Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors
Abstract
1. Introduction
2. Pathophysiology of PPP
3. Mechanism of Action of IL-23 Inhibitors
4. Clinical Evidence for IL-23 Inhibitors in PPP
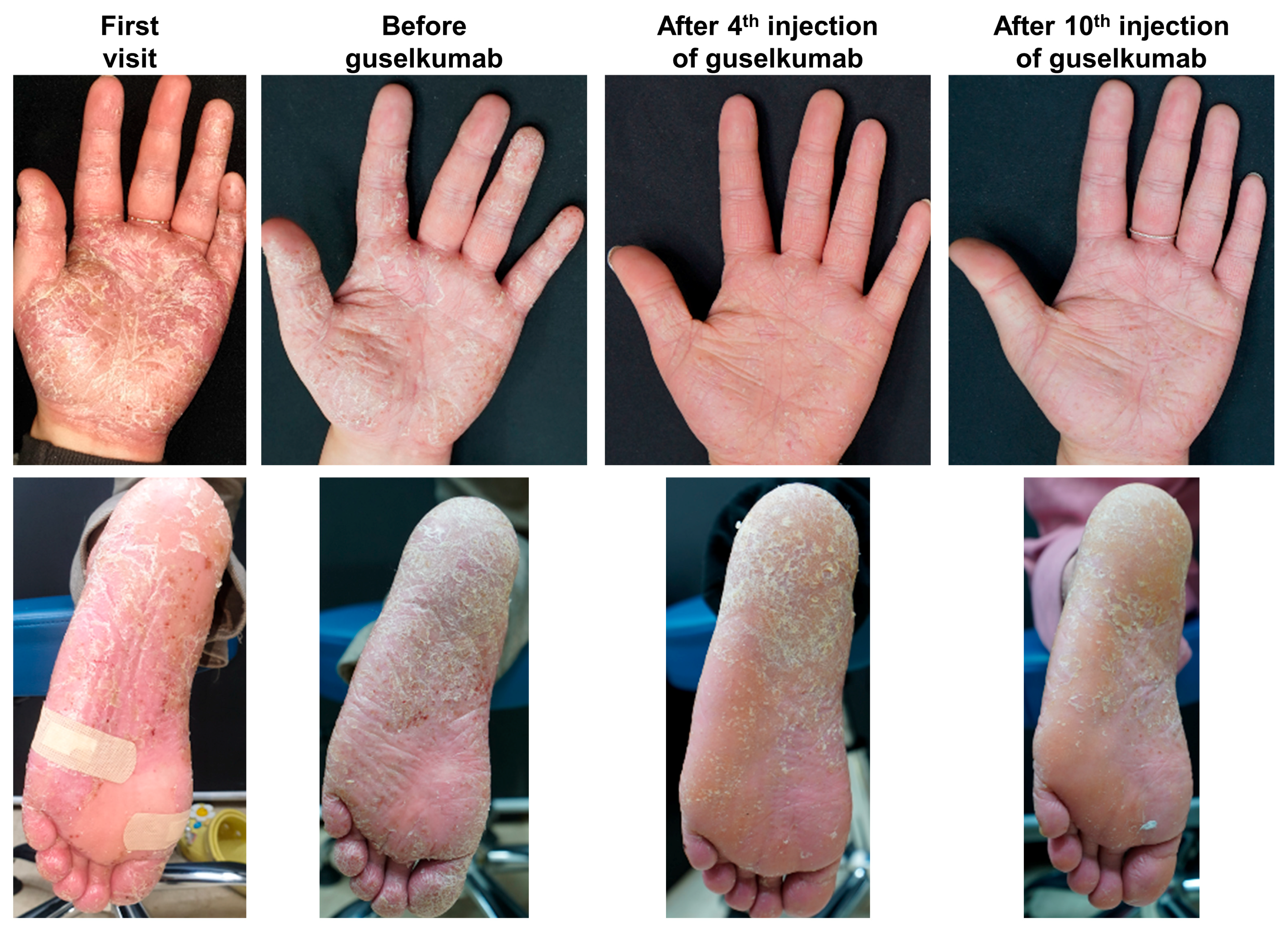
4.1. Guselkumab
4.2. Risankizumab
4.3. Others
4.4. Summary
5. Other Biologics for PPP
5.1. IL-12/23 Inhibitors (Ustekinumab)
5.2. IL-17 Inhibitors (Secukinumab, Brodalumab)
5.3. IL-36 Inhibitors (Spesolimab)
5.4. IL-1 Inhibitors (Anakinra)
5.5. Dual IL-17A/F Inhibitors (Bimekizumab)
6. Small Molecules for PPP
6.1. Apremilast
6.2. Others
6.3. Summary
7. Limitations and Future Directions
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murakami, M.; Terui, T. Palmoplantar pustulosis: Current understanding of disease definition and pathomechanism. J. Dermatol. Sci. 2020, 98, 13–19. [Google Scholar] [CrossRef]
- Kharawala, S.; Golembesky, A.K.; Bohn, R.L.; Esser, D. The clinical, humanistic, and economic burden of palmoplantar pustulosis: A structured review. Expert. Rev. Clin. Immunol. 2020, 16, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Trattner, H.; Blüml, S.; Steiner, I.; Plut, U.; Radakovic, S.; Tanew, A. Quality of life and comorbidities in palmoplantar pustulosis—A cross-sectional study on 102 patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Van Voorhees, A.S.; Hsu, S. Pustular Psoriasis: A Narrative Review of Recent Developments in Pathophysiology and Therapeutic Options. Dermatol. Ther. 2021, 11, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Haxhinasto, S.; Garcet, S.; Kunjravia, N.; Cueto, I.; Gonzalez, J.; Rambhia, D.; Harari, O.; Sleeman, M.A.; Hamilton, J.D.; et al. Comparison of the Inflammatory Circuits in Psoriasis Vulgaris, Non–Pustular Palmoplantar Psoriasis, and Palmoplantar Pustular Psoriasis. J. Investig. Dermatol. 2023, 143, 87–97.e14. [Google Scholar] [CrossRef]
- Twelves, S.; Mostafa, A.; Dand, N.; Burri, E.; Farkas, K.; Wilson, R.; Cooper, H.L.; Irvine, A.D.; Oon, H.H.; Kingo, K.; et al. Clinical and genetic differences between pustular psoriasis subtypes. J. Allergy Clin. Immunol. 2019, 143, 1021–1026. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.R.; Ban, M.S.; Lee, H.; Cho, J.Y.; Jo, S.J. Metabolic profiling of psoriasis vulgaris and palmoplantar pustulosis. Exp. Dermatol. 2024, 33, e15159. [Google Scholar] [CrossRef]
- Hernandez-Cordero, A.; Thomas, L.; Smail, A.; Lim, Z.Q.; Saklatvala, J.R.; Chung, R.; Curtis, C.J.; Baum, P.; Visvanathan, S.; Burden, A.D.; et al. A genome-wide meta-analysis of palmoplantar pustulosis implicates TH2 responses and cigarette smoking in disease pathogenesis. J. Allergy Clin. Immunol. 2024, 154, 657–665.e659. [Google Scholar] [CrossRef]
- van Straalen, K.R.; Kirma, J.; Yee, C.M.; Bugada, L.F.; Rizvi, S.M.; Wen, F.; Wasikowski, R.; Fox, J.; Do, T.H.; Schuler, C.F.; et al. Disease heterogeneity and molecular classification of inflammatory palmoplantar diseases. J. Allergy Clin. Immunol. 2024, 154, 1204–1215.e1209. [Google Scholar] [CrossRef]
