CrossRef] [PubMed]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R-Core-Team: Vienna, Austria, 2021. [Google Scholar]
- R-Studio-Team. Integrated Development for R; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ’ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe COVID-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef]
- Farooqi, M.; Khan, A.; Jacobs, A.; D’Souza, V.; Consiglio, F.; Karmen, C.L.; Dornbush, R.; Hasnat, G.S.; Ferrando, S.J. Examining the long-term sequelae of SARS-CoV-2 infection in patients seen in an outpatient psychiatric department. Neuropsychiatr. Dis. Treat. 2022, 18, 1259–1268. [Google Scholar] [CrossRef]
- Kazi, A.W.; Summer, R.; Sundaram, B.; George, G. Lung recovery with prolonged ECMO following fibrotic COVID-19 acute respiratory distress syndrome. Am. J. Med. Sci. 2023, 365, 307–312. [Google Scholar] [CrossRef]
- Palakshappa, J.A.; Krall, J.T.W.; Belfield, L.T.; Files, D.C. Long-term outcomes in acute respiratory distress syndrome: Epidemiology, mechanisms, and patient evaluation. Crit. Care Clin. 2021, 37, 895–911. [Google Scholar] [CrossRef]
- Rajajee, V.; Fung, C.M.-C.; Seagly, K.S.; Park, P.K.; Raghavendran, K.; Machado-Aranda, D.A.; Scott, J.W.; Delano, M.J.; El Ela, A.S.A.A.M.A.; Haft, J.W.; et al. One-year functional, cognitive, and psychological outcomes following the use of extracorporeal membrane oxygenation in coronavirus disease 2019: A prospective study. Crit. Care Explor. 2021, 3, e0537. [Google Scholar] [CrossRef]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; I Lone, N.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Whiteson, J.H. Pulmonary sequelae of coronavirus disease 2019. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 573–584. [Google Scholar] [CrossRef]
- Horn, M.; Wathelet, M.; Fovet, T.; Amad, A.; Vuotto, F.; Faure, K.; Astier, T.; Noël, H.; Duhem, S.; Vaiva, G.; et al. Is COVID-19 associated with posttraumatic stress disorder? J. Clin. Psychiatry 2020, 82, 20m13641. [Google Scholar] [CrossRef]
- Nagarajan, R.; Krishnamoorthy, Y.; Basavarachar, V.; Dakshinamoorthy, R. Prevalence of post-traumatic stress disorder among survivors of severe COVID-19 infections: A systematic review and meta-analysis. J. Affect. Disord. 2022, 299, 52–59. [Google Scholar] [CrossRef]
- Yang, T.; Yan, M.Z.; Li, X.; Lau, E.H.Y. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: A systematic review and meta-analysis. Infection 2022, 50, 1067–1109. [Google Scholar] [CrossRef] [PubMed]
- Khitab, A.; Reid, J.; Bennett, V.; Adams, G.C.; Balbuena, L. Late onset and persistence of post-traumatic stress disorder symptoms in survivors of critical care. Can. Respir. J. 2013, 20, 429–433. [Google Scholar] [CrossRef]
- Parker, A.M.; Sricharoenchai, T.; Raparla, S.; Schneck, K.W.; Bienvenu, O.J.; Needham, D.M. Posttraumatic stress disorder in critical illness survivors: A metaanalysis. Crit. Care Med. 2015, 43, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Righy, C.; Rosa, R.G.; da Silva, R.T.A.; Kochhann, R.; Migliavaca, C.B.; Robinson, C.C.; Teche, S.P.; Teixeira, C.; Bozza, F.A.; Falavigna, M. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: A systematic review and meta-analysis. Crit. Care 2019, 23, 213. [Google Scholar] [CrossRef]
- Carola, V.; Vincenzo, C.; Morale, C.; Pelli, M.; Rocco, M.; Nicolais, G. Psychological health in COVID-19 patients after discharge from an intensive care unit. Front. Public Health 2022, 10, 951136. [Google Scholar] [CrossRef]
