Clinical Periodontal Evaluation and Assessment of Dipeptidyl-Peptidase-4 and Galectin-3 in Gingival Crevicular Fluid of Periodontitis Patients with Heart Failure and Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Periodontal Examination and Diagnosis
2.4. Gingival Crevicular Fluid Sampling
2.5. Immunological Analysis of DPP-4 and Gal-3
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Patients from the Four Groups
3.2. Differences Between the Assessed Parameters
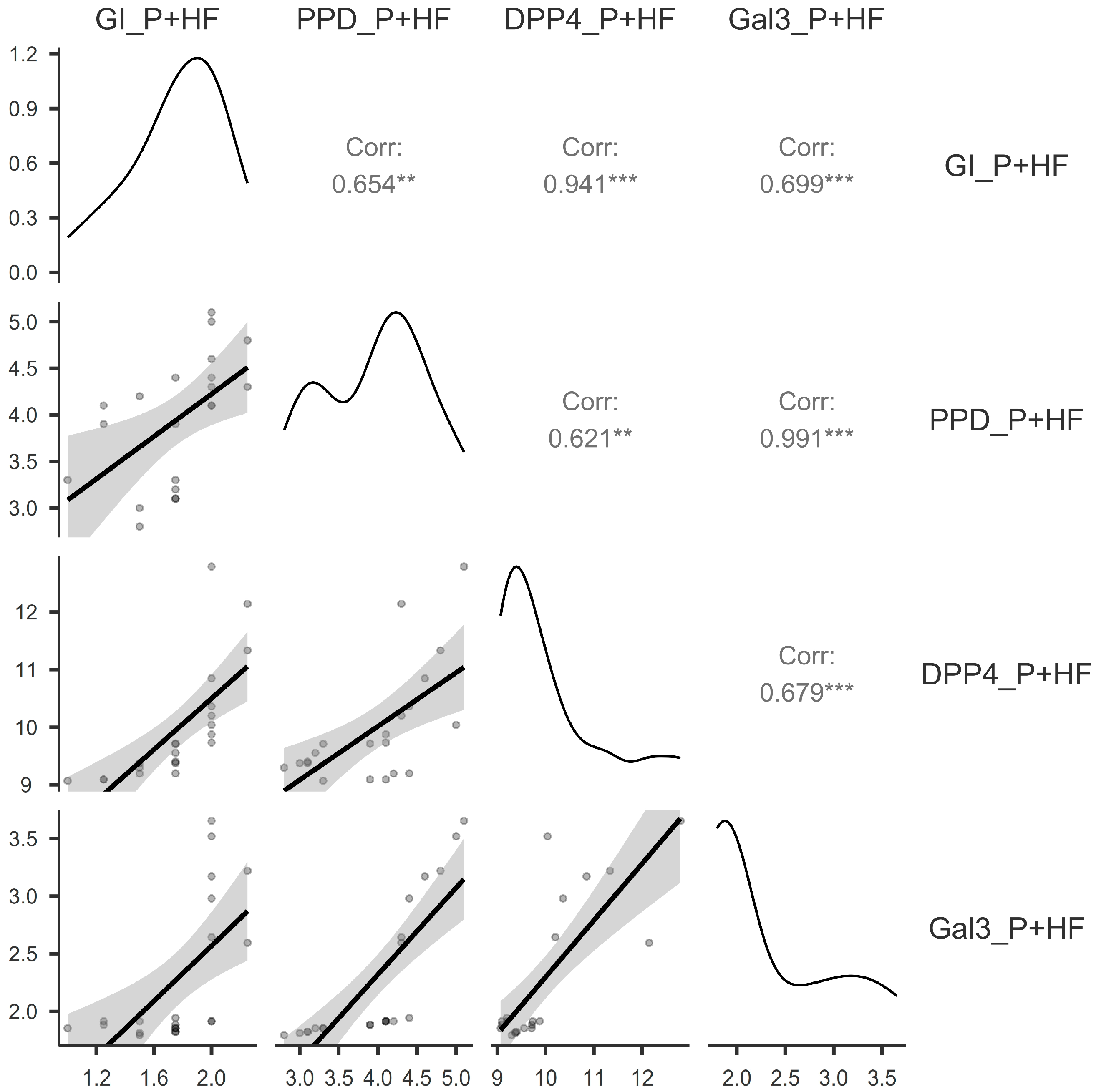
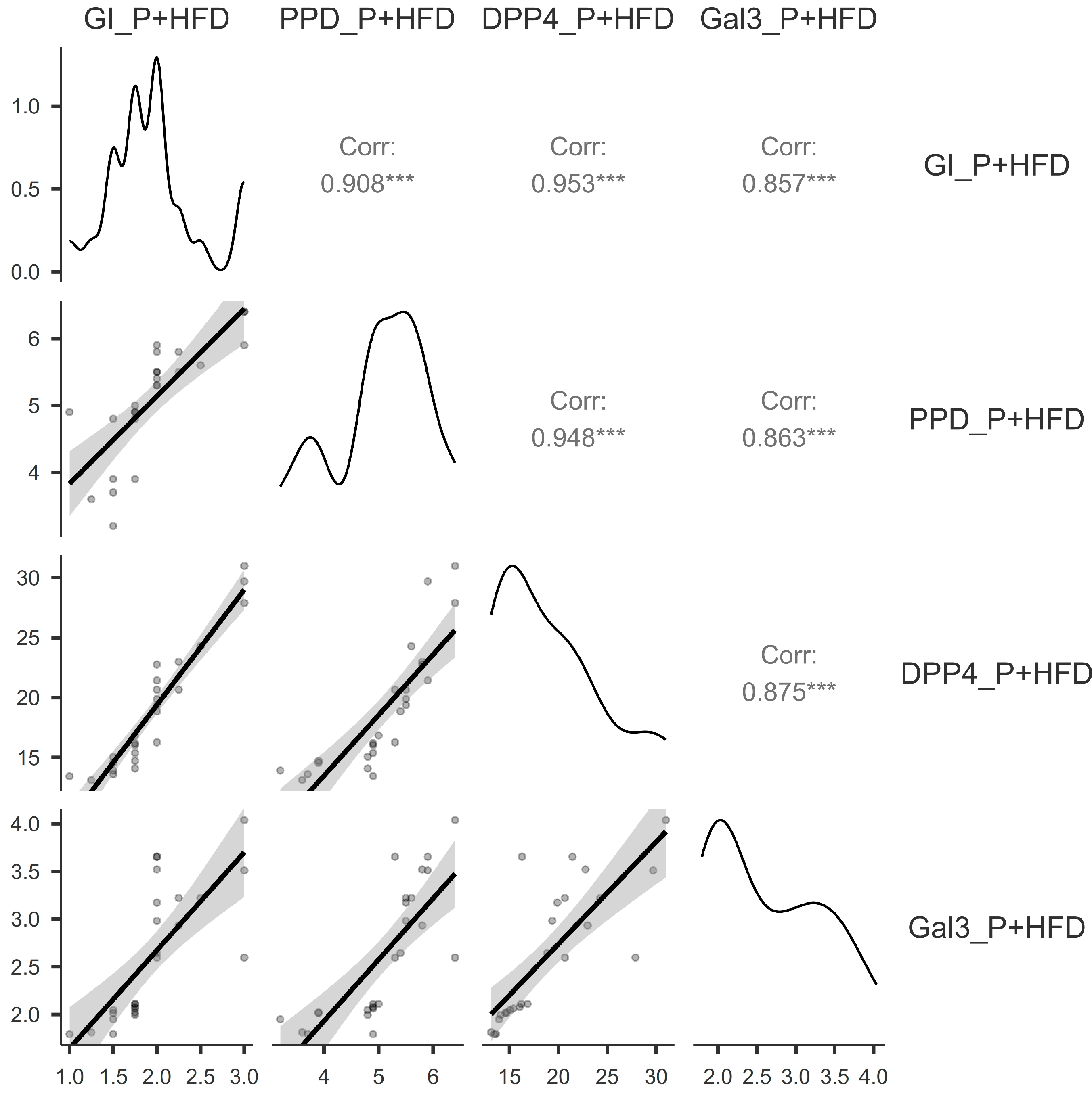
3.3. Correlations Between the Assessed Parameters
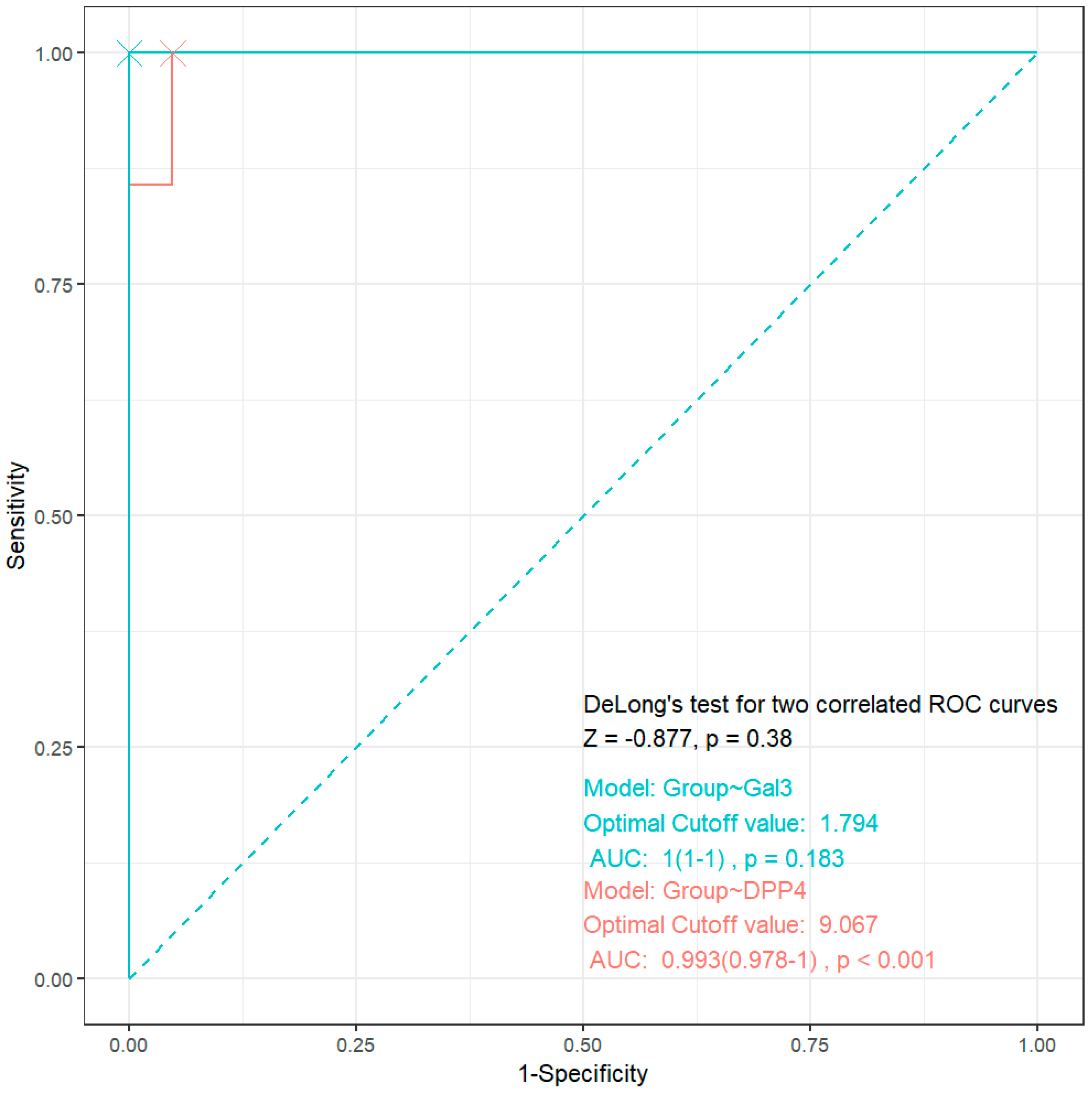
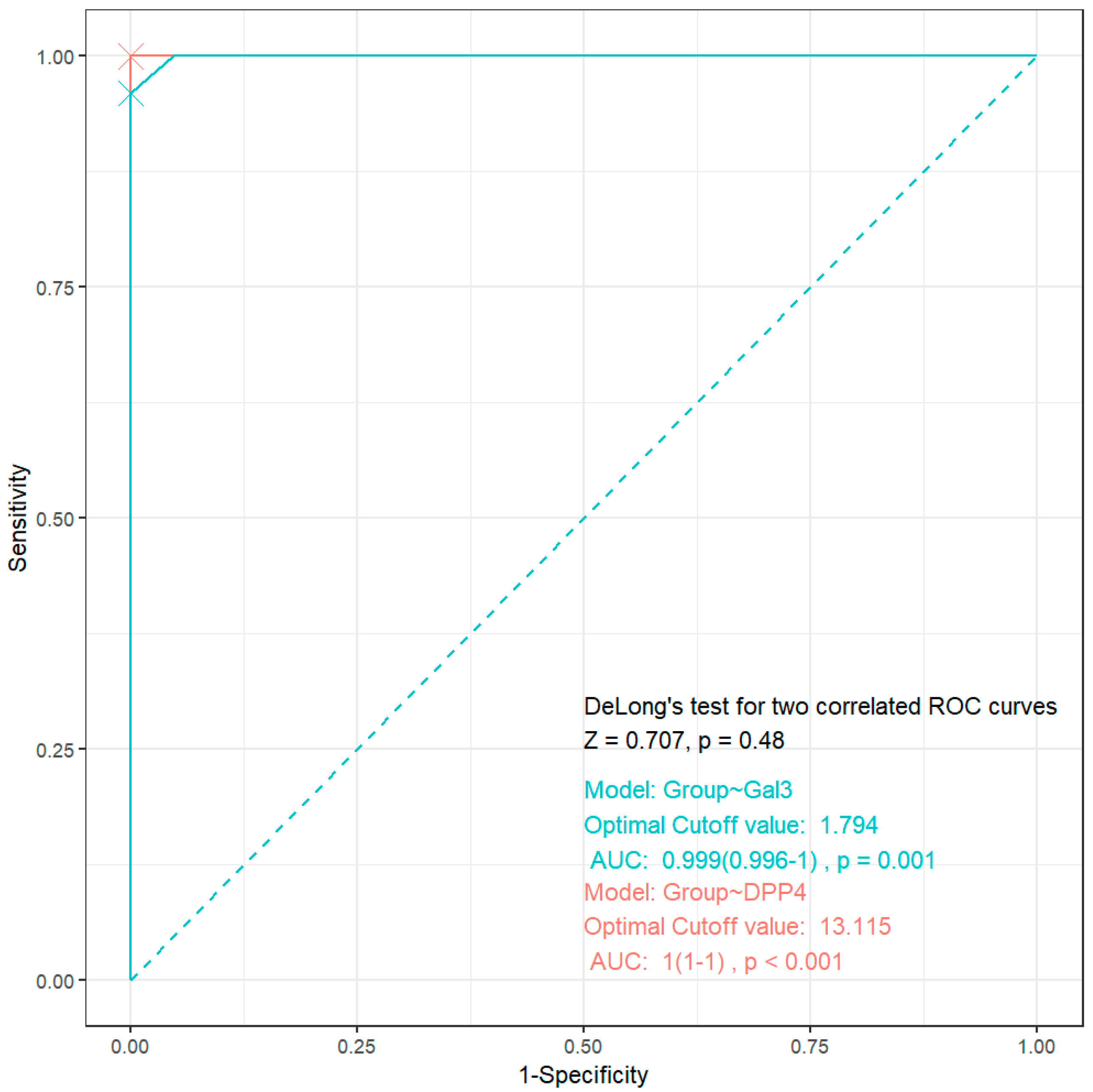
3.4. ROC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Ohara-Nemoto, Y.; Shimoyama, Y.; Nakasato, M.; Nishimata, H.; Ishikawa, T.; Sasaki, M.; Kimura, S.; Nemoto, T.K. Distribution of dipeptidyl peptidase (DPP) 4, DPP5, DPP7 and DPP11 in human oral microbiota-potent biomarkers indicating presence of periodontopathic bacteria. FEMS Microbiol. Lett. 2018, 365, fny221. [Google Scholar] [CrossRef]
- Kennett, C.N.; Cox, S.W.; Eley, B.M. Investigations into the cellular contribution to host tissue proteases and inhibitors in gingival crevicular fluid. J. Clin. Periodontol. 1997, 24, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Elgün, S.; Ozmeriç, N.; Demirtaş, S. Alanine aminopeptidase and dipeptidylpeptidase IV in saliva: The possible role in periodontal disease. Clin. Chim. Acta 2000, 298, 187–191. [Google Scholar] [CrossRef]
- Gheonea, T.C.; Șurlin, P.; Nicolae, F.M.; Gheorghe, D.N.; Popescu, D.M.; Rogoveanu, I. Dipeptidyl-Peptidase-4 and Glucagon-like-Peptide-1, a Link in the Connection between Periodontitis and Diabetes Mellitus-What Do We Know So Far?—A Scoping Review. J. Clin. Med. 2024, 13, 903. [Google Scholar] [CrossRef]
- Eley, B.M.; Cox, S.W. Cathepsin B/L-, elastase-, tryptase-, trypsin- and dipeptidyl peptidase IV-like activities in gingival crevicular fluid: Correlation with clinical parameters in untreated chronic periodontitis patients. J. Periodont. Res. 1992, 27, 62–69. [Google Scholar] [CrossRef]