- Ju, S.; Park, C.J.; Kim, Y.-S. A case of palmoplantar pustulosis following adalimumab therapy in a patient with ulcerative colitis. J. Korean Soc. Psoriasis 2024, 21, 61–64. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.Y.; Cho, S.I.; Jo, S.J. Risks of Comorbidities in Patients with Palmoplantar Pustulosis vs Patients with Psoriasis Vulgaris or Pompholyx in Korea. JAMA Dermatol. 2022, 158, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Kamijima, Y.; Sato, T.; Ooba, N.; Koide, D.; Iizuka, H.; Nakagawa, H. Epidemiology of psoriasis and palmoplantar pustulosis: A nationwide study using the Japanese national claims database. BMJ Open 2015, 5, e006450. [Google Scholar] [CrossRef] [PubMed]
- Sevrain, M.; Richard, M.A.; Barnetche, T.; Rouzaud, M.; Villani, A.P.; Paul, C.; Beylot-Barry, M.; Jullien, D.; Aractingi, S.; Aubin, F.; et al. Treatment for palmoplantar pustular psoriasis: Systematic literature review, evidence-based recommendations and expert opinion. J. Eur. Acad. Dermatol. Venereol. 2014, 28 (Suppl. S5), 13–16. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): Results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef]
- Reich, K.; Armstrong, A.W.; Langley, R.G.; Flavin, S.; Randazzo, B.; Li, S.; Hsu, M.C.; Branigan, P.; Blauvelt, A. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): Results from a phase 3, randomised controlled trial. Lancet 2019, 394, 831–839. [Google Scholar] [CrossRef]
- Terui, T.; Kobayashi, S.; Okubo, Y.; Murakami, M.; Zheng, R.; Morishima, H.; Goto, R.; Kimura, T. Efficacy and Safety of Guselkumab in Japanese Patients with Palmoplantar Pustulosis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2019, 155, 1153–1161. [Google Scholar] [CrossRef]
- Okubo, Y.; Murakami, M.; Kobayashi, S.; Tsuji, S.; Kishimoto, M.; Ikeda, K.; Jibiki, M.; Neimark, E.; Padilla, B.; Shen, J.; et al. Risankizumab in Japanese patients with moderate-to-severe palmoplantar pustulosis: Results from the randomized, phase 3 JumPPP study. J. Dermatol. 2025, 52, 593–602. [Google Scholar] [CrossRef]
- Zhang, M.; Hua, L.; Hong, S.; Sun, X.; Zhou, Y.; Luo, Y.; Liu, L.; Wang, J.; Wang, C.; Lin, N.; et al. Efficacy and safety of biological agents to treat patients with palmoplantar pustulosis: A systematic scoping review. Int. Immunopharmacol. 2023, 122, 110553. [Google Scholar] [CrossRef]
- Leong, H.F.; Wang, W.H.; Peng, F. Biologics as a novel treatment option for palmoplantar pustulosis: A comprehensive review. Postepy Dermatol. Alergol. 2024, 41, 262–269. [Google Scholar] [CrossRef]
- Huang, I.H.; Wu, P.C.; Chiu, H.Y.; Huang, Y.H. Small-Molecule Inhibitors and Biologics for Palmoplantar Psoriasis and Palmoplantar Pustulosis: A Systematic Review and Network Meta-Analysis. Am. J. Clin. Dermatol. 2024, 25, 347–358. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hagforsen, E.; Morhenn, V.; Ishida-Yamamoto, A.; Iizuka, H. Patients with palmoplantar pustulosis have increased IL-17 and IL-22 levels both in the lesion and serum. Exp. Dermatol. 2011, 20, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Fuentes-Duculan, J.; Mashiko, S.; Li, X.; Bonifacio, K.M.; Cueto, I.; Suárez-Fariñas, M.; Maari, C.; Bolduc, C.; Nigen, S.; et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J. Dermatol. Sci. 2017, 85, 20–26. [Google Scholar] [CrossRef]