- Damico, V.; Murano, L.; Margosio, V.; Tognoni, N.; Dal Molin, A.; Bassi, E.; Busca, E.; Crimella, F. Long-term effects of Coronavirus 2 infection after intensive care: A prospective study. Minerva Anestesiol. 2023, 89, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Ferreira, A.R.; Fernandes, J.; Vieira, T.; Fontes, L.; Coimbra, I.; Paiva, J.A.; Fernandes, L. Depressive and anxiety symptoms in severe COVID-19 survivors: A prospective cohort study. Psychiatr. Q. 2022, 93, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Neville, T.H.; Hays, R.D.; Tseng, C.-H.; Gonzalez, C.A.; Chen, L.; Hong, A.; Yamamoto, M.; Santoso, L.; Kung, A.; Schwab, K.; et al. Survival after severe COVID-19: Long-term outcomes of patients admitted to an intensive care unit. J. Intensive Care Med. 2022, 37, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Pappa, S.; Barmparessou, Z.; Athanasiou, N.; Sakka, E.; Eleftheriou, K.; Patrinos, S.; Sakkas, N.; Pappas, A.; Kalomenidis, I.; Katsaounou, P. Depression, insomnia and post-traumatic stress disorder in COVID-19 survivors: Role of gender and impact on quality of life. J. Pers. Med. 2022, 12, 486. [Google Scholar] [CrossRef]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L.; ELSO member centers. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Iacobucci, D.; Posavac, S.S.; Kardes, F.R.; Schneider, M.J.; Popovich, D.L. Toward a more nuanced understanding of the statistical properties of a median split. J. Consum. Psychol. 2015, 25, 652–665. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, T.; Wang, C.T.; Xu, L.; Gao, X.J. Assessment of 1-year outcomes in survivors of severe acute respiratory distress syndrome receiving extracorporeal membrane oxygenation or mechanical ventilation: A prospective observational study. Chin. Med. J. 2017, 130, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Dreier, E.; Malfertheiner, M.V.; Dienemann, T.; Fisser, C.; Foltan, M.; Geismann, F.; Graf, B.; Lunz, D.; Maier, L.S.; Müller, T.; et al. ECMO in COVID-19-prolonged therapy needed? A retrospective analysis of outcome and prognostic factors. Perfusion 2021, 36, 582–591. [Google Scholar] [CrossRef]
- Shah, P.; Miller, C.; Parilla, G.; Daneshmand, M.; Creel-Bulos, C. Outcomes associated with prolonged ECMO in COVID-19 associated ARDS: A single center experience. Perfusion 2024, 39, 1213–1221. [Google Scholar] [CrossRef]
- Rai, A.; Malviya, M.; Jacobs, A.; Nadile, V.; Ahmad, M.; Thomas, S.V. Extra Corporeal Membrane Oxygenation and Long Term Neurological Function Among COVID-19 Patients. Am. J. Respir. Crit. Care Med. 2021, 203, A2557. [Google Scholar]
- Martínez, E.; Aguilera, C.; Márquez, D.; Ziegler, G.; Plumet, J.; Tschopp, L.; Cominotti, C.; Sturzenegger, V.; Cimino, C.; Escobar, H.; et al. Post intensive care syndrome in survivors of COVID-19 who required mechanical ventilation during the third wave of the pandemic: A prospective study. Heart Lung 2023, 62, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Liu, K.; Kawakami, D.; Kawai, Y.; Morisawa, T.; Nishida, T.; Sumita, H.; Unoki, T.; Hifumi, T.; Iida, Y.; et al. Post-intensive care syndrome and its new challenges in coronavirus disease 2019 (COVID-19) pandemic: A review of recent advances and perspectives. J. Clin. Med. 2021, 10, 3870. [Google Scholar] [CrossRef] [PubMed]
- Kosilek, R.P.; Schmidt, K.; Baumeister, S.E.; Gensichen, J. Frequency and risk factors of post-intensive care syndrome components in a multicenter randomized controlled trial of German sepsis survivors. J. Crit. Care 2021, 65, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.; Rodríguez, N.; Diaz-Vieito, M.; Domínguez, M.J.; Casal, A.; Riveiro, V.; Cariñena, A.; Moreno, E.; Pose, A.; Valdés, L.; et al. Quality of life and persistent symptoms after hospitalization for COVID-19. A prospective observational study comparing ICU with non-ICU patients. Rev. Esp. Anestesiol. Reanim. 2022, 69, 326–335. [Google Scholar] [CrossRef]