- Beighton, D.; Life, J.S. Trypsin-like, chymotrypsin-like and glycylprolyl dipeptidase activities in gingival crevicular fluid from human periodontal sites with gingivitis. Arch. Oral Biol. 1989, 34, 843–846. [Google Scholar] [CrossRef]
- Aemaimanan, P.; Sattayasai, N.; Wara-Aswapati, N.; Pitiphat, W.; Suwannarong, W.; Prajaneh, S.; Taweechaisupapong, S. Alanine aminopeptidase and dipeptidyl peptidase IV in saliva of chronic periodontitis patients. J. Periodontol. 2009, 80, 1809–1814. [Google Scholar] [CrossRef]
- Sano, M. Mechanism by which dipeptidyl peptidase-4 inhibitors increase the risk of heart failure and possible differences in heart failure risk. J. Cardiol. 2019, 73, 28–32. [Google Scholar] [CrossRef]
- Chen, S.Y.; Kong, X.Q.; Zhang, K.F.; Luo, S.; Wang, F.; Zhang, J.J. DPP4 as a Potential Candidate in Cardiovascular Disease. J. Inflamm. Res. 2022, 15, 5457–5469. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Genco, R.; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: Consensus report. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Van Dyke, T.E.; Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S24–S29. [Google Scholar] [CrossRef]
- Cloete, L. Diabetes mellitus: An overview of the types, symptoms, complications and management. Nurs. Stand. 2022, 37, 61–66. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhou, Z.; Xiao, Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: A snap-shot. Rev. Endocr. Metab. Disord. 2022, 23, 569–577. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontol. 2000 2020, 82, 214–224. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Fonarow, G.C.; Greene, S.J.; Holmes, D.N.; Alhanti, B.; Devore, A.D.; Butler, J.; Heidenreich, P.A.; Huang, J.C.; Kittleson, M.M.; et al. Medical Therapy Before, During and After Hospitalization in Medicare Beneficiaries with Heart Failure and Diabetes: Get With The Guidelines—Heart Failure Registry. J. Card. Fail. 2024, 30, 319–328. [Google Scholar] [CrossRef]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.M., Jr.; Haught, W.H. Evaluation of heart failure patients: Objective parameters to assess functional capacity. Clin. Cardiol. 1996, 19, 455–460. [Google Scholar] [CrossRef]
