Measuring Short-Term Outcomes Following Primary Total Hip Arthroplasty: A Value-Based Healthcare Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Quality Assessment
- -
- Nonresponders (Did not achieve the MCID):
- -
- Moderate responders (Achieved the MCID but did not reach the PASS):
- -
- High responders (Achieved the MCID and reached the PASS):
2.2. Cost Assessment
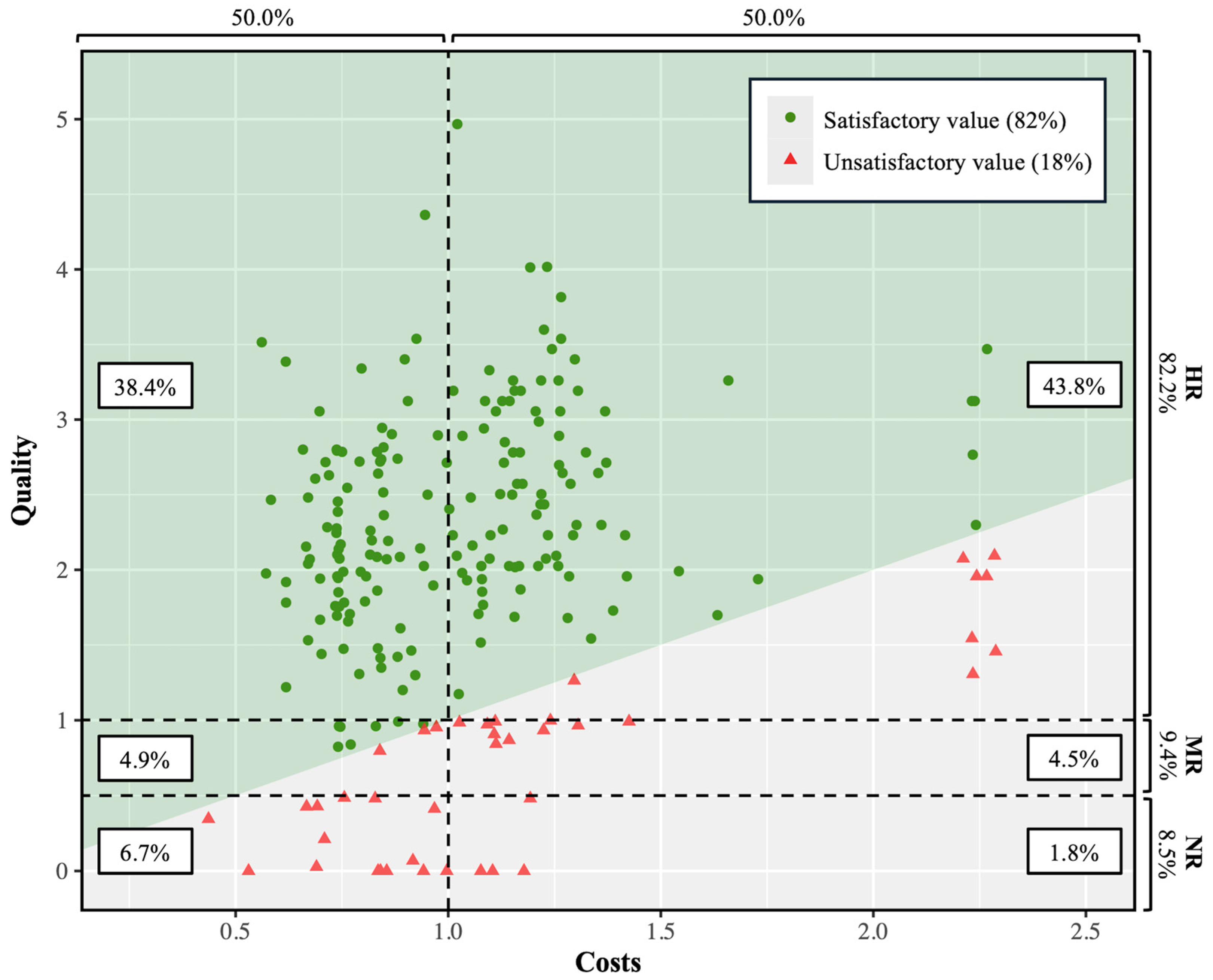
2.3. Value Assessment
2.4. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THA | total hip arthroplasty |
| VBHC | value-based healthcare |
| PROM | patient-reported outcome measure |
| MCID | Linear dichroism |
| PASS | patient acceptable symptom state |
| OA | osteoarthritis |
| SCB | substantial clinical benefit |
| USB | University Hospital Basel |
| DRG | diagnosis-related group |
| CCER | Commission Cantonale d’Ethique de la Recherche |
| BMI | body mass index |
| ASA | American Society of Anesthesiologists |
| LOS | length of stay |
| mHHS | modified Harris Hip Score |
| pVAS | pain on a visual analogue scale |
| HOOS-PS | Hip disability and Osteoarthritis Outcome Score—Physical function Short form |
| CHF | Swiss francs |
| quantile–quantile | |
| IQR | interquartile range |
| SD | standard deviation |
| VIF | Variance Inflation Factor |
| β | regression coefficient |
| EQ-5D | EuroQol 5-dimensions |
| COVID-19 | COVID-19 |
| OECD | Organisation for Economic Co-operation and Development |
| SIRIS | Swiss Implant Registry |
References
- W-Dahl, A.; Kärrholm, J.; Rogmark, C.; Nåtman, J.; Bülow, E.; Arani, P.I.; Mohaddes, M.; Rolfson, O. The Swedish Arthroplasty Register—Annual Report 2023; Ola Rolfson: Göteborg, Sweden, 2023. [Google Scholar]
- Evans, J.T.; Evans, J.P.; Walker, R.W.; Blom, A.W.; Whitehouse, M.R.; Sayers, A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Patel, I.; Nham, F.; Zalikha, A.K.; El-Othmani, M.M. Epidemiology of total hip arthroplasty: Demographics, comorbidities and outcomes. Arthroplasty 2023, 5, 2. [Google Scholar] [CrossRef]
- OECD. Health at a Glance 2023: OECD Indicators; OECD Publishing: Paris, France, 2023. [Google Scholar]
- McPherson, K.; Gon, G.; Scott, M. International Variations in a Selected Number of Surgical Procedures. In OECD Health Working Papers; OECD Publishing: Paris, France, 2013; p. 61. [Google Scholar] [CrossRef]
