Proximal Landing Zone’s Impact on Outcomes of Branched and Fenestrated Aortic Arch Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Data Collection
2.4. Definitions
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Previous Aortic History and Current Disease
3.3. Device Configuration
3.4. Thirty-Day Outcomes
3.5. Follow-Up Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenorio, E.R.; Oderich, G.S.; Kölbel, T.; Dias, N.V.; Sonesson, B.; Karelis, A.; Farber, M.; Parodi, F.E.; Timaran, C.H.; Scott, C.K.; et al. Multicenter global early feasibility study to evaluate total endovascular arch repair using three-vessel inner branch stent-grafts for aneurysms and dissections. J. Vasc. Surg. 2021, 74, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Detter, C.; Law, Y.; Rohlffs, F.; Heidemann, F.; Brickwedel, J.; von Kodolitsch, Y.; Debus, S.E.; Kölbel, T. Single-center experience with an inner branched arch endograft. J. Vasc. Surg. 2019, 69, 977–985.e1. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Law, Y.; Rohlffs, F.; Spanos, K.; Debus, E.S.; Kölbel, T. Fenestrated endovascular repair for diseases involving the aortic arch. J. Vasc. Surg. 2020, 71, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Debus, E.S.; von Kodolitsch, Y.; Wipper, S.; Rohlffs, F.; Detter, C.; Roeder, B.; Kölbel, T. Branched versus fenestrated endografts for endovascular repair of aortic arch lesions. J. Vasc. Surg. 2016, 64, 592–599. [Google Scholar] [CrossRef]
- DiBartolomeo, A.D.; Ding, L.; Weaver, F.A.; Han, S.M.; Magee, G.A. Risk of Stroke with Thoracic Endovascular Aortic Repair of the Aortic Arch. Ann. Vasc. Surg. 2023, 97, 37–48. [Google Scholar] [CrossRef]
- Fichadiya, A.; Gregory, A.J.; Kotha, V.K.; Herget, E.J.; Smith, H.N.; Tai, E.; Guo, M.; Mina, F.; Appoo, J.J. Extended-arch repair for acute type-A aortic dissection: Perioperative and mid-term results. Eur. J. Cardiothorac. Surg. 2019, 56, 714–721. [Google Scholar] [CrossRef]
- Kölbel, T.; Rohlffs, F.; Wipper, S.; Carpenter, S.W.; Debus, E.S.; Tsilimparis, N. Carbon Dioxide Flushing Technique to Prevent Cerebral Arterial Air Embolism and Stroke During TEVAR. J. Endovasc. Ther. 2016, 23, 393–395. [Google Scholar] [CrossRef]
- Rohlffs, F.; Trepte, C.; Ivancev, K.; Tsilimparis, N.; Makaloski, V.; Debus, E.S.; Kölbel, T. Air Embolism During TEVAR: Liquid Perfluorocarbon Absorbs Carbon Dioxide in a Combined Flushing Technique and Decreases the Amount of Gas Released From Thoracic Stent-Grafts During Deployment in an Experimental Setting. J. Endovasc. Ther. 2019, 26, 76–80. [Google Scholar] [CrossRef]