- Dekker, R.L.; Lennie, T.A.; Moser, D.K.; Miller, C.S.; Ebersole, J.L.; Chung, M.L.; Campbell, C.L.; Bailey, A.; Tovar, E.G. Salivary Biomarkers, Oral Inflammation, and Functional Status in Patients With Heart Failure. Biol. Res. Nurs. 2017, 19, 153–161. [Google Scholar] [CrossRef]
- Seropian, I.M.; Cassaglia, P.; Miksztowicz, V.; González, G.E. Unraveling the role of galectin-3 in cardiac pathology and physiology. Front. Physiol. 2023, 14, 1304735. [Google Scholar] [CrossRef]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure-The Diagnostic, Prognostic and Therapeutic Potential-Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.; Johnson, R.B. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J. Clin. Periodontol. 2004, 31, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a Glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef]
- Mithradas, N.; Ram, V.S.; Ravindran, N.; Warrier, V.D.; Asirvatham, S.L.; Saket, P. Evaluation of Salivary Galectin-3 Level and Its Potential Role in Increasing the Severity of COVID-19 Infection in Patients with Periodontitis. World J. Dent. 2023, 14, 3–8. [Google Scholar] [CrossRef]
- Afacan, B.; Ilhan, H.A.; Köse, T.; Emingil, G. Gingival Crevicular Fluid Galectin-3 and Interleukin-1 Beta Levels in Stage 3 Periodontitis with Grade B and C. Clin. Oral Investig. 2023, 27, 3749–3758. [Google Scholar] [CrossRef]
- Ali, M.; Shemais, N.; Shaker, O.; Ghallab, N. Gingival Crevicular Fluid and Salivary Levels of Galectin-3 in Patients with Gingivitis and Patients with Stage III Periodontitis: An Observational Study. Adv. Dent. J. 2023, 5, 742–751. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins as Pattern Recognition Receptors: Structure, Function, and Evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Lo Giudice, A. Analysis of Galectin-3 Levels as a Source of Coronary Heart Disease Risk during Periodontitis. J. Periodont. Res. 2021, 56, 597–605. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-Induced Gingivitis: Case Definition and Diagnostic Considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S44–S67. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2004, 27 (Suppl. S1), S5–S10. [Google Scholar] [CrossRef]
