Prevalence and Risk Factors of Oral Lesions in a Portuguese Subpopulation: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Data Collection and Variables
2.3. Data Analysis
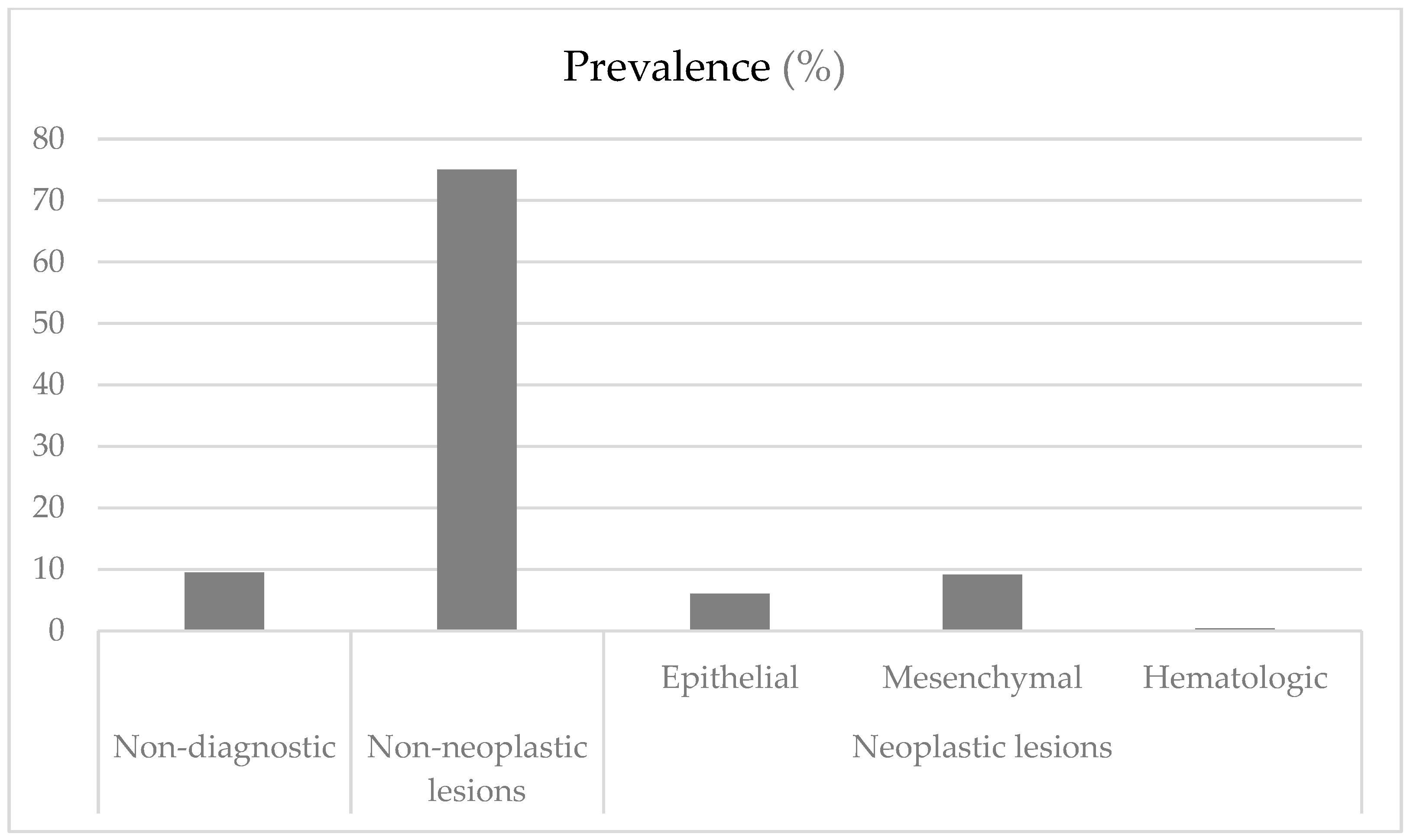
3. Results
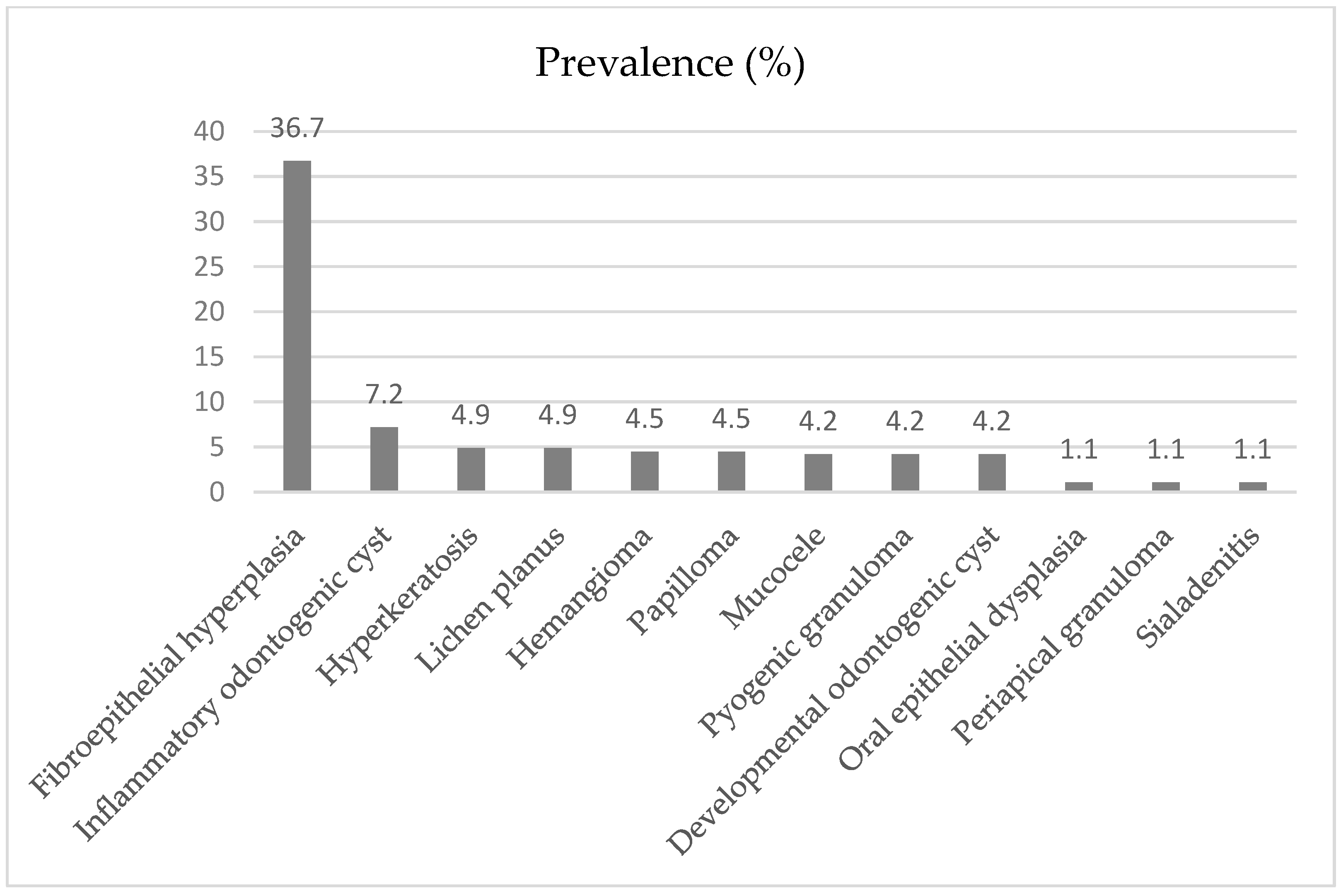
3.1. Non-Neoplastic Lesions
3.2. Neoplastic Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMDC | Egas Moniz Dental Clinic |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| HPV | Human Papillomavirus |
| SDGs | Sustainable Development Goals |
| WHO | World Health Organization |
References
- Shanti, R.M.; Tanaka, T.; Stanton, D.C. Oral Biopsy Techniques. Dermatol. Clin. 2020, 38, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.M.; Rimal, J.; Zhang, P.; Johnson, N.W. Stark Differences in Cancer Epidemiological Data Between GLOBOCAN and GBD: Emphasis on Oral Cancer and Wider Implications. EClinicalMedicine 2022, 54, 101673. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC)/World Health Organization (WHO). Global Cancer Observatory: Cancer Today. Portugal. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/620-portugal-fact-sheet.pdf (accessed on 5 March 2025).