- Nana, P.; Houérou, T.L.; Guihaire, J.; Gaudin, A.; Fabre, D.; Haulon, S. Early Outcomes on Triple-Branch Arch Device with Retrograde Left Common Carotid Branch: A Case Series. J. Endovasc. Ther. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Abisi, S.; Elnemr, M.; Clough, R.; Alotaibi, M.; Gkoutzios, P.; Modarai, B.; Haulon, S. The Development of Totally Percutaneous Aortic Arch Repair with Inner-Branch Endografts: Experience from 2 Centers. J. Endovasc. Ther. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, 334–482. [Google Scholar] [CrossRef]
- Haulon, S.; Greenberg, R.K.; Spear, R.; Eagleton, M.; Abraham, C.; Lioupis, C.; Verhoeven, E.; Ivancev, K.; Kölbel, T.; Stanley, B.; et al. Global experience with an inner branched arch endograft. J. Thorac. Cardiovasc. Surg. 2014, 148, 1709–1716. [Google Scholar] [CrossRef]
- Verscheure, D.; Haulon, S.; Tsilimparis, N.; Resch, T.; Wanhainen, A.; Mani, K.; Dias, N.; Sobocinski, J.; Eagleton, M.; Ferreira, M.; et al. Endovascular Treatment of Post Type A Chronic Aortic Arch Dissection with a Branched Endograft: Early Results from a Retrospective International Multicenter Study. Ann. Surg. 2021, 273, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Stana, J.; Prendes, C.F.; Konstantinou, N.; Öz, T.; Pilchmaier, M.; Peterss, S.; Tsilimparis, N. Endovascular arch repair of anastomotic aneurysm and pseudoaneurysm in patients after open repair of the ascending aorta and aortic arch: A case series. Eur. J. Cardiothorac. Surg. 2023, 64, ezad345. [Google Scholar] [CrossRef] [PubMed]
- Milne, C.P.E.; Amako, M.; Spear, R.; Clough, R.A.; Hertault, A.; Sobocinski, J.; Brown, W.; Haulon, S. Inner-Branched Endografts for the Treatment of Aortic Arch Aneurysms After Open Ascending Aortic Replacement for Type A Dissection. Ann. Thorac. Surg. 2016, 102, 2028–2035. [Google Scholar] [CrossRef]
- Nana, P.; Spanos, K.; Panuccio, G.; Rohlffs, F.; Detter, C.; von Kodolitsch, Y.; Torrealba, J.I.; Kölbel, T. Branched and fenestrated endovascular aortic arch repair in patients with native proximal aortic landing zone. J. Vasc. Surg. 2024, 80, 621–629.e3. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vanderbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Olsson, K.W.; Mani, K.; Burdess, A.; Patterson, S.; Scali, S.T.; Kölbel, T.; Panuccio, G.; Eleshra, A.; Bertoglio, L.; Ardita, V.; et al. Outcomes After Endovascular Aortic Intervention in Patients with Connective Tissue Disease. JAMA Surg. 2023, 158, 832–839. [Google Scholar] [CrossRef]