- Panagakos, F.S.; Davies, R.M. Gingival Diseases—Their Aetiology, Prevention and Treatment. In State of the Art; IntechOpen: London, UK, 2011; pp. 41–54. [Google Scholar]
- Kumagai, Y.; Yagishita, H.; Yajima, A.; Okamoto, T.; Konishi, K. Molecular Mechanism for Connective Tissue Destruction by Dipeptidyl Aminopeptidase IV Produced by the Periodontal Pathogen Porphyromonas gingivalis. Infect. Immun. 2005, 73, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Eley, B.M.; Cox, S.W. Cathepsin B/L-, Elastase-, Tryptase-, Trypsin- and Dipeptidyl Peptidase IV-Like Activities in Gingival Crevicular Fluid: A Comparison of Levels Before and After Periodontal Surgery in Chronic Periodontitis Patients. J. Periodontol. 1992, 63, 412–417. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, H.C.; Zhu, W.; Kim, S.M.; Sabet, M.; Handfield, M.; Hillman, J.; Progulske-Fox, A.; Lee, S.W. Identification of Tannerella forsythia Antigens Specifically Expressed in Patients with Periodontal Disease. FEMS Microbiol. Lett. 2007, 275, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.W.; Gazi, M.I.; Eley, B.M. Dipeptidyl Peptidase II- and IV-Like Activities in Gingival Tissue and Crevicular Fluid from Human Periodontitis Lesions. Arch. Oral Biol. 1992, 37, 167–173. [Google Scholar] [CrossRef]
- Kennett, C.N.; Cox, S.W.; Eley, B.M. Histochemical and Immunocytochemical Localization of Dipeptidyl Peptidases II and IV in Human Gingiva. J. Periodontol. 1996, 67, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Eley, B.M.; Cox, S.W. Correlation Between Gingival Crevicular Fluid Dipeptidyl Peptidase II and IV Activity and Periodontal Attachment Loss: A 2-Year Longitudinal Study in Chronic Periodontitis Patients. Oral Dis. 1995, 1, 201–213. [Google Scholar] [CrossRef]