- Feng, J.; Zhou, Z.; Shen, X.; Wang, Y.; Shi, L.; Wang, Y.; Hu, Y.; Sun, H.; Liu, W. Prevalence and distribution of oral mucosal lesions: A cross-sectional study in Shanghai, China. J. Oral. Pathol. Med. 2015, 44, 490–494. [Google Scholar] [CrossRef]
- El Toum, S.; Cassia, A.; Bouchi, N.; Kassab, I. Prevalence and distribution of oral mucosal lesions by sex and age categories: A retrospective study of patients attending Lebanese School of Dentistry. Int. J. Dent. 2018, 2018, 4030134. [Google Scholar] [CrossRef]
- Ertem, S.Y.; Uz, F. An assessment of the distribution and prevalence of benign intraoral pathologies. Diagnostics 2025, 15, 350. [Google Scholar] [CrossRef]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral cancer and precancer: A narrative review on the relevance of early diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J. Oral. Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef]
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Business Council for Sustainable Development. Objetivo 3: Saúde de Qualidade. 2024. Available online: https://ods.pt/objectivos/3-vida-saudavel/ (accessed on 15 June 2024).
- Glick, M.; Williams, D.M.; Kleinman, D.V.; Vujicic, M.; Watt, R.G.; Weyant, R.J. A New Definition for Oral Health Developed by the FDI World Dental Federation Opens the Door to a Universal Definition of Oral Health. J. Am. Dent. Assoc. 2016, 147, 915–917. [Google Scholar] [CrossRef]
- Huang, Y.K.; Chang, Y.C. Oral Health: The First Step to Sustainable Development Goal 3. J. Formos. Med. Assoc. 2022, 121, 1348–1350. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2025, 25th ed.; Norwegian Institute of Public Health: Oslo, Norway, 2024. [Google Scholar]
- World Health Organization, Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2023. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Área Metropolitana de Lisboa. Municípios AML. 2023. Available online: www.aml.pt/index.php (accessed on 4 November 2024).
- Monteiro, L.S.; Albuquerque, R.; Paiva, A.; de la Peña-Moral, J.; Amaral, J.B.; Lopes, C.A. A Comparative Analysis of Oral and Maxillofacial Pathology Over a 16-Year Period, in the North of Portugal. Int. Dent. J. 2017, 67, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Oivio, U.M.; Pesonen, P.; Ylipalosaari, M.; Kullaa, A.; Salo, T. Prevalence of Oral Mucosal Normal Variations and Lesions in a Middle-Aged Population: A Northern Finland Birth Cohort 1966 Study. BMC Oral. Health 2020, 20, 357. [Google Scholar] [CrossRef]
- Saraswathi, T.R.; Ranganathan, K.; Shanmugam, S.; Sowmya, R.; Narasimhan, P.D.; Gunaseelan, R. Prevalence of Oral Lesions in Relation to Habits: Cross-Sectional Study in South India. Indian J. Dent. Res. 2006, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Al-Mobeeriek, A.; AlDosari, A.M. Prevalence of oral lesions among Saudi dental patients. Ann. Saudi Med. 2009, 29, 365–368. [Google Scholar] [CrossRef]
- Parlak, A.H.; Koybasi, S.; Yavuz, T.; Yesildal, N.; Anul, H.; Aydogan, I.; Cetinkaya, R.; Kavak, A. Prevalence of oral lesions in 13- to 16-year-old students in Duzce, Turkey. Oral. Dis. 2006, 12, 553–558. [Google Scholar] [CrossRef]