- Porter, M.E.; Teisberg, E.O. Redefining Health Care: Creating Value-Based Competition on Results; Press, H.B.R., Ed.; Harvard Business School Press: Brighton, MA, USA, 2006. [Google Scholar]
- Bernstein, D.N.; Nwachukwu, B.U.; Bozic, K.J. Value-based Health Care: Moving Beyond “Minimum Clinically Important Difference” to a Tiered System of Evaluating Successful Clinical Outcomes. Clin. Orthop. Relat. Res. 2019, 477, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Ladermann, A.; Eurin, R.; Alibert, A.; Bensouda, M.; Bothorel, H. Measuring Patient Value after Total Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 5700. [Google Scholar] [CrossRef]
- Reilly, C.A.; Doughty, H.P.; Werth, P.M.; Rockwell, C.W.; Sparks, M.B.; Jevsevar, D.S. Creating a Value Dashboard for Orthopaedic Surgical Procedures. J. Bone Jt. Surg. Am. 2020, 102, 1849–1856. [Google Scholar] [CrossRef]
- Goh, G.S.; Tarabichi, S.; Baker, C.M.; Qadiri, Q.S.; Austin, M.S. Should We Aim to Help Patients “Feel Better” or “Feel Good” After Total Hip Arthroplasty? Determining Factors Affecting the Achievement of the Minimal Clinically Important Difference and Patient Acceptable Symptom State. J. Arthroplasty 2023, 38, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bothorel, H.; Pernoud, A.; Christofilopoulos, P. Translation and Cross-Cultural Adaptation into French of the Harris Hip Score and the Modified Harris Hip Score. Patient Relat. Outcome Meas. 2024, 15, 81–91. [Google Scholar] [CrossRef]
- Lawrie, C.M.; Bechtold, D.; Schwabe, M.; Clohisy, J.C. Primary total hip arthroplasty via the direct anterior approach in the lateral decubitus position: Surgical technique, learning curve, complications, and early results. Bone Jt. J. 2021, 103-B, 53–58. [Google Scholar] [CrossRef]
- Parilla, F.W.; Freiman, S.; Pashos, G.E.; Thapa, S.; Clohisy, J.C. Comparison of modern periacetabular osteotomy for hip dysplasia with total hip arthroplasty for hip osteoarthritis-10-year outcomes are comparable in young adult patients. J. Hip Preserv. Surg. 2022, 9, 178–184. [Google Scholar] [CrossRef]
- Navas, L.; Faller, J.; Schmidt, S.; Streit, M.; Hauschild, M.; Zimmerer, A. Sports Activity and Patient-Related Outcomes after Cementless Total Hip Arthroplasty in Patients Younger than 40 Years. J. Clin. Med. 2021, 10, 4644. [Google Scholar] [CrossRef]