- Cox, S.W.; Eley, B.M. Cathepsin B/L-, Elastase-, Tryptase-, Trypsin- and Dipeptidyl Peptidase IV-Like Activities in Gingival Crevicular Fluid: A Comparison of Levels Before and After Basic Periodontal Treatment of Chronic Periodontitis Patients. J. Clin. Periodontol. 1992, 19, 333–339. [Google Scholar] [CrossRef]
- Eley, B.M.; Cox, S.W. Correlation of gingival crevicular fluid proteases with clinical and radiological measurements of periodontal attachment loss. J. Dent. 1992, 20, 90–99. [Google Scholar] [CrossRef]
- Bando, Y.K.; Murohara, T. Heart Failure as a Comorbidity of Diabetes: Role of Dipeptidyl Peptidase 4. J. Atheroscler. Thromb. 2016, 23, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Liang, L.; Khazanie, P.; Hammill, B.G.; Fonarow, G.C.; Yancy, C.W.; Bhatt, D.L.; Curtis, L.H.; Hernandez, A.F. Antihyperglycemic Medication Use Among Medicare Beneficiaries With Heart Failure, Diabetes Mellitus, and Chronic Kidney Disease. Circ. Heart Fail. 2016, 9, e002638. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomized controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Braunwald, E.; Raz, I.; Cavender, M.A.; Morrow, D.A.; Jarolim, P.; Udell, J.A.; Mosenzon, O.; Im, K.; Umez-Eronini, A.A.; et al. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014, 130, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Bhatt, D.L.; Braunwald, E.; Cavender, M.A.; Mosenzon, O.; Steg, P.G.; Davidson, J.A.; Nicolau, J.C.; Corbalan, R.; Hirshberg, B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: Observations from the SAVOR-TIMI 53 Trial. Diabetes Care 2015, 38, 696–705. [Google Scholar] [CrossRef]
- Salim, H.M.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Dipeptidyl peptidase-4 inhibitor, linagliptin, ameliorates endothelial dysfunction and atherogenesis in normoglycemic apolipoprotein-E deficient mice. Vasc. Pharmacol. 2016, 79, 16–23. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 8, e9392. [Google Scholar] [CrossRef]
- Velickovic, M.; Arsenijevic, A.; Acovic, A.; Arsenijevic, D.; Milovanovic, J.; Dimitrijevic, J.; Todorovic, Z.; Milovanovic, M.; Kanjevac, T.; Arsenijevic, N. Galectin-3, possible role in pathogenesis of periodontal diseases and potential therapeutic target. Front. Pharmacol. 2021, 12, 638258. [Google Scholar] [CrossRef]