- Demko, C.A.; Sawyer, D.; Slivka, M.; Smith, D.; Wotman, S. Prevalence of oral lesions in the dental office. Gen. Dent. 2009, 57, 504–509. [Google Scholar] [PubMed]
- Jahanbani, J.; Morse, D.E.; Alinejad, H. Prevalence of oral lesions and normal variants of the oral mucosa in 12 to 15-year-old students in Tehran, Iran. Arch. Iran. Med. 2012, 15, 142–145. [Google Scholar]
- Blanco, D.; Rodrigues, R.; Lemos, C.; Bussadori, S.; Júnior, J.; Teixeira, V. A retrospective study of the incidence of oral lesions of soft tissue referenced in a Brazilian town. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2017, 124, e128. [Google Scholar] [CrossRef]
- Amaral, S.; Miranda, Á.; Netto, J.; Pires, F. Prevalence of oral and maxillofacial diseases diagnosed in an Oral Medicine service during a 7-year period. J. Oral. Diagn. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, V.S.; Ananda, S.R.; Kavitha, A.P.; Krupashankar, R. Prevalence and risk indicators of oral mucosal lesions in adult population visiting primary health centers and community health centers in Kodagu district. J. Fam. Med. Prim. Care 2019, 8, 2337–2342. [Google Scholar] [CrossRef]
- Collins, J.R.; Brache, M.; Ogando, G.; Veras, K.; Rivera, H. Prevalence of oral mucosal lesions in an adult population from eight communities in Santo Domingo, Dominican Republic. Acta Odontol. Latinoam. 2021, 34, 249–256. [Google Scholar] [CrossRef]
- Bajracharya, D.; Gupta, S.; Ojha, B.; Baral, R. Prevalence of oral mucosal lesions in a tertiary care dental hospital of Kathmandu. JNMA J. Nepal. Med. Assoc. 2017, 56, 362–366. [Google Scholar] [CrossRef]
- Kelloway, E.; Ha, W.N.; Dost, F.; Farah, C.S. A retrospective analysis of oral and maxillofacial pathology in an Australian adult population. Aust. Dent. J. 2014, 59, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Margiotta, V. Oral mucosal lesions and risk habits among men in an Italian study population. J. Oral. Pathol. Med. 2001, 30, 22–28. [Google Scholar] [CrossRef]
- Shulman, J.D.; Beach, M.M.; Rivera-Hidalgo, F. The prevalence of oral mucosal lesions in U.S. adults: Data from the Third National Health and Nutrition Examination Survey, 1988–1994. J. Am. Dent. Assoc. 2004, 135, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Institute of Health Metrics and Evaluation. Global Burden of Disease 2019 (GBD 2019) Results; IHME: Seattle, WA, USA, 2020; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 12 August 2024).
- Gupta, A.; Shrestha, P.; Poudyal, S.; Kumar, S.; Lamichhane, R.S.; Acharya, S.K.; Shivhare, P. Prevalence and distribution of oral mucosal lesions and normal variants among Nepalese population. Biomed. Res. Int. 2023, 2023, 9375084. [Google Scholar] [CrossRef]
- Gambhir, R.; Veeresha, K.L.; Sohi, R.; Kakkar, H.; Aggarwal, A.; Gupta, D. The prevalence of oral mucosal lesions in the patients visiting a dental school in Northern India in relation to sex, site and distribution: A retrospective study. J. Clin. Exp. Dent. 2011, 3, e10–e17. [Google Scholar] [CrossRef]
- Ferreira, A.M.; de Souza Lucena, E.E.; de Oliveira, T.C.; da Silveira, É.; de Oliveira, P.T.; de Lima, K.C. Prevalence and factors associated with oral potentially malignant disorders in Brazil’s rural workers. Oral. Dis. 2016, 22, 536–542. [Google Scholar] [CrossRef]