- Kölbel, T.; Eleshra, A.; Aldaq, M.; Rohlffs, F.; Debus, S.E.; Honig, S.; Dtter, C.; von Kodolitsch, Y.; Tsilimparis, N.; Panuccio, G. Endovascular Treatment of Aortic Pathologies in Patients with Marfan Syndrome: Single-Center Experience. J. Endovasc. Ther. 2022, 29, 602–610. [Google Scholar] [CrossRef]
- Law, Y.; Kölbel, T.; Detter, C.; Rohlffs, F.; von Kodolitsch, Y.; Makaloski, V.; Debus, S.E.; Tsilimparis, N. Emergency Use of Branched Thoracic Endovascular Repair in the Treatment of Aortic Arch Pathologies. Ann. Thorac. Surg. 2019, 107, 1799–1806. [Google Scholar] [CrossRef]
- Kölbel, T.; Detter, C.; Carpenter, S.W.; Rohlffs, F.; von Kodolitsch, Y.; Wipper, S.; Reichenspurner, H.; Debus, S.E.; Tsilimparis, N. Acute Type A Aortic Dissection Treated Using a Tubular Stent-Graft in the Ascending Aorta and a Multibranched Stent-Graft in the Aortic Arch. J. Endovasc. Ther. 2017, 24, 75–80. [Google Scholar] [CrossRef]
- Torrealba, J.I.; Spanos, K.; Panuccio, G.; Rohlffs, F.; Gandet, T.; Heidemann, F.; Tsilimparis, N.; Kölbel, T. Non-Standard Management of Target Vessels with the Inner Branch Arch Endograft: A Single-Center Retrospective Study. J. Endovasc. Ther. 2022, 29, 555–564. [Google Scholar] [CrossRef]
- Madhwal, S.; Rahagopal, V.; Bhatt, D.L.; Baizer, C.T.; Whitlow, P.; Kapadia, S.R. Predictors of difficult carotid stenting as determined by aortic arch angiography. J. Invasive Cardiol. 2008, 20, 200–204. [Google Scholar] [PubMed]
- Mitchell, R.S.; Ishimaru, S.; Ehrlich, M.P.; Iwase, T.; Lauterjung, L.; Shimono, T.; Fattori, R.; Yutani, C. First International Summit on Thoracic Aortic Endografting: Roundtable on thoracic aortic dissection as an indication for endografting. J. Endovasc. Ther. 2002, 9, 98–105. [Google Scholar] [CrossRef]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y.; Writing Committee Group. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73, 4–52. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.R.; Kupferthaler, A.; Kern, M.; Rousseau, H.; Ferrer, C.; Iwakoshi, S.; Sakaguchi, S.; Stelzmüller, M.-E.; Ehrlich, M.; Loewe, C.; et al. Branched versus fenestrated thoracic endovascular aortic repair in the aortic arch: A multicenter comparison. J. Thorac. Cardiovasc. Surg. 2022, 164, 1379–1389. [Google Scholar] [CrossRef]