- de Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and autoimmune diseases. Exp. Biol. Med. 2015, 240, 1019–1028. [Google Scholar] [CrossRef]
- Akkaya, H.Ü.; Yılmaz, H.E.; Narin, F.; Sağlam, M. Evaluation of Galectin-3, Peptidylarginine Deiminase-4, and Tumor Necrosis Factor-α Levels in Gingival Crevicular Fluid for Periodontal Health, Gingivitis, and Stage III Grade C Periodontitis: A Pilot Study. J. Periodontol. 2022, 93, 80–88. [Google Scholar] [CrossRef]
- Tarrad, N.A.F.; Shaker, O.G.; Elbanna, R.M.H.; AbdelKawy, M. Outcome of Non-Surgical Periodontal Treatment on Gal-1 and Gal-3 GCF Levels in Periodontitis Patients: A Case-Control Study. Clin. Oral Investig. 2024, 28, 309. [Google Scholar] [CrossRef]
- Karsiyaka Hendek, M.; Olgun, E.; Kisa, U. The Effect of Initial Periodontal Treatment on Gingival Crevicular Fluid Galectin-3 Levels in Participants with Periodontal Disease. Aust. Dent. J. 2021, 66, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Ao, M.; Furusho, H.; Chea, C.; Nagasaki, A.; Sakamoto, S.; Ando, T.; Inubushi, T.; Kozai, K.; Takata, T. Galectin-3 Plays an Important Role in Preterm Birth Caused by Dental Infection of Porphyromonas gingivalis. Sci. Rep. 2018, 8, 2867. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- McCullough, P.A.; Olobatoke, A.; Vanhecke, T.E. Galectin-3: A Novel Blood Test for the Evaluation and Management of Patients with Heart Failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bhambhani, V.; Christenson, R.H.; Meijers, W.C.; De Boer, R.A.; Levy, D.; Larson, M.G.; Ho, J.E. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2018, 72, 3246–3254. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Almorós, A.; Pello, A.; Aceña, Á.; Martínez-Milla, J.; Lorenzo, Ó.; Tarín, N.; Cristóbal, C.; Blanco-Colio, L.M.; Martín-Ventura, J.-L.; Huelmos, A.; et al. Galectin-3 is Associated with Cardiovascular Events in Post-Acute Coronary Syndrome Patients with Type-2 Diabetes. J. Clin. Med. 2020, 9, 1105. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Wettersten, N.; van Veldhuisen, D.J.; Mueller, C.