- Carrard, V.; Haas, A.; Rados, P.; Filho, M.; Oppermann, R.; Albandar, J.; Susin, C. Prevalence and risk indicators of oral mucosal lesions in an urban population from South Brazil. Oral. Dis. 2011, 17, 171–179. [Google Scholar] [CrossRef]
- Pentenero, M.; Broccoletti, R.; Carbone, M.; Conrotto, D.; Gandolfo, S. The prevalence of oral mucosal lesions in adults from the Turin area. Oral. Dis. 2008, 14, 356–366. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Rai, S.; Bhatnagar, G.; Kaur, M.; Goel, S.; Prabhat, M. Prevalence study of oral mucosal lesions, mucosal variants, and treatment required for patients reporting to a dental school in North India: In accordance with WHO guidelines. J. Fam. Community Med. 2013, 20, 41–48. [Google Scholar] [CrossRef]
- Mehrotra, R.; Thomas, S.; Nair, P.; Pandya, S.; Singh, M.; Nigam, N.S.; Shukla, P. Prevalence of oral soft tissue lesions in Vidisha. BMC Res. Notes 2010, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Yang, Y.H.; Wang, T.Y.; Shieh, T.Y.; Warnakulasuriya, S. Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern Taiwan. J. Oral. Pathol. Med. 2005, 34, 460–466. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Sixto-Requeijo, R.; Diniz-Freitas, M.; Torreira-Lorenzo, J.C.; García-García, A.; Gándara-Rey, J.M. An analysis of oral biopsies extracted from 1995 to 2009, in an oral medicine and surgery unit in Galicia (Spain). Med. Oral Patol. Oral Cir. Bucal 2012, 17, e16–e22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef]
- Gandolfo, S.; Richiardi, L.; Carrozzo, M.; Broccoletti, R.; Carbone, M.; Pagano, M.; Vestita, C.; Rosso, S.; Merletti, F. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: A follow-up study in an Italian population. Oral Oncol. 2004, 40, 77–83. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Harper, J.I.; Hoeger, P.H. Infantile haemangioma. Lancet 2017, 390, 85–94. [Google Scholar] [CrossRef]
- Chowdhury, R.; Satoskar, R. Surveillance for hepatocellular carcinoma. In Primary Liver Cancer: Surveillance, Diagnosis and Treatment; Humana Press Inc.: Clifton, NJ, USA, 2012; pp. 17–43. [Google Scholar] [CrossRef]
- Lin, H.C.; Corbet, E.F.; Lo, E.C. Oral mucosal lesions in adult Chinese. J. Dent. Res. 2001, 80, 1486–1490. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística. Censos 2021 Resultados Definitivos—Portugal. 2024. Available online: https://www.sgeconomia.gov.pt/noticias/ine-censos-resultados-definitivos-2021.aspx (accessed on 14 June 2024).
- Su, S.; Lipsky, M.S.; Licari, F.W.; Hung, M. Comparing oral health behaviours of men and women in the United States. J. Dent. 2022, 122, 104157. [Google Scholar] [CrossRef]
- Espinoza, I.; Rojas, R.; Aranda, W.; Gamonal, J. Prevalence of oral mucosal lesions in elderly people in Santiago, Chile. J. Oral Pathol. Med. 2003, 32, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and cancer: Epidemiology and biological mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Liu, S.; Goloubeva, O.; Ord, R.; Strome, S.E.; Suntharalingam, M.; Taylor, R.; Morales, R.E.; Wolf, J.S.; Zimrin, A.; et al. Oropharyngeal cancer as a driver of racial outcome disparities in squamous cell carcinoma of the head and neck: 10-year experience at the University of Maryland Greenebaum Cancer Center. Head Neck 2016, 38, 564–572. [Google Scholar] [CrossRef]