- Bissonnette, R.; Nigen, S.; Langley, R.G.; Lynde, C.W.; Tan, J.; Fuentes-Duculan, J.; Krueger, J.G. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1298–1305. [Google Scholar] [CrossRef]
- McCluskey, D.; Benzian-Olsson, N.; Mahil, S.K.; Hassi, N.K.; Wohnhaas, C.T.; Burden, A.D.; Griffiths, C.E.M.; Ingram, J.R.; Levell, N.J.; Parslew, R.; et al. Single-cell analysis implicates T(H)17-to-T(H)2 cell plasticity in the pathogenesis of palmoplantar pustulosis. J. Allergy Clin. Immunol. 2022, 150, 882–893. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Xu, W.D.; Liu, X.Y.; Liu, X.Y.; Huang, A.F.; Su, L.C. Biology of IL-36 Signaling and Its Role in Systemic Inflammatory Diseases. Front. Immunol. 2019, 10, 2532. [Google Scholar] [CrossRef]
- Körber, A.; Mössner, R.; Renner, R.; Sticht, H.; Wilsmann-Theis, D.; Schulz, P.; Sticherling, M.; Traupe, H.; Hüffmeier, U. Mutations in IL36RN in patients with generalized pustular psoriasis. J. Investig. Dermatol. 2013, 133, 2634–2637. [Google Scholar] [CrossRef]
- Navarini, A.A.; Simpson, M.A.; Borradori, L.; Yawalkar, N.; Schlapbach, C. Homozygous missense mutation in IL36RN in generalized pustular dermatosis with intraoral involvement compatible with both AGEP and generalized pustular psoriasis. JAMA Dermatol. 2015, 151, 452–453. [Google Scholar] [CrossRef]
- Bachelez, H.; Choon, S.E.; Marrakchi, S.; Burden, A.D.; Tsai, T.F.; Morita, A.; Turki, H.; Hall, D.B.; Shear, M.; Baum, P.; et al. Inhibition of the Interleukin-36 Pathway for the Treatment of Generalized Pustular Psoriasis. N. Engl. J. Med. 2019, 380, 981–983. [Google Scholar] [CrossRef]
- Tauber, M.; Bal, E.; Pei, X.Y.; Madrange, M.; Khelil, A.; Sahel, H.; Zenati, A.; Makrelouf, M.; Boubridaa, K.; Chiali, A.; et al. IL36RN Mutations Affect Protein Expression and Function: A Basis for Genotype-Phenotype Correlation in Pustular Diseases. J. Investig. Dermatol. 2016, 136, 1811–1819. [Google Scholar] [CrossRef]
- Mahil, S.K.; Catapano, M.; Di Meglio, P.; Dand, N.; Ahlfors, H.; Carr, I.M.; Smith, C.H.; Trembath, R.C.; Peakman, M.; Wright, J.; et al. An analysis of IL-36 signature genes and individuals with IL1RL2 knockout mutations validates IL-36 as a psoriasis therapeutic target. Sci. Transl. Med. 2017, 9, eaan2514. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Kübelbeck, T.; Kolb, A.; Ringen, J.; Waisman, A.; Wittmann, M.; Karbach, S.; Kölsch, S.M.; Kramer, D. IL-17A-driven psoriasis is critically dependent on IL-36 signaling. Front. Immunol. 2023, 14, 1256133. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Alam, M.A.; Ansari, A.W.; Jochebeth, A.; Leo, R.; Al-Abdulla, M.N.; Al-Khawaga, S.; AlHammadi, A.; Al-Malki, A.; Al Naama, K.; et al. Emerging Role of the IL-36/IL-36R Axis in Multiple Inflammatory Skin Diseases. J. Investig. Dermatol. 2024, 144, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Madonna, S.; Girolomoni, G.; Dinarello, C.A.; Albanesi, C. The Significance of IL-36 Hyperactivation and IL-36R Targeting in Psoriasis. Int. J. Mol. Sci. 2019, 20, 3318. [Google Scholar] [CrossRef]