- Nana, P.; Spanos, K.; Dakis, K.; Giannoukas, A.; Kölbel, T.; Haulon, S. Systematic Review on Customized and Non-customized Device Techniques for the Endovascular Repair of the Aortic Arch. J. Endovasc. Ther. 2024, 31, 505–521. [Google Scholar] [CrossRef]
- Rohlffs, F.; Nana, P.; Panuccio, G.; Torrealba, J.I.; Tsilimparis, N.; Rybczynski, M.; Detter, C.; Kölbel, T. A Retrospective Analysis of 10-year Experience on Branched and Fenestrated Endovascular Aortic Arch Repair. Ann. Surg. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Gaudino, M.; Girardi, L.N.; Rahouma, M.; Leonard, J.R.; Di Franco, A.; Lau, C.; Mehta, N.; Abouarab, A.; Schwann, A.N.; Scuderi, G.; et al. Editor's Choice—Aortic Re-operation After Replacement of the Proximal Aorta: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 515–523. [Google Scholar] [CrossRef]
- Spath, P.; Campana, F.; Tsilimparis, N.; Gallitto, E.; Pini, R.; Faggioli, G.; Caputo, S.; Gargiulo, M. Outcomes of Fenestrated and Branched Endografts for Partial and Total Endovascular Repair of the Aortic Arch—A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 106–116. [Google Scholar] [CrossRef]
- Budtz-Lilly, J.; Vikholm, P.; Wanhainen, A.; Astudillo, R.; Thelin, S.; Mani, K. Technical eligibility for endovascular treatment of the aortic arch after open type A aortic dissection repair. J. Thorac. Cardiovasc. Surg. 2021, 162, 770–777. [Google Scholar] [CrossRef]
- Lou, X.; Leshnower, B.G.; Binongo, J.; Beckerman, Z.; McPherson, L.; Chen, E.P. Re-Operative Aortic Arch Surgery in a Contemporary Series. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 377–382. [Google Scholar] [CrossRef]
- Gao, F.; Ge, Y.; Zhong, Y.; Zhuang, X.; Zhu, J. Redo Total Aortic Arch Replacement in Patients with Aortic Dissection After Open-Heart Surgery and Long-Term Follow-Up Results. Braz. J. Cardiovasc. Surg. 2023, 38, 265–270. [Google Scholar] [CrossRef]
- Sandhu, H.K.; Tanaka, A.; Zaidi, S.T.; Perlick, A.; Miller, C.C., 3rd; Safi, H.J.; Estrera, A.L. Impact of redo sternotomy on proximal aortic repair: Does previous aortic repair affect outcomes? Thorac. Cardiovasc. Surg. 2020, 159, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Ali Taruq, M.; Malik, M.K.; Uddin, Q.S.; Altaf, Z.; Zafar, M. Minimally Invasive Procedure versus Conventional Redo Sternotomy for Mitral Valve Surgery in Patients with Previous Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Chest Surg. 2023, 56, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, H.; Ge, Y.; Guo, W. Avoiding Stroke in Patients Undergoing Endovascular Aortic Arch Repair: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 82, 265–277. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Liu, L.; Lu, Q.; Zhang, T.; Jing, Z. Retrograde Type A Aortic Dissection After Thoracic Endovascular Aortic Repair: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e004649. [Google Scholar] [CrossRef]
- Canaud, L.; Ozdemir, B.A.; Patterson, B.O.; Holt, P.J.E.; Loftus, I.M.; Thompson, M.M. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann. Surg. 2014, 260, 389–395. [Google Scholar] [CrossRef]
- Nana, P.; Panuccio, G.; Rohlffs, F.; Torrealba, J.I.; Tsilimparis, N.; Kölbel, T. Early and midterm outcomes of fenestrated and branched endovascular aortic repair in thoracoabdominal aneurysm types I through III. J. Vasc. Surg. 2023; In press. [Google Scholar] [CrossRef]
- Beckman, E.; Martens, A.; Korte, W.; Kaufeld, T.; Krueger, H.; Haverich, A.; Shrestha, M. Open total arch replacement with trifurcated graft and frozen elephant trunk. Ann. Cardiothorac. Surg. 2020, 9, 170–177. [Google Scholar] [CrossRef]
- Coselli, J.S.; Rosalli, E.E.; Preventza, O.; Malisrie, S.C.; Stewart, A.; Stelzer, P.; Takayama, H.; Chen, E.P. Total aortic arch replacement using a frozen elephant trunk device: Results of a 1-year US multicenter trial. J. Thorac. Cardiovasc. Surg. 2024, 167, 1680–1692.e2. [Google Scholar] [CrossRef]
- Hoshima, K.; Kato, M.; Ishimaru, S.; Michihata, N.; Yasunaga, H.; Komori, K.; Japanese Committee for Stentgraft Management. Effect of the urgency and landing zone on rates of in-hospital death, stroke, and paraplegia after thoracic endovascular aortic repair in Japan. J. Vasc. Surg. 2021, 74, 556–568. [Google Scholar] [CrossRef]
- Gaudry, M.; Porto, A.; Guivier-Curien, C.; Blanchard, A.; Bal, L.; Ressequier, N.; Omnes, V.; De Masi, M.; Ejargue, M.; Jacquier, A.; et al. Results of a prospective follow-up study after type A aortic dissection repair: A high rate of distal aneurysmal evolution and reinterventions. Eur. J. Cardiothorac. Surg. 2021, 61, 152–159. [Google Scholar] [CrossRef]
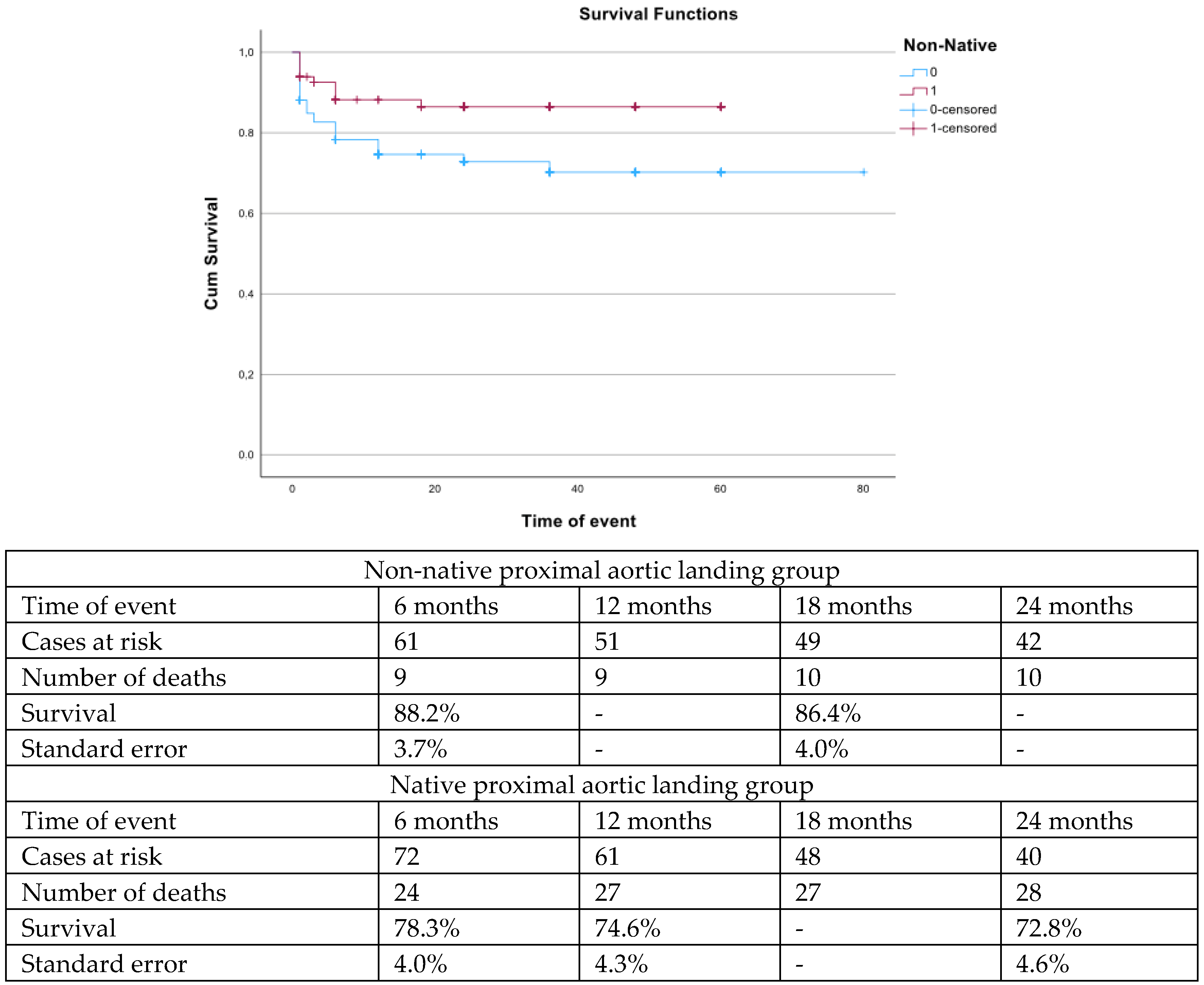


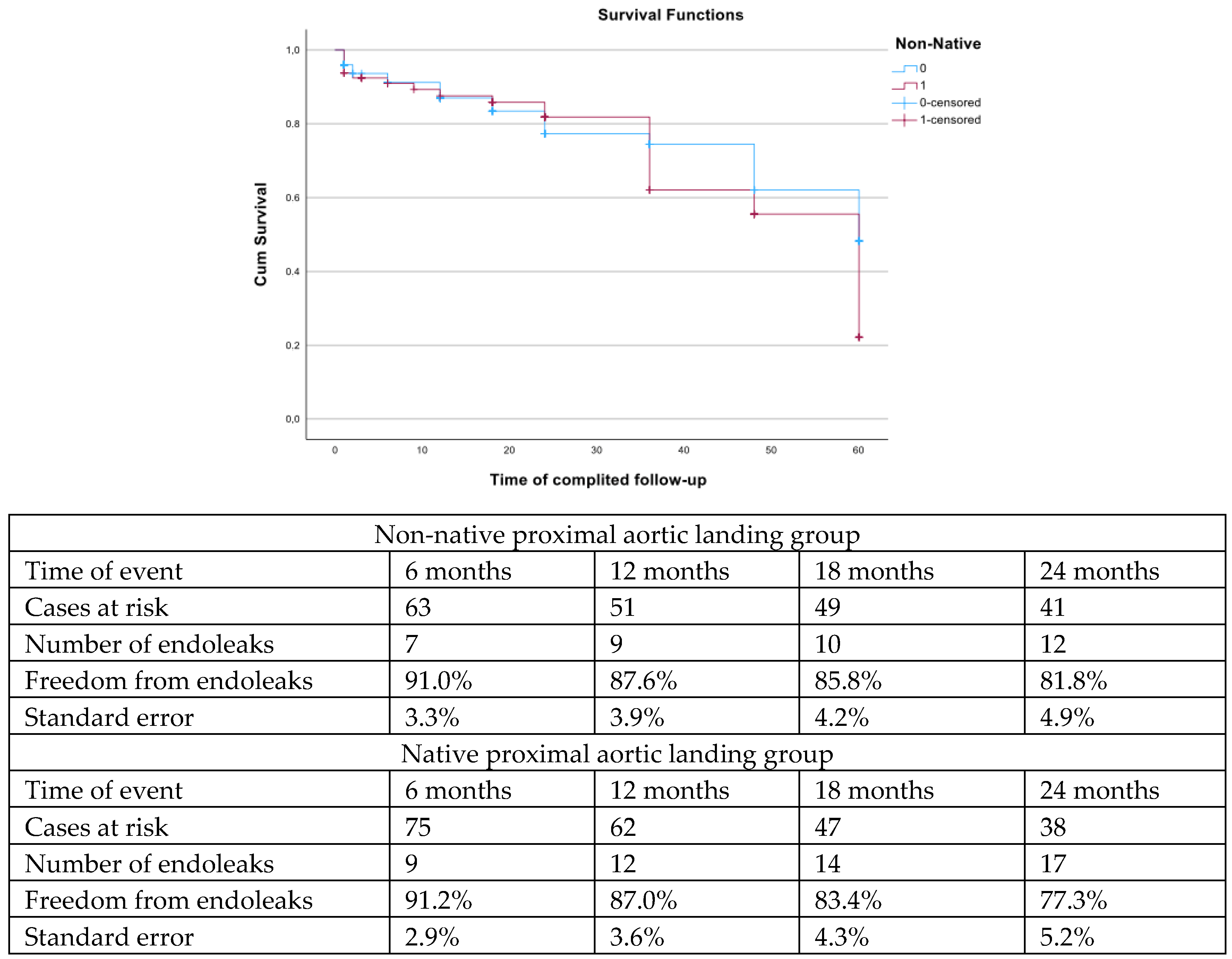