- Danoff, J.R.; Goel, R.; Sutton, R.; Maltenfort, M.G.; Austin, M.S. How Much Pain Is Significant? Defining the Minimal Clinically Important Difference for the Visual Analog Scale for Pain After Total Joint Arthroplasty. J. Arthroplasty 2018, 33, S71–S75.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, W.N.; Ding, Z.C.; Yuan, M.C.; Cai, Y.R.; Zhou, Z.K. Duloxetine reduces pain after Total hip arthroplasty: A prospective, randomized controlled study. BMC Musculoskelet. Disord. 2021, 22, 492. [Google Scholar] [CrossRef]
- Rojanasopondist, P.; Galea, V.P.; Connelly, J.W.; Matuszak, S.J.; Rolfson, O.; Bragdon, C.R.; Malchau, H. What Preoperative Factors are Associated With Not Achieving a Minimum Clinically Important Difference After THA? Findings from an International Multicenter Study. Clin. Orthop. Relat. Res. 2019, 477, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Tubach, F.; Ravaud, P.; Baron, G.; Falissard, B.; Logeart, I.; Bellamy, N.; Bombardier, C.; Felson, D.; Hochberg, M.; van der Heijde, D.; et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: The patient acceptable symptom state. Ann. Rheum. Dis. 2005, 64, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Emara, A.K.; Klika, A.; Piuzzi, N.S.; The Cleveland Clinic OME Arthroplasty Group. Does Implant Selection Affect Patient-Reported Outcome Measures After Primary Total Hip Arthroplasty? J. Bone Jt. Surg. Am. 2021, 103, 2306–2317. [Google Scholar] [CrossRef]
- Sutton, R.M.; Baker, C.M.; D’Amore, T.; Krueger, C.A.; Courtney, P.M. The Appropriateness of Preoperative Patient Reported Outcome Measures as an Indication for Total Hip Arthroplasty. J. Arthroplasty 2023, 38, S252–S257. [Google Scholar] [CrossRef]
- Fortin, P.R.; Penrod, J.R.; Clarke, A.E.; St-Pierre, Y.; Joseph, L.; Bélisle, P.; Liang, M.H.; Ferland, D.; Phillips, C.B.; Mahomed, N.; et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002, 46, 3327–3330. [Google Scholar] [CrossRef]
- Peters, R.M.; van Steenbergen, L.N.; Stewart, R.E.; Stevens, M.; Rijk, P.C.; Bulstra, S.K.; Zijlstra, W.P. Which patients improve most after total hip arthroplasty? Influence of patient characteristics on patient-reported outcome measures of 22,357 total hip arthroplasties in the Dutch Arthroplasty Register. Hip Int. 2021, 31, 593–602. [Google Scholar] [CrossRef]
- Ibaseta, A.; Pasqualini, I.; Khan, S.T.; Zhang, C.; Klika, A.K.; Piuzzi, N.S.; Cleveland Clinic Adult Reconstruction Research Group. Contralateral THAs More Than 1 Year Apart: Do PROMs and Healthcare Utilization Differ After Each Surgery? Clin. Orthop. Relat. Res. 2025, 483, 832–842. [Google Scholar] [CrossRef]