- Johnston, A.; Xing, X.; Wolterink, L.; Barnes, D.H.; Kahlenberg, J.M.; Harms, P.W.; Gudjonsson, J.E. IL-1 and IL-36 are the dominant cytokines in Palmar Plantar Pustulosis. J. Dermatol. Sci. 2016, 84, e99. [Google Scholar] [CrossRef]
- Skov, L.; Beurskens, F.J.; Zachariae, C.O.; Reitamo, S.; Teeling, J.; Satijn, D.; Knudsen, K.M.; Boot, E.P.; Hudson, D.; Baadsgaard, O.; et al. IL-8 as antibody therapeutic target in inflammatory diseases: Reduction of clinical activity in palmoplantar pustulosis. J. Immunol. 2008, 181, 669–679. [Google Scholar] [CrossRef]
- Czerwińska, J.; Owczarczyk-Saczonek, A. The Role of the Neutrophilic Network in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2022, 23, 1840. [Google Scholar] [CrossRef]
- Xiaoling, Y.; Chao, W.; Wenming, W.; Feng, L.; Hongzhong, J. Interleukin (IL)-8 and IL-36γ but not IL-36Ra are related to acrosyringia in pustule formation associated with palmoplantar pustulosis. Clin. Exp. Dermatol. 2019, 44, 52–57. [Google Scholar] [CrossRef]
- Niaouris, A.; Hernández-Cordero, A.; Haddad, S.; Hassi, N.K.; Benzian-Olsson, N.; Bugarin Diz, C.; Burden, A.D.; Cooper, H.L.; Griffiths, C.E.M.; Parslew, R.; et al. Damaging Alleles Affecting Multiple CARD14 Domains Are Associated with Palmoplantar Pustulosis. J. Investig. Dermatol. 2023, 143, 504–508.e505. [Google Scholar] [CrossRef]
- Setta-Kaffetzi, N.; Simpson, M.A.; Navarini, A.A.; Patel, V.M.; Lu, H.C.; Allen, M.H.; Duckworth, M.; Bachelez, H.; Burden, A.D.; Choon, S.E.; et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am. J. Hum. Genet. 2014, 94, 790–797. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Țiburcă, L.; Bembea, M.; Zaha, D.C.; Jurca, A.D.; Vesa, C.M.; Rațiu, I.A.; Jurca, C.M. The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Curr. Issues Mol. Biol. 2022, 44, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Costache, D.O.; Feroiu, O.; Ghilencea, A.; Georgescu, M.; Căruntu, A.; Căruntu, C.; Țiplica, S.G.; Jinga, M.; Costache, R.S. Skin Inflammation Modulation via TNF-α, IL-17, and IL-12 Family Inhibitors Therapy and Cancer Control in Patients with Psoriasis. Int. J. Mol. Sci. 2022, 23, 5198. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- Navarro-Compán, V.; Puig, L.; Vidal, S.; Ramírez, J.; Llamas-Velasco, M.; Fernández-Carballido, C.; Almodóvar, R.; Pinto, J.A.; Galíndez-Aguirregoikoa, E.; Zarco, P.; et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front. Immunol. 2023, 14, 1191782. [Google Scholar] [CrossRef]
- Terui, T.; Kobayashi, S.; Okubo, Y.; Murakami, M.; Hirose, K.; Kubo, H. Efficacy and Safety of Guselkumab, an Anti-interleukin 23 Monoclonal Antibody, for Palmoplantar Pustulosis: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 309–316. [Google Scholar] [CrossRef]