| Variables (N, %, Mean ± SD, Median; IQR) | nNPAL Group (83 Patients) | NPAL Group (126 Patients) | p |
|---|---|---|---|
| Age (years) | 68.3 ± 5.5 | 70.8 ± 4.2 | 0.08 |
| Males | 41 (49.4) | 88 (69.8) | 0.003 |
| Tobacco use | 31 (37.3) | 55 (43.7) | 0.37 |
| - Active tobacco use | 13 (15.7) | 32 (25.4) | 0.09 |
| Hypertension | 75 (90.3) | 116 (92.1) | 0.68 |
| Diabetes mellitus | 5 (6.0) | 21 (16.7) | 0.02 |
| Dyslipidemia | 38 (45.8) | 66 (53.2) | 0.35 |
| Coronary artery disease | 20 (24.1) | 41 (32.5) | 0.19 |
| Chronic heart failure | 8 (9.6) | 12 (9.5) | 0.97 |
| Chronic obstructive pulmonary disease | 15 (18.1) | 25 (19.8) | 0.75 |
| Chronic kidney disease | 7 (8.4) | 17 (13.5) | 0.26 |
| - Creatinine (mg/dL) | 1.1 ± 0.2 | 1.2 ± 0.3 | 0.06 |
| - eGFR (mL/min) | 72.0 ± 11.0 | 67.2 ± 9.7 | 0.13 |
| - Dialysis | 0 (0.0) | 2 (1.6) | 0.36 |
| Stroke | 10 (12.0) | 10 (7.9) | 0.32 |
| Transient ischemic attack | 7 (8.4) | 6 (4.8) | 0.26 |
| Peripheral arterial disease | 5 (6.0) | 18 (14.3) | 0.06 |
| ASA score | 3 (IQR 0) | 3 (IQR 0) | 0.68 |
| ASA score ≥ III | 71 (85.5) | 104 (82.5) | 0.56 |
| Urgent setting | 7 (8.4) | 23 (18.3) | 0.04 |
| - Ruptured aneurysm | 3 (3.6) | 12 (9.5) | 0.11 |
| - Symptomatic | 4 (4.8) | 11 (8.7) | 0.28 |
| Variable (N, %) | nNPAL Group (83 Patients) | NPAL Group (126 Patients) | p |
|---|---|---|---|
| Previous aortic repair | |||
| Aortic valve repair | 30 (36.1) | 2 (1.6) | <0.001 |
| - Mechanical aortic valve | 12 (14.4) | 1 (0.8) | <0.001 |
| f/bTEVAR | 0 (0.0) | 1 (0.8) | 0.54 |
| TEVAR | 6 (7.2) | 19 (15.1) | 0.09 |
| Open thoracic aortic repair | 2 (2.4) | 4 (3.2) | 0.75 |
| f/bEVAR | 1 (1.2) | 6 (4.7) | 0.16 |
| EVAR | 6 (7.2) | 5 (3.9) | 0.30 |
| Open abdominal aortic repair | 6 (7.2) | 13 (10.2) | 0.45 |
| Aortic lesion distribution | |||
| Ascending aneurysms | 3 (3.6) | 1 (0.8) | 0.15 |
| Arch aneurysms | 51 (61.4) | 37 (29.4) | <0.001 |
| - Degenerative | 15 (29.4) | 29 (78.4) | 0.39 |
| - Dissection | 36 (70.6) | 8 (21.6) | <0.001 |
| Penetrating aortic ulcer | 1 (1.2) | 33 (26.2) | <0.001 |
| - Related to aortic dissection | 0 (0.0) | 1 (3.0) | 0.54 |
| Intramural hematoma | 2 (2.4) | 1 (0.8) | 0.37 |
| - Related to aortic dissection | 0 (0.0) | 1 (100.0) | 0.54 |
| Pseudoaneurysms | 5 (6.0) | 11 (8.7) | 0.47 |
| - Related to aortic dissection | 1 (20.0) | 2 (18.2) | 0.82 |
| TAAA type I and II | 19 (22.9) | 39 (31.0) | 0.20 |