- Rolfson, O.; Donahue, G.S.; Hallsten, M.; Garellick, G.; Karrholm, J.; Nemes, S. Patient-reported outcomes in cemented and uncemented total hip replacements. Hip Int. 2016, 26, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.M.; Khan, F.J.; Feathers, J.R.; Lewis, M.H.; Morris, K.H.; Waddell, J.P. Uncemented total hip arthroplasty can be used safely in the elderly population. Bone Jt. Open 2021, 2, 293–300. [Google Scholar] [CrossRef]
- Toro, G.; Bothorel, H.; Saffarini, M.; Jacquot, L.; Chouteau, J.; Rollier, J.C. Uncemented total hip arthroplasty in octogenarian and nonagenarian patients. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bloch, B.V.; White, J.J.E.; Matar, H.E.; Berber, R.; Manktelow, A.R.J. Should patient age thresholds dictate fixation strategy in total hip arthroplasty? Bone Jt. J. 2022, 104-B, 206–211. [Google Scholar] [CrossRef]
- Meding, J.B.; Ritter, M.A.; Davis, K.E.; Hillery, M. Cemented and uncemented total hip arthroplasty using the same femoral component. Hip Int. 2016, 26, 62–66. [Google Scholar] [CrossRef]
- SIRIS. SIRIS Report 2024. Annual Report of the Swiss National Joint Registry, Hip and Knee; SIRIS: Bern, Switzerland, 2024. [Google Scholar]
- Call, C.M.; Lachance, A.D.; Zink, T.M.; Stoddard, H.; Babikian, G.M.; Rana, A.J.; McGrory, B.J. Variation in Demographics, Hospital, and Patient-Reported Outcomes Following Total Hip Arthroplasty According to Biological Sex. J. Arthroplasty 2025, 40, 127–135.e1. [Google Scholar] [CrossRef] [PubMed]
- Rossi, N.; Nannini, A.; Ulivi, M.; Sirtori, P.; Banfi, G.; Tomaiuolo, R.; de Girolamo, L.; Mangiavini, L.; Peretti, G.M. Men and women undergoing total hip arthroplasty have different clinical presentations before surgery and different outcomes at 1-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 2635–2643. [Google Scholar] [CrossRef]
- Aggarwal, A.; Naylor, J.M.; Adie, S.; Liu, V.K.; Harris, I.A. Preoperative Factors and Patient-Reported Outcomes After Total Hip Arthroplasty: Multivariable Prediction Modeling. J. Arthroplasty 2022, 37, 714–720.e714. [Google Scholar] [CrossRef]
- Prentice, H.A.; Inacio, M.C.S.; Singh, A.; Namba, R.S.; Paxton, E.W. Preoperative Risk Factors for Opioid Utilization After Total Hip Arthroplasty. J. Bone Jt. Surg. Am. 2019, 101, 1670–1678. [Google Scholar] [CrossRef]