- Wilsmann-Theis, D.; Patt, S.; Pinter, A.; Gerdes, S.; Magnolo, N.; Németh, R.; Schmitz, J.; Paul, C.; Augustin, M.; Staubach, P.; et al. Efficacy and safety of guselkumab in European patients with palmoplantar pustulosis: A multi-center, single-arm clinical trial (GAP study). JAAD Int. 2025, 18, 69–78. [Google Scholar] [CrossRef]
- Okubo, Y.; Morishima, H.; Zheng, R.; Terui, T. Sustained efficacy and safety of guselkumab in patients with palmoplantar pustulosis through 1.5 years in a randomized phase 3 study. J. Dermatol. 2021, 48, 1838–1853. [Google Scholar] [CrossRef]
- Morita, A.; Chen, Y.; Leung, M.W.L.; Kawashima, N.; Terui, T. Effect of guselkumab on serum biomarkers in Japanese palmoplantar pustulosis patients in a randomized phase 3 study. JEADV Clin. Pract. 2023, 2, 59–72. [Google Scholar] [CrossRef]
- Cho, M.K.; Kim, D.H. Real-world experience of the efficacy and safety of guselkumab 100 mg in patients with palmoplantar pustulosis in Korea: A retrospective single-center study. JAAD Int. 2025, 18, 117–118. [Google Scholar] [CrossRef]
- Poortinga, S.; Balakirski, G.; Kromer, C.; Mössner, R.; Schön, M.P.; Bieber, T.; Wilsmann-Theis, D. The challenge of palmoplantar pustulosis therapy: Are Interleukin-23 inhibitors an option? J. Eur. Acad. Dermatol. Venereol. 2021, 35, e907–e911. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Bachelez, H.; Burden, A.D.; Rissler, M.; Sieder, C.; Orsenigo, R.; Chaouche-Teyara, K. Secukinumab for moderate-to-severe palmoplantar pustular psoriasis: Results of the 2PRECISE study. J. Am. Acad. Dermatol. 2019, 80, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Kobayashi, S.; Murakami, M.; Sano, S.; Kikuta, N.; Ouchi, Y.; Terui, T. Efficacy and Safety of Brodalumab, an Anti-interleukin-17 Receptor A Monoclonal Antibody, for Palmoplantar Pustulosis: 16-Week Results of a Randomized Clinical Trial. Am. J. Clin. Dermatol. 2024, 25, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Burden, A.D.; Bissonnette, R.; Navarini, A.A.; Murakami, M.; Morita, A.; Haeufel, T.; Ye, B.; Baehner, F.; Terui, T. Spesolimab Efficacy and Safety in Patients with Moderate-to-Severe Palmoplantar Pustulosis: A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase IIb, Dose-Finding Study. Dermatol. Ther. 2023, 13, 2279–2297. [Google Scholar] [CrossRef]
- Cro, S.; Cornelius, V.R.; Pink, A.E.; Wilson, R.; Pushpa-Rajah, A.; Patel, P.; Abdul-Wahab, A.; August, S.; Azad, J.; Becher, G.; et al. Anakinra for palmoplantar pustulosis: Results from a randomized, double-blind, multicentre, two-staged, adaptive placebo-controlled trial (APRICOT). Br. J. Dermatol. 2021, 186, 245–256. [Google Scholar] [CrossRef]
- Reich, K.; Warren, R.B.; Lebwohl, M.; Gooderham, M.; Strober, B.; Langley, R.G.; Paul, C.; De Cuyper, D.; Vanvoorden, V.; Madden, C.; et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N. Engl. J. Med. 2021, 385, 142–152. [Google Scholar] [CrossRef]