- Gheno, J.N.; Martins, M.A.; Munerato, M.C.; Hugo, F.N.; Sant’ana Filho, M.; Weissheimer, C.; Carrard, V.C.; Martins, M.D. Oral mucosal lesions and their association with sociodemographic, behavioral, and health status factors. Braz. Oral Res. 2015, 29, S1806-83242015000100289. [Google Scholar] [CrossRef]
- Silva, M.F.; Barbosa, K.G.; Pereira, J.V.; Bento, P.M.; Godoy, G.P.; Gomes, D.Q. Prevalence of oral mucosal lesions among patients with diabetes mellitus types 1 and 2. An. Bras. Dermatol. 2015, 90, 49–53. [Google Scholar] [CrossRef]
- Vasconcelos, B.C.; Novaes, M.; Sandrini, F.A.; Maranhão Filho, A.W.; Coimbra, L.S. Prevalence of oral mucosa lesions in diabetic patients: A preliminary study. Braz. J. Otorhinolaryngol. 2008, 74, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Guggenheimer, J.; Moore, P.A.; Rossie, K.; Myers, D.; Mongelluzzo, M.B.; Block, H.M.; Weyant, R.; Orchard, T. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: II. Prevalence and characteristics of Candida and candidal lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 89, 570–576. [Google Scholar] [CrossRef]
- Mei, Z.; Liang, M.; Li, L.; Zhang, Y.; Wang, Q.; Yang, W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer 2017, 140, 1068–1081. [Google Scholar] [CrossRef]
- Cantini, L.; Pecci, F.; Hurkmans, D.P.; Belderbos, R.A.; Lanese, A.; Copparoni, C.; Aerts, S.; Cornelissen, R.; Dumoulin, D.W.; Fiordoliva, I.; et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur. J. Cancer 2021, 144, 41–48. [Google Scholar] [CrossRef]
- Bertl, K.; Parllaku, A.; Pandis, N.; Buhlin, K.; Klinge, B.; Stavropoulos, A. The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy-a systematic review and meta-analysis. J. Dent. 2017, 67, 18–28. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Carbonate apatite containing statin enhances bone formation in healing incisal extraction sockets in rats. Materials 2018, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, N.K.; Kim, Y.G.; Kim, J.Y.; Kim, H.H.; Lee, Y. Fluvastatin inhibits osteoclast differentiation and Porphyromonas gingivalis lipopolysaccharide-induced alveolar bone erosion in mice. J. Periodontol. 2017, 88, 390–398. [Google Scholar] [CrossRef]
- Moraschini, V.; Almeida, D.C.F.; Calasans-Maia, J.A.; Diuana Calasans-Maia, M. The ability of topical and systemic statins to increase osteogenesis around dental implants: A systematic review of histomorphometric outcomes in animal studies. Int. J. Oral Maxillofac. Surg. 2018, 47, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Varalakshmi, P.R.; Kavitha, M.; Govindan, R.; Narasimhan, S. Effect of statins with α-tricalcium phosphate on proliferation, differentiation, and mineralization of human dental pulp cells. J. Endod. 2013, 39, 806–812. [Google Scholar] [CrossRef]