| - Degenerative | 8 (42.1) | 21 (53.8) | 0.15 |
| - Dissections | 11 (57.9) | 18 (46.2) | 0.83 |
| Thoracic aortic aneurysms | 2 (2.4) | 4 (3.2) | . |
| - Degenerative | 1 (50.0) | 2 (50.0) | 0.82 |
| - Dissection | 1 (50.0) | 2 (50.0) | 0.82 |
| Any aortic dissection | 53 (63.9) | 32 (25.4) | <0.001 |
| Chronic aortic dissection | 49 (59.0) | 30 (23.8) | <0.001 |
| - Chronic TAAD | 1 (2.0) | 4 (3.2) | 0.36 |
| - Chronic TBAD | 48 (98.0) | 26 (20.6) | <0.001 |
| Acute aortic dissection | 4 (4.8) | 2 (1.6) | 0.17 |
| - Acute TAAD | 0 (0.0) | 0 (0.0) | - |
| - Acute TBAD | 4 (100.0) | 2 (100.0) | 0.17 |
| Proximal extension of the disease | |||
| Zone 0 | 23 (27.7) | 9 (7.1) | <0.001 |
| Zone 1 | 42 (50.6) | 13 (10.3) | <0.001 |
| - Zone 0 and 1 | 65 (78.3) | 22 (17.3) | <0.001 |
| Zone 2 | 12 | 72 (57.1) | <0.001 |
| Zone 3 | 6 | 32 (25.4) | <0.001 |
| Arch type | |||
| Type I | 45 (54.2) | 39 (31.0) | <0.001 |
| Type II | 34 (41.0) | 69 (54.8) | 0.06 |
| Type III | 4 (4.8) | 18 (14.2) | 0.03 |
| Variable (N, %) | nNPAL (83 Patients) | NPAL (126 Patients) | p |
|---|---|---|---|
| fTEVAR | 11 (13.3) | 72 (57.1) | <0.001 |
| Only fenestrations | 6 (7.2) | 10 (13.9) | 0.85 |
| Only scallop | 0 (0.0) | 5 (6.9) | 0.16 |
| Fenestration and scallop | 5 (6.0) | 57 (79.2) | <0.001 |
| bTEVAR | 72 (86.7) | 44 (34.9) | <0.001 |
| - Triple branch devices | 14 (16.9) | 4 (9.0) | <0.001 |
| - Double branch devices | 52 (62.7) | 37 (29.7) | <0.001 |
| - Single branch devices | 6 (7.2) | 3 (2.4) | 0.09 |
| -LSA branch device | 0 (0.0) | 10 (13.9) | 0.03 |
| Adjacent procedures | |||
| Debranching of supra-aortic trunks | 65 (78.3) | 68 (54.0) | <0.001 |
| - LCCA-LSA bypass | 57 (68.7) * | 60 (47.6) ** | - |
| - LSA transposition | 2 (2.4) | 6 (4.8) | - |
| - RCCA-RSA | 2 (2.4) | 2 (1.6) | - |
| - Axillo-axillary bypass | 4 (4.8) | 1 (0.8) | - |
| Ascending thoracic component | 0 (0.0) | 7 (5.5) | 0.08 |
| Distal thoracic extension | 65 (78.3) | 68 (54.0) | <0.001 |
| FLE (among patients managed for aortic dissection) | 27/53 (50.9) | 11/32 (34.4) | 0.14 |
| Variable (N, %) | nNPAL Group (83 Patients) | NPAL Group (126 Patients) | p |
|---|---|---|---|
| Technical success | 82 (98.8) | 119 (94.4) | 0.07 |
| Mortality | 5 (6.0) | 15 (11.9) | 0.16 |
| Stroke | 4 (4.8) | 17 (13.5) | 0.04 |
| - Major stroke | 2 (2.4) | 10 (7.9) | 0.09 |
| - Minor stroke | 2 (2.4) | 7 (5.5) | 0.27 |
| Spinal cord ischemia | 3 (3.6) | 7 (5.5) | 0.52 |