- Passeron, T.; Perrot, J.L.; Jullien, D.; Goujon, C.; Ruer, M.; Boyé, T.; Villani, A.P.; Quiles Tsimaratos, N. Treatment of Severe Palmoplantar Pustular Psoriasis with Bimekizumab. JAMA Dermatol. 2024, 160, 199–203. [Google Scholar] [CrossRef]
- Terui, T.; Okubo, Y.; Kobayashi, S.; Sano, S.; Morita, A.; Imafuku, S.; Tada, Y.; Abe, M.; Yaguchi, M.; Uehara, N.; et al. Efficacy and Safety of Apremilast for the Treatment of Japanese Patients with Palmoplantar Pustulosis: Results from a Phase 2, Randomized, Placebo-Controlled Study. Am. J. Clin. Dermatol. 2023, 24, 837–847. [Google Scholar] [CrossRef]
- Wilsmann-Theis, D.; Kromer, C.; Gerdes, S.; Linker, C.; Magnolo, N.; Sabat, R.; Reich, K.; Mössner, R. A multicentre open-label study of apremilast in palmoplantar pustulosis (APLANTUS). J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2045–2050. [Google Scholar] [CrossRef]
- Ständer, S.; Syring, F.; Ludwig, R.J.; Thaçi, D. Successful Treatment of Refractory Palmoplantar Pustular Psoriasis With Apremilast: A Case Series. Front. Med. 2020, 7, 543944. [Google Scholar] [CrossRef]
- Kato, N.; Takama, H.; Ando, Y.; Yanagishita, T.; Ohshima, Y.; Ohashi, W.; Akiyama, M.; Watanabe, D. Immediate response to apremilast in patients with palmoplantar pustulosis: A retrospective pilot study. Int. J. Dermatol. 2021, 60, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Kt, S.; Thakur, V.; Narang, T.; Dogra, S.; Handa, S. Apremilast in treatment of palmoplantar pustulosis—A case series. Int. J. Dermatol. 2021, 60, e247–e248. [Google Scholar] [CrossRef] [PubMed]
- Terui, T.; Okubo, Y.; Kobayashi, S.; Morita, A.; Imafuku, S.; Tada, Y.; Abe, M.; Strober, B.; Gooderham, M.; Zhang, W.; et al. Apremilast Versus Placebo in Japanese Patients with Palmoplantar Pustulosis: A 52-Week, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. SSRN 2024. [Google Scholar] [CrossRef]
- Du, N.; Yang, J.; Zhang, Y.; Lv, X.; Cao, L.; Min, W. Successful treatment of refractory palmoplantar pustulosis by upadacitinib: Report of 28 patients. Front. Med. 2024, 11, 1476793. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Wang, H.; Yang, R.; Luo, S. Clinical efficacy and safety of upadacitinib in the treatment of palmoplantar pustulosis: A single-center retrospective study. Indian. J. Dermatol. Venereol. Leprol. 2024, 29, 1–4. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Mi, W.Y.; Wang, Y.Y.; Li, W. Palmoplantar pustulosis with psoriatic arthritis ineffective to interleukin-17 inhibitors: Two patients successfully treated with upadacitinib. J. Dermatol. Treat. 2023, 34, 2280508. [Google Scholar] [CrossRef]
- Rahbar Kooybaran, N.; Tsianakas, A.; Assaf, K.; Mohr, J.; Wilsmann-Theis, D.; Mössner, R. Response of palmoplantar pustulosis to upadacitinib: A case series of five patients. J. Dtsch. Dermatol. Ges. 2023, 21, 1387–1392. [Google Scholar] [CrossRef]
- De Luca, D.A.; Papara, C.; Hawro, T.; Thaçi, D.; Ständer, S. Exploring the effect of deucravacitinib in patients with palmoplantar pustular psoriasis. J. Dermatol. Treat. 2024, 35, 2399220. [Google Scholar] [CrossRef]