- Wang, W.; Morrison, T.A.; Geller, J.A.; Yoon, R.S.; Macaulay, W. Predicting short-term outcome of primary total hip arthroplasty:a prospective multivariate regression analysis of 12 independent factors. J. Arthroplasty 2010, 25, 858–864. [Google Scholar] [CrossRef]
- Lin, E.; Bozic, K.J.; Ibrahim, S.; O’Connor, M.I.; Nelson, C.L. Does Value-Based Care Threaten Joint Arthroplasty Access for Vulnerable Patient Populations?: AOA Critical Issues. J. Bone Jt. Surg. Am. 2022, 104, e92. [Google Scholar] [CrossRef]
- MacDonald, D.J.; Clement, N.D.; Howie, C.R.; Scott, C.E.H. The effect of COVID-19 restrictions on rehabilitation and functional outcome following total hip and knee arthroplasty during the first wave of the pandemic. Bone Jt. Open 2021, 2, 380–387. [Google Scholar] [CrossRef]
- Hanna, S.A.; Sarraf, K.M.; Ramachandran, M.; Achan, P. Systematic review of the outcome of total hip arthroplasty in patients with sequelae of Legg-Calve-Perthes disease. Arch. Orthop. Trauma Surg. 2017, 137, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Flecher, X.; Pioger, C.; Fabre-Aubrespy, M.; Ollivier, M.; Argenson, J.N. Long-term results of custom-made femoral stems. Orthopade 2020, 49, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Nogier, A.; Tourabaly, I.; Ramos-Pascual, S.; Muller, J.H.; Saffarini, M.; Courtin, C. Outcomes of primary total hip arthroplasty using 3D image-based custom stems in unselected patients: A systematic review. EFORT Open Rev. 2021, 6, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Devane, P.; Horne, G.; Gehling, D.J. Oxford hip scores at 6 months and 5 years are associated with total hip revision within the subsequent 2 years. Clin. Orthop. Relat. Res. 2013, 471, 3870–3874. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Cleveland Clinic, O.M.E. Arthroplasty Group. Patient-reported outcomes at 1 and 2 years after total hip and knee arthroplasty: What is the minimum required follow-up? Arch. Orthop. Trauma Surg. 2022, 142, 2121–2129. [Google Scholar] [CrossRef]
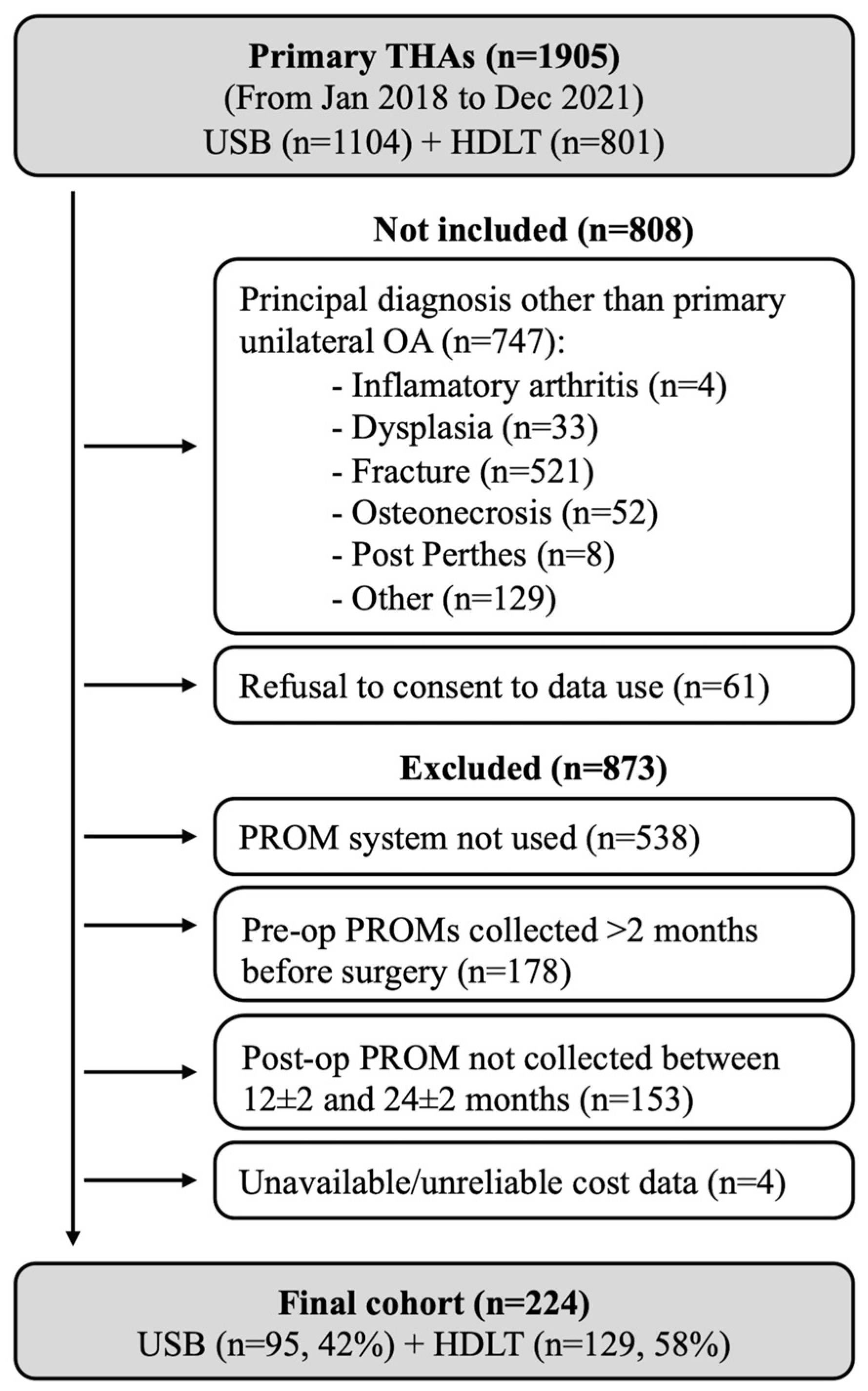
| Total (n = 224 Patients) | ||
|---|---|---|
| Patient characteristics | ||
| Age, mean (SD) | 70 | (10) |
| BMI, mean (SD) | 26 | (4) |
| Male sex, N (%) | 102 | (46) |
| Previous hip surgery, N (%) * | 8 | (4) |
| Charnley grade, N (%) ** | ||
| A | 119 | (53) |