| - Grade 1 | 0 (0.0) | 3 (2.4) | 0.36 |
| - Grade 2 | 3 (3.6) | 3 (2.4) | 0.60 |
| - Grade 3 | 0 (0.0) | 1 (0.8) | 0.77 |
| - Complete recovery | 1 (1.2) | 4 (3.2) | 0.36 |
| - Late evolution | 0 (0.0) | 1 (0.8) | 0.77 |
| - Cerebrospinal fluid drainage | 9 (10.4) | 28 (22.2) | 0.03 |
| Retrograde type A dissection | NA | 5 (3.9) | - |
| Congestive heart failure | 2 (2.4) | 5 (3.9) | 0.54 |
| Pericardial effusion | 2 (2.4) | 7 (5.5) | 0.27 |
| Acute kidney injury | 12 (14.4) | 7 (5.5) | 0.03 |
| - Permanent Dialysis | 1 (1.2) | 0 (0.0) | 0.76 |
| Reinterventions | 25 (30.1) | 24 (19.0) | 0.06 |
| Access complications | 19 (22.9) | 28 (22.2) | 0.90 |
| - UEA complications | 6 (7.2) | 11 (8.7) | 0.70 |
| - Access-related reinterventions | 14 (16.9) | 16 (12.7) | 0.40 |
| Myocardial infarction | 0 (0.0) | 3 (2.4) | 0.36 |
| Respiratory failure | 4 (4.8) | 9 (7.1) | 0.49 |
| Endoleak at pre-discharge CTA | |||
| Type I | 27 (32.5) | 14 (11.1) | <0.001 |
| - Type Ia | 14 (16.9) | 8 (6.3) | 0.02 |
| - Type Ib | 10 (12.0) | 5 (3.9) | 0.03 |
| - Type Ic | 3 (3.6) | 1 (0.8) | 0.06 |
| Type II | 4 (4.8) | 6 (4.8) | 0.98 |
| Type III | 4 (4.8) | 9 (7.1) | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nana, P.; Spanos, K.; Panuccio, G.; Torrealba, J.I.; Rohlffs, F.; Detter, C.; von Kodolitsch, Y.; Kölbel, T. Proximal Landing Zone’s Impact on Outcomes of Branched and Fenestrated Aortic Arch Repair. J. Clin. Med. 2025, 14, 3288. https://doi.org/10.3390/jcm14103288
Nana P, Spanos K, Panuccio G, Torrealba JI, Rohlffs F, Detter C, von Kodolitsch Y, Kölbel T. Proximal Landing Zone’s Impact on Outcomes of Branched and Fenestrated Aortic Arch Repair. Journal of Clinical Medicine. 2025; 14(10):3288. https://doi.org/10.3390/jcm14103288
Chicago/Turabian StyleNana, Petroula, Konstantinos Spanos, Giuseppe Panuccio, José I. Torrealba, Fiona Rohlffs, Christian Detter, Yskert von Kodolitsch, and Tilo Kölbel. 2025. "Proximal Landing Zone’s Impact on Outcomes of Branched and Fenestrated Aortic Arch Repair" Journal of Clinical Medicine 14, no. 10: 3288. https://doi.org/10.3390/jcm14103288
APA StyleNana, P., Spanos, K., Panuccio, G., Torrealba, J. I., Rohlffs, F., Detter, C., von Kodolitsch, Y., & Kölbel, T. (2025). Proximal Landing Zone’s Impact on Outcomes of Branched and Fenestrated Aortic Arch Repair. Journal of Clinical Medicine, 14(10), 3288. https://doi.org/10.3390/jcm14103288