- Bang, C.-H.; Park, C.-J.; Kim, Y.-S. The Expanding Therapeutic Potential of Deucravacitinib Beyond Psoriasis: A Narrative Review. J. Clin. Med. 2025, 14, 1745. [Google Scholar] [CrossRef]

| Ref. | Patients (n) | Intervention | Main Efficacy Results |
|---|---|---|---|
| [46] | 24-week phase 2 RCT in Japanese patients with moderate-to-severe PPP (n = 49) | Guselkumab 200 mg or placebo at W0 and 4 | PPPASI improvement at 16 weeks was significantly higher in guselkumab vs. placebo (p = 0.009). PPPASI-50 at 16 weeks was significantly higher in guselkumab vs. placebo (p = 0.009). |
| [47] | 24-week single-arm, phase 2 study in Caucasian patients with moderate-to-severe PPP (n = 50) | Guselkumab 100 mg at week 0, 4, 12, and 20 | Median PPPASI reduction by 59.6% at week 24 compared to baseline (p < 0.001) PPPASI-50 and PPPASI-75 at week 24: 66.0% and 34.0%. |
| [16] | 60-week phase 3 RCT in Japanese patients with moderate-to-severe PPP (n = 159) | Guselkumab 100 or 200 mg or placebo at week 0, 4, 12; all guselkumab thereafter every 8 weeks | Both guselkumab groups demonstrated significant PPPASI improvement vs. placebo (p < 0.001) The guselkumab 100 mg group (57.4%) achieved a significantly higher PPPASI-50 response at week 16 vs. placebo (34.0%; p = 0.02); however, it was not significant for the guselkumab 200 mg group (36.5%, p = 0.78). The efficacy endpoint improved consistently through week 52 |
| [48] | 82-week long-term extension of the phase 3 RCT in a Japanese patient with moderate-to-severe PPP (n = 133) | After a 60-week trial, the patients were followed up until 84 weeks | The mean improvement in the guselkumab groups from baseline in the PPPASI at week 84 was ~79%. |
| [50] | A retrospective, single-center RWE study in Korean patients with moderate-to-severe PPP (n = 17) | More than 4 cycles of guselkumab 100 mg 58.8% used concurrent systemic therapy with acitretin or cyclosporin | At week 28, PPPASI-50 (82.4%) and PPPASI-75 (47.1%). |
| [17] | 68-week phase 3 RCT in Japanese patients with moderate-to-severe PPP (n = 119) | Risankizumab 150 mg or placebo at week 0, 4, 16; all risankizumab thereafter every 12 weeks | PPPASI improvement at week 16 was significantly higher in risankizumab vs. placebo (p < 0.05) At week 16, PPPASI-50 was significantly higher in risankizumab (41.0%) vs. placebo (24.1%) (p < 0.05), but not for PPPASI-75 (13.1% vs. 15.5%, p = 0.74) At week 68, PPPASI-50 (87.0%; 90.9%) and PPPASI-75 (57.4%; 69.1%) of patients in the continuous risankizumab;placebo-to-risankizumab groups, respectively. |
| [51] | A retrospective, two-center RWE study in German patients with moderate-to-severe PPP (n = 16) | Guselkumab (n = 12), Risankizuamb (n = 3), Tildrakizumab (n = 1) as per label in psoriasis | At week 12, PPPASI-50 (56.3%) and PPPASI-75 (25.0%) At week 48, PPPASI-50 (62.5%) and PPPASI-75 (43.8%) |
| Ref. | Patients (n) | Intervention | Main Efficacy Results |
|---|---|---|---|
| [24] | 16-week RCT in Caucasian