- Jahanbin, A.; Abtahi, M.; Namdar, P.; Heravi, F.; Sadeghi, F.; Arab, H.; Shafaee, H. Evaluation of the effects of subgingival injection of simvastatin on space re-opening after orthodontic space closure in adults. J. Dent. Res. Dent. Clin. Dent. Prospects 2016, 10, 3–7. [Google Scholar] [CrossRef]
- Madi, M.; Kassem, A. Topical simvastatin gel as a novel therapeutic modality for palatal donor site wound healing following free gingival graft procedure. Acta Odontol. Scand. 2018, 76, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, X.; Chen, J.; Ge, X.; Qin, Q.; Zhu, H.; Zhang, C.; Sun, X. Simvastatin attenuates radiation-induced salivary gland dysfunction in mice. Drug Des. Devel. Ther. 2016, 10, 2271–2278. [Google Scholar] [CrossRef]
- Gutkind, J.S.; Molinolo, A.A.; Wu, X.; Wang, Z.; Nachmanson, D.; Harismendy, O.; Alexandrov, L.B.; Wuertz, B.R.; Ondrey, F.G.; Laronde, D.; et al. Inhibition of mTOR signaling and clinical activity of metformin in oral premalignant lesions. JCI Insight 2021, 6, e147096. [Google Scholar] [CrossRef]
- Wei, J.; Huang, J.; Kuang, Y.; Li, Y.; Zhong, D.; Song, J. Metformin inhibits proliferation of oral squamous cell carcinoma cells by suppressing proteolysis of nerve growth factor receptor. Arch. Oral Biol. 2021, 121, 104971. [Google Scholar] [CrossRef]
| Variable | n (%) | |
|---|---|---|
| Gender | Female | 155 (58.7) |
| Male | 109 (41.3) | |
| Ager (years) | 20–44 | 72 (27.3) |
| 45–64 | 101 (38.3) | |
| ≥65 | 91 (34.5) | |
| Employment status | Student | 19 (7.2) |
| Employed | 117 (44.3) | |
| Unemployed | 36 (13.6) | |
| Retired | 92 (34.8) | |
| Toothbrushing frequency | 2–3 times/daily | 205 (77.7) |
| 1 time/daily | 50 (18.9) | |
| 2–6 times/weekly | 9 (3.4) | |
| Smoking status | Smoker | 70 (26.5) |
| Non-smoker | 194 (73.5) | |
| Active smokers (Cigarettes per day) (n = 70) | Light (<10) | 32 (45.7) |
| Medium (10–20) | 27 (38.6) | |
| Heavy (<20) | 11 (15.7) | |
| Systemic diseases | Yes | 172 (65.2) |
| No | 92 (34.8) | |
| History of oncological disease | Yes | 13 (4.9) |
| No | 251 (95.1) | |
| Medication | Yes | 158 (59.8) |
| No | 106 (40.2) | |
| Use of oral prosthesis | Yes | 89 (33.7) |
| No | 175 (66.3) | |
| Variates/Diagnostic Category | Non-Neoplastic Lesion (%) | Neoplastic Lesion (%) | Epithelial (%) | Mesenchymal (%) | Hematologic (%) | |
|---|---|---|---|---|---|---|
| Gender | F | 119 (60.1) | 20 (48.8) | 7 (43.8) | 12 (50.0) | 1 (100.0) |
| M | 79 (39.9) | 21 (51.2) | 9 (56.3) | 12 (50.0) | 0 (0.0) | |
| Age | 20–44 | 53 (26.7) | 14 (34.1) | 7 (43.8) | 7 (29.2) | 0 (0.0) |
| 45–64 | 81 (40.9) | 9 (22.0) | 3 (18.8) | 6 (25.0) | 0 (0.0) | |
| ≥65 | 64 (32.3) | 18 (43.9) | 6 (37.5) | 11 (45.8) | 1 (100.0) | |
| Employment status | Student | 10 (5.1) | 7 (17.1) | 4 (25.0) | 3 (12.5) | 0 (0.0) |
| Employed | 96 (48.5) | 11 (26.8) | 4 (25.0) | 7 (29.2) | 0 (0.0) | |
| Unemployed | 30 (15.2) | 3 (7.3) | 1 (6.3) | 2 (8.3) | 0 (0.0) | |
| Retired | 62 (31.3) | 20 (48.8) | 7 (43.8) | 12 (50.0) | 1 (100.0) | |
| Toothbrushing frequency | 2–3 times/daily | 151 (76.3) | 31 (75.6) | 8 (50.0) | 22 (91.7) | 1 (100.0) |
| 1 time/daily | 40 (20.2) | 8 (19.5) | 7 (43.8) | 1 (4.2) | 0 (0.0) | |
| 2–6 times/weekly | 7 (3.5) | 2 (4.9) | 1 (6.3) | 1 (4.2) | 0 (0.0) | |
| Smoking status | Smoker | 53 (26.8) | 10 (24.4) | 4 (25.0) | 6 (25.0) | 0 (0.0) |