- Watson, N.; Firman, D.; Baade, P.; Ring, I. Telephone administration of the SF-36 health survey: Validation studies and population norms for adults in Queensland. Aust. N. Z. J. Public Health 1996, 20, 359–363. [Google Scholar] [CrossRef]

| SF-36 Subscale | Current Study Population | Turgeon et al. [38] | |
|---|---|---|---|
| Mean (SD) | Pooled Weighted Mean Scores | p | |
| n = 18 | n = 289 | ||
| Physical functioning | 62.22 [±32.95] | 70.31 | 0.02 |
| Role limitation due to physical problems | 37.50 [±48.70] | 49.62 | 0.72 |
| Role limitation due to emotional problems | 47.92 [±50.14] a | 69.82 c | 0.09 |
| Energy/fatigue (vitality) | 51.02 [±33.97] | 54.46 | 0.66 |
| Emotional well-being | 70.89 [±22.83] | 68.8 d | 0.54 |
| Social functioning | 38.14 [±41.45] | 71.42 b | <0.01 |
| Pain | 75.14 [±32.95] | 70.41 | 0.82 |
| General health | 49.24 [±30.03] | 56.47 | <0.01 |
| Demographic | Mean (SD); No, % | n = 18 |
| Age, years | 52 [±9.5] | |
| Sex, male (%) | 9 (50) | |
| BMI, kg/m2 | 30 [±5] | |
| Marital status, married (%) | 14 (78) | |
| Return to work, yes (%) | 8 (44) | |
| Early retirement (%) | 3 (17) | |
| Inability to work at time of the interview (%) | 7 (39) | |
| ICU Data | Mean (SD) | |
| SAPS III | 66.35 [±7.87] | n = 17 |
| ICU LOS, days | 41.6 [±21.6] | |
| ECMO duration, days | 21.4 [±12.2] | |
| IMV pre-ECMO duration, days | 8.93 [±7.96] | |
| IMV duration, days | 25.2 [±11.83] | |
| Days on sedatives * | 31.8 [±13.6] | |
| Days on benzodiazepines ° | 19.9 [±10.4] | |
| Days on neuroleptics ^ | 20.2 [±11.4] | |
| Days on NMBA | 5.06 [±3.1] | |
| Comorbidities | no. (%) | |
| Underlying pulmonary disease, no. (%) | 4 (22) | |
| Arterial hypertension, no. (%) | 10 (56) | |
| Obesity, no. (%) | 8 (44) | |
| Diabetes mellitus, no. (%) | 4 (22) | |
| Chronic heart disease, no. (%) | 1 (6) | |
| Immunosuppression, no. (%) | 1 (6) | |
| Chronic kidney disease, no. (%) | 1 (6) | |
| Mental or cognitive problems, no. (%) | 0 (0) | |
| No underlying disease, no. (%) | 4 (22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermann, M.; Filipsky, R.; Bukowski, N.; Gerger, G.; Hermann, A.; Krenn, K.; Teufel, A.; Kimberger, O.; Laxar, D.; Maleczek, M.; et al. Long-Term Health-Related Quality of Life Following Survival of Acute Respiratory Distress Syndrome and Extracorporeal Membrane Oxygenation Due to COVID-19. J. Clin. Med. 2025, 14, 3358. https://doi.org/10.3390/jcm14103358
Hermann M, Filipsky R, Bukowski N, Gerger G, Hermann A, Krenn K, Teufel A, Kimberger O, Laxar D, Maleczek M, et al. Long-Term Health-Related Quality of Life Following Survival of Acute Respiratory Distress Syndrome and Extracorporeal Membrane Oxygenation Due to COVID-19. Journal of Clinical Medicine. 2025; 14(10):3358. https://doi.org/10.3390/jcm14103358
Chicago/Turabian StyleHermann, Martina, Rebecca Filipsky, Nils Bukowski, Gernot Gerger, Alexander Hermann, Katharina Krenn, Anna Teufel, Oliver Kimberger, Daniel Laxar, Mathias Maleczek, and et al. 2025. "Long-Term Health-Related Quality of Life Following Survival of Acute Respiratory Distress Syndrome and Extracorporeal Membrane Oxygenation Due to COVID-19" Journal of Clinical Medicine 14, no. 10: 3358. https://doi.org/10.3390/jcm14103358
APA StyleHermann, M., Filipsky, R., Bukowski, N., Gerger, G., Hermann, A., Krenn, K., Teufel, A., Kimberger, O., Laxar, D., Maleczek, M., Schaden, E., Wiegele, M., Willschke, H., & Tiboldi, A. (2025). Long-Term Health-Related Quality of Life Following Survival of Acute Respiratory Distress Syndrome and Extracorporeal Membrane Oxygenation Due to COVID-19. Journal of Clinical Medicine, 14(10), 3358. https://doi.org/10.3390/jcm14103358