| B1 | 60 | (27) |
| B2 | 33 | (15) |
| C | 2 | (1) |
| ASA score, N (%) | ||
| 1 | 30 | (13) |
| 2 | 133 | (59) |
| 3 | 59 | (26) |
| 4 | 2 | (1) |
| Hospitalization characteristics | ||
| LOS (days), mean (SD) | 4 | (2) |
| Surgical approach, N (%) | ||
| Anterior | 132 | (59) |
| Anterolateral | 88 | (39) |
| Posterior | 4 | (2) |
| Custom-made THA, N (%) | 16 | (7) |
| Cementation type, N (%) | ||
| Uncemented | 141 | (63) |
| Hybrid *** | 75 | (33) |
| Fully cemented | 8 | (4) |
| Total (n = 224 Patients) | ||
|---|---|---|
| Preoperative PROM, mean (SD) | 57 | (17) |
| Postoperative PROM, mean (SD) | 91 | (15) |
| ≥PASS, N (%) | 188 | (84) |
| PROM improvement, mean (SD) | 34 | (18) |
| ≥MCID, N (%) | 206 | (92) |
| PROM interpretation, N (%) | ||
| Nonresponders (<MCID) | 19 | (8) |
| Moderate responders (≥MCID AND <PASS) | 21 | (9) |
| High responders (≥MCID AND ≥PASS) | 184 | (82) |
| Quality | Cost | Value | ||||||||||
| β | 95% C.I. | p | β | 95% C.I. | p | β | 95% C.I. | p | ||||
| Age (yrs) * | 0.08 | (−0.07 to 0.22) | 0.293 | −0.01 | (−0.03 to 0.01) | 0.443 | 0.08 | (−0.08 to 0.24) | 0.311 | |||
| BMI | 0.00 | (−0.03 to 0.02) | 0.765 | 0.00 | (−0.00 to 0.01) | 0.123 | −0.01 | (−0.04 to 0.02) | 0.451 | |||
| Male sex | 0.21 | (−0.02 to 0.45) | 0.076 | −0.01 | (−0.04 to 0.02) | 0.576 | 0.27 | (0.00 to 0.53) | 0.047 | |||
| Preoperative PROM * | −0.35 | (−0.43 to −0.27) | <0.001 | 0.00 | (−0.01 to 0.01) | 0.957 | −0.36 | (−0.45 to −0.26) | <0.001 | |||
| Previous hip surgery | −0.96 | (−1.56 to 0.37) | 0.002 | 0.05 | (−0.03 to 0.14) | 0.221 | −1.41 | (−2.09 to −0.74) | <0.001 | |||
| ASA score | ||||||||||||
| 1 | REF | REF | REF | |||||||||
| 2 | −0.18 | (−0.53 to 0.17) | 0.308 | 0.02 | (−0.03 to 0.07) | 0.392 | −0.04 | (−0.44 to 0.36) | 0.851 | |||
| 3 | −0.39 | (−0.80 to 0.03) | 0.066 | 0.03 | (−0.03 to 0.09) | 0.361 | −0.27 | (−0.74 to 0.20) | 0.263 | |||
| 4 | −0.55 | (−1.72 to 0.61) | 0.350 | 0.12 | (−0.05 to 0.29) | 0.152 | −0.89 | (−2.22 to 0.43) | 0.185 | |||
| Charnley grade | ||||||||||||
| A | REF | REF | REF | |||||||||
| B1 | −0.04 | (−0.30 to 0.21) | 0.737 | 0.02 | (−0.01 to 0.06) | 0.195 | −0.12 | (−0.41 to 0.17) | 0.431 | |||
| B2 | −0.31 | (−0.63 to 0.01) | 0.060 | 0.01 | (−0.04 to 0.06) | 0.714 | −0.36 | (−0.73 to −0.00) | 0.049 | |||
| C | 0.10 | (−1.04 to 1.24) | 0.864 | −0.07 | (−0.23 to 0.10) | 0.432 | 0.09 | (−1.20 to 1.38) | 0.892 | |||