| Non-smoker | 145 (73.2) | 31 (75.6) | 12 (75.0) | 18 (75.0) | 1 (100.0) | |
| Active smokers (Cigarettes per day) | Light (<10) | 22 (41.5) | 7 (70.0) | 4 (100.0) | 3 (50.0) | 0 (0.0) |
| Medium (10–20) | 22 (41.5) | 1 (10.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | |
| Heavy (<20) | 9 (17.0) | 2 (20.0) | 0 (0.0) | 2 (33,3) | 0 (0.0) | |
| Drugs | Yes | 3 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 195 (98.5) | 41 (100.0) | 16 (100.0) | 24 (100.0) | 1 (100.0) | |
| Alcoholism | Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 198 (100.0) | 41 (100.0) | 16 (100.0) | 24 (100.0) | 1 (100.0) | |
| Systemic diseases | Yes | 125 (63.1) | 29 (70.7) | 10 (62.5) | 18 (75.0) | 1 (100.0) |
| No | 73 (36.9) | 12 (29.3) | 6 (37.5) | 6 (25.0) | 0 (0.0) | |
| History of oncological disease | Yes | 7 (3.5) | 5 (12.2) | 1 (6.3) | 4 (16.7) | 0 (0.0) |
| No | 191 (96.5) | 36 (87.8) | 15 (93.8) | 20 (83.3) | 1 (100.0) | |
| Medication | Yes | 116 (58.6) | 24 (58.5) | 6 (37.5) | 17 (70.8) | 1 (100.0) |
| No | 82 (41.4) | 17 (41.5) | 10 (62.5) | 7 (29.2) | 0 (0.0) | |
| Use of dental prosthesis | Yes | 66 (33.3) | 14 (34.1) | 3 (18.8) | 10 (41.7) | 1 (100.0) |
| No | 132 (66.7) | 27 (65.9) | 13 (81.3) | 14 (58.3) | 0 (0.0) | |
| Neoplastic Lesion (%) | p-Value | Epithelial (%) | p-Value | Mesenchymal (%) | p-Value | Hematologic (%) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Antihypertensive (C09) | No | 16.0 | 0.768 * | 6.3 | 0.830 * | 9.7 | 0.621 * | 0.0 | - |
| Yes | 14.6 | 5.6 | 7.9 | 1.1 | |||||
| Statins (C10AA) | No | 17.4 | 0.046 * | 7.1 | - | 9.8 | 0.549 ** | 0.4 | - |
| Yes | 5.0 | 0.0 | 5.0 | 0.0 | |||||
| Antidepressant (N06A) | No | 15.5 | 1.00 ** | 6.5 | 0.702 ** | 8.6 | 0.508 ** | 0.4 | - |
| Yes | 15.6 | 3.1 | 12.5 | 0.0 | |||||
| Antidiabetics (A10) | No | 12.7 | 0.001 ** | 5.1 | 0.075 ** | 7.2 | 0.007 ** | 0.4 | - |
| Yes | 39.3 | 14.3 | 25.0 | 0.0 | |||||
| History of oncological disease | No | 14.3 | 0.035 ** | 6.0 | 0.565 ** | 8.0 | 0.022 ** | 0.4 | - |
| Yes | 38.5 | 7.7 | 30.8 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroteia, C.; Pereira, G.M.; Proença, L.; Mendes, J.J.; Cavacas, M.A. Prevalence and Risk Factors of Oral Lesions in a Portuguese Subpopulation: A Retrospective Study. J. Clin. Med. 2025, 14, 3294. https://doi.org/10.3390/jcm14103294
Doroteia C, Pereira GM, Proença L, Mendes JJ, Cavacas MA. Prevalence and Risk Factors of Oral Lesions in a Portuguese Subpopulation: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(10):3294. https://doi.org/10.3390/jcm14103294
Chicago/Turabian StyleDoroteia, Carolina, Gonçalo Martins Pereira, Luís Proença, José João Mendes, and Maria Alzira Cavacas. 2025. "Prevalence and Risk Factors of Oral Lesions in a Portuguese Subpopulation: A Retrospective Study" Journal of Clinical Medicine 14, no. 10: 3294. https://doi.org/10.3390/jcm14103294
APA StyleDoroteia, C., Pereira, G. M., Proença, L., Mendes, J. J., & Cavacas, M. A. (2025). Prevalence and Risk Factors of Oral Lesions in a Portuguese Subpopulation: A Retrospective Study. Journal of Clinical Medicine, 14(10), 3294. https://doi.org/10.3390/jcm14103294