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Birkhahn, R.; Vilke, G.M.; et al. The Influence of Body Mass Index on Clinical Interpretation of Established and Novel Biomarkers in Acute Heart Failure. J. Card. Fail. 2023, 29, 1121–1131. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-Regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Health Effects of Cigarettes: Cardiovascular Disease. Available online: https://www.cdc.gov/tobacco/about/cigarettes-and-cardiovascular-disease.html (accessed on 10 March 2025).
- Reed, Z.E.; Sallis, H.M.; Richmond, R.C.; Attwood, A.S.; Lawlor, D.A.; Munafò, M.R. Investigating Whether Smoking and Alcohol Behaviors Influence Risk of Type 2 Diabetes Using a Mendelian Randomisation Study. Sci. Rep. 2025, 15, 7985. [Google Scholar] [CrossRef]

| C (n = 21) | P (n = 21) | P+HF (n = 21) | P+HF+D (n = 25) | |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 47.7 ± 6.16 | 50.5 ± 5.93 | 55.4 ± 5.72 | 59.4 ± 5.63 |
| Median (IQR) | 48 (42–52) | 51 (46–55) | 55 (50–61) | 59 (56–64) |
| Min–max | 38–61 | 43–62 | 47–66 | 49–67 |
| Gender | ||||
| Male (%) | 12 (57%) | 12 (57%) | 11 (52%) | 16 (64%) |
| Female (%) | 9 (43%) | 9 (43%) | 10 (48%) | 9 (36%) |
| Smoking, yes (%) | 8 (38%) | 15 (71%) | 15 (71%) | 16 (64%) |
| Parameters (ng/mL) | C (n = 21) | P (n = 21) | P+HF (n = 21) | P+HF+D (n = 25) |
|---|---|---|---|---|
| DPP4 | ||||
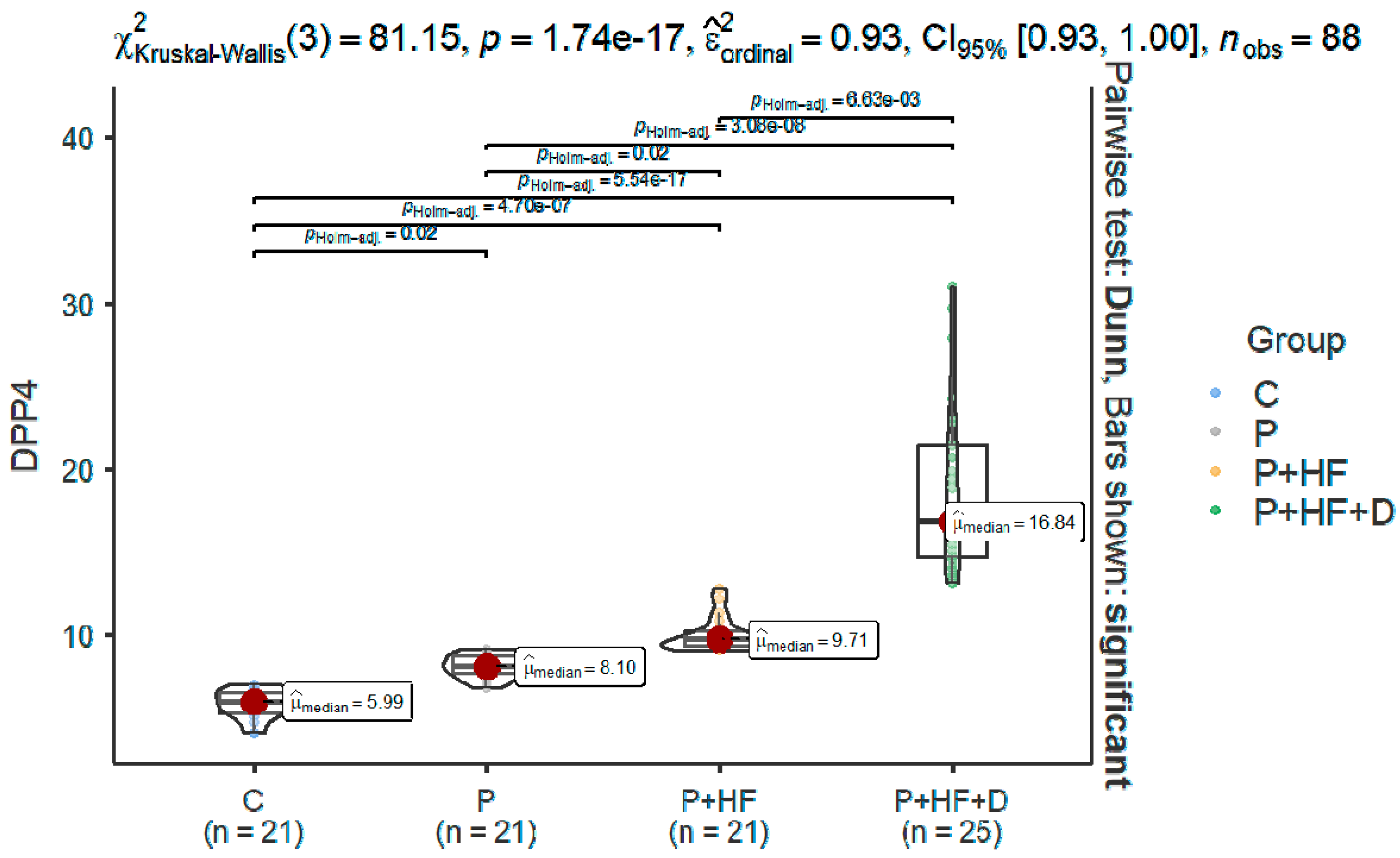
| Mean ± SD | 5.84 ± 0.86 | 8.11 ± 0.69 | 9.97 ± 1.02 | 18.9 ± 5.19 |
| Median | 5.99 (5.3–6.5) | 8.1 (7.6–8.7) | 9.7 (9.3–10.2) | 16.8 (14.7–21.4) |
| Min–max | 4.09–7 | 6.81–9.16 | 9.07–12.8 | 13.1–31.0 |
| Normality | 0.19 | 0.578 | <0.001 | 0.011 |
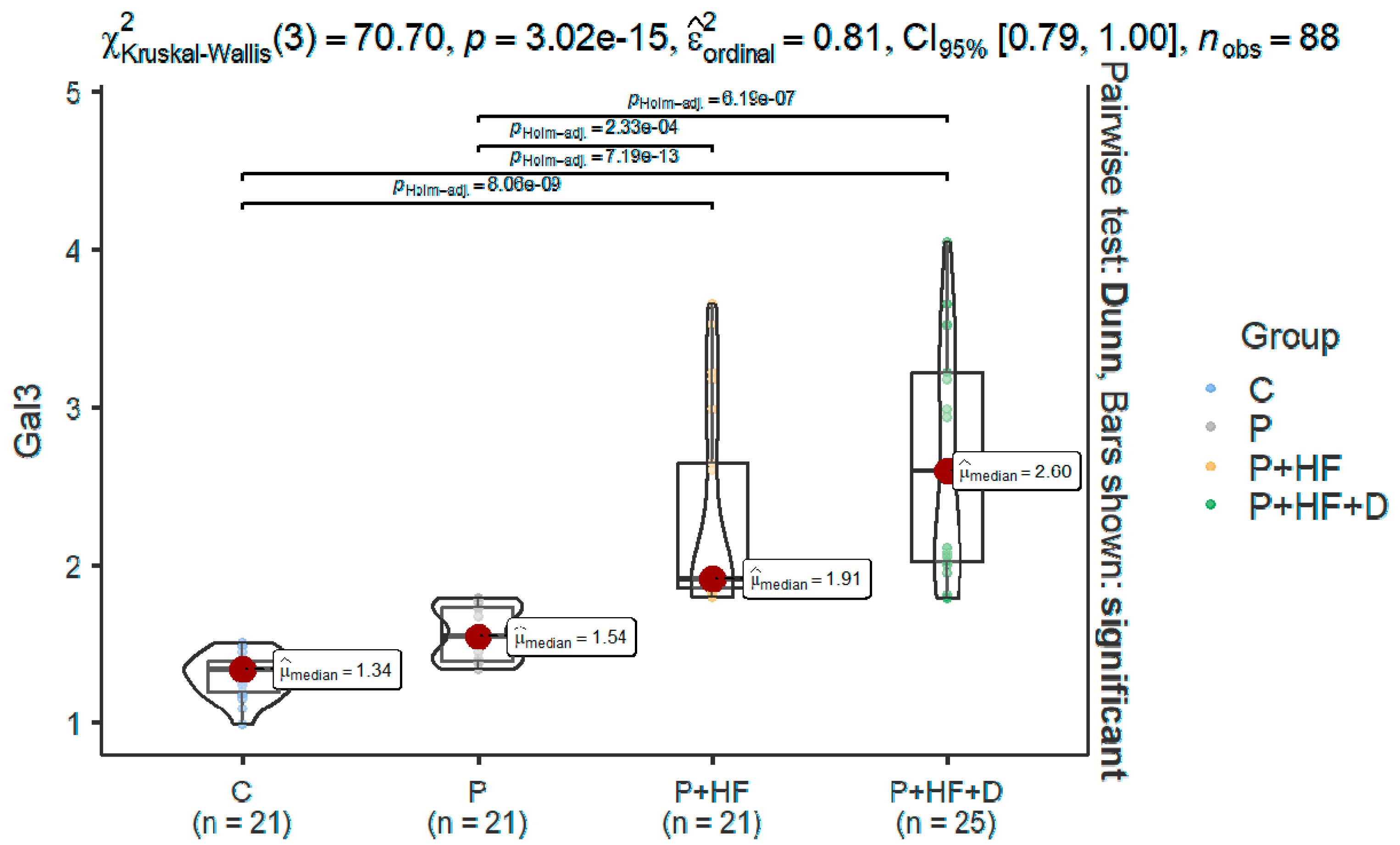
| Gal3 | ||||
| Mean ± SD | 1.3 ± 0.14 | 1.57 ± 0.17 | 2.48 ± 0.64 | 2.62 ± 0.71 |
| Median | 1.3 (1.2–1.4) | 1.5 (1.4–1.7) | 1.9 (1.8–2.6) | 2.60 (2.02–3.22) |
| Min–max | 0.99–1.50 | 1.34–1.79 | 1.79–3.65 | 1.79–4.04 |
| Normality | 0.682 | 0.006 | <0.001 | 0.010 |
| Parameters (ng/mL) | Groups | Smoking | Non-Smoking | p-Value |
|---|---|---|---|---|
| DPP4 | C | 5.79 ± 0.96 | 5.86 ± 0.83 | 0.870 |