| Institution—THA type | ||||||||||||
| La Tour—standard | REF | REF | REF | |||||||||
| La Tour—custom | −0.04 | (−0.56 to 0.47) | 0.864 | 0.81 | (0.74 to 0.89) | <0.001 | −0.76 | (−1.35 to −0.18) | 0.011 | |||
| USB—standard | −0.80 | (−1.15 to −0.44) | <0.001 | −0.24 | (−0.29 to −0.19) | <0.001 | −0.15 | (−0.56 to 0.25) | 0.453 | |||
| LOS (days) | −0.03 | (−0.12 to 0.06) | 0.508 | 0.01 | (−0.00 to 0.02) | 0.090 | −0.05 | (−0.15 to 0.05) | 0.354 | |||
| Cementation type | ||||||||||||
| None | REF | REF | REF | |||||||||
| Hybrid ** | −0.08 | (−0.37 to 0.21) | 0.595 | −0.06 | (−0.11 to −0.02) | 0.003 | 0.13 | (−0.20 to 0.46) | 0.438 | |||
| Fully cemented | −0.71 | (−1.33 to −0.09) | 0.025 | 0.00 | (−0.09 to 0.09) | 0.970 | −0.83 | (−1.53 to −0.12) | 0.021 | |||
| Year of operation | ||||||||||||
| 2018 | REF | REF | REF | |||||||||
| 2019 | −0.07 | (−0.53 to 0.38) | 0.749 | 0.01 | (−0.06 to 0.08) | 0.792 | −0.04 | (−0.56 to 0.48) | 0.878 | |||
| 2020 | −0.57 | (−0.97 to −0.16) | 0.006 | −0.20 | (−0.26 to −0.14) | <0.001 | −0.27 | (−0.73 to 0.19) | 0.256 | |||
| 2021 | −0.46 | (−0.86 to −0.07) | 0.022 | −0.30 | (−0.36 to −0.25) | <0.001 | 0.05 | (−0.40 to 0.50) | 0.822 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christofilopoulos, P.; Bothorel, H.; Bilger, S.; Rüter, F.; Cody, R.; Stoffel, K. Measuring Short-Term Outcomes Following Primary Total Hip Arthroplasty: A Value-Based Healthcare Approach. J. Clin. Med. 2025, 14, 3310. https://doi.org/10.3390/jcm14103310
Christofilopoulos P, Bothorel H, Bilger S, Rüter F, Cody R, Stoffel K. Measuring Short-Term Outcomes Following Primary Total Hip Arthroplasty: A Value-Based Healthcare Approach. Journal of Clinical Medicine. 2025; 14(10):3310. https://doi.org/10.3390/jcm14103310
Chicago/Turabian StyleChristofilopoulos, Panayiotis, Hugo Bothorel, Selina Bilger, Florian Rüter, Robyn Cody, and Karl Stoffel. 2025. "Measuring Short-Term Outcomes Following Primary Total Hip Arthroplasty: A Value-Based Healthcare Approach" Journal of Clinical Medicine 14, no. 10: 3310. https://doi.org/10.3390/jcm14103310
APA StyleChristofilopoulos, P., Bothorel, H., Bilger, S., Rüter, F., Cody, R., & Stoffel, K. (2025). Measuring Short-Term Outcomes Following Primary Total Hip Arthroplasty: A Value-Based Healthcare Approach. Journal of Clinical Medicine, 14(10), 3310. https://doi.org/10.3390/jcm14103310