| P | 8.25 ± 0.57 | 7.77 ± 0.9 | 0.368 | |
| P+HF | 10.2 ± 1.14 | 9.48 ± 0.37 | 0.132 | |
| P+HF+D | 20.2 ± 5.66 | 16.6 ± 3.38 | 0.128 | |
| Gal3 | C | 1.28 ± 0.16 | 1.31 ± 0.13 | 0.787 |
| P | 1.59 ± 0.16 | 1.53 ± 0.19 | 0.518 | |
| P+HF | 2.34 ± 0.64 | 2.14 ± 0.68 | 0.387 | |
| P+HF+D | 2.68 ± 0.66 | 2.52 ± 0.83 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păvălan, A.; Boldeanu, M.V.; Nicolae, F.M.; Gheonea, T.C.; Rogoveanu, I.; Florescu, C.; Turcu-Știolică, A.; Gheorghe, D.N.; Popescu, D.M.; Șoancă, A.; et al. Clinical Periodontal Evaluation and Assessment of Dipeptidyl-Peptidase-4 and Galectin-3 in Gingival Crevicular Fluid of Periodontitis Patients with Heart Failure and Diabetes. J. Clin. Med. 2025, 14, 3345. https://doi.org/10.3390/jcm14103345
Păvălan A, Boldeanu MV, Nicolae FM, Gheonea TC, Rogoveanu I, Florescu C, Turcu-Știolică A, Gheorghe DN, Popescu DM, Șoancă A, et al. Clinical Periodontal Evaluation and Assessment of Dipeptidyl-Peptidase-4 and Galectin-3 in Gingival Crevicular Fluid of Periodontitis Patients with Heart Failure and Diabetes. Journal of Clinical Medicine. 2025; 14(10):3345. https://doi.org/10.3390/jcm14103345
Chicago/Turabian StylePăvălan, Ana, Mihail Virgil Boldeanu, Flavia Mirela Nicolae, Theodora Claudia Gheonea, Ion Rogoveanu, Cristina Florescu, Adina Turcu-Știolică, Dorin Nicolae Gheorghe, Dora Maria Popescu, Andrada Șoancă, and et al. 2025. "Clinical Periodontal Evaluation and Assessment of Dipeptidyl-Peptidase-4 and Galectin-3 in Gingival Crevicular Fluid of Periodontitis Patients with Heart Failure and Diabetes" Journal of Clinical Medicine 14, no. 10: 3345. https://doi.org/10.3390/jcm14103345
APA StylePăvălan, A., Boldeanu, M. V., Nicolae, F. M., Gheonea, T. C., Rogoveanu, I., Florescu, C., Turcu-Știolică, A., Gheorghe, D. N., Popescu, D. M., Șoancă, A., Roman, A., & Șurlin, P. (2025). Clinical Periodontal Evaluation and Assessment of Dipeptidyl-Peptidase-4 and Galectin-3 in Gingival Crevicular Fluid of Periodontitis Patients with Heart Failure and Diabetes. Journal of Clinical Medicine, 14(10), 3345. https://doi.org/10.3390/jcm14103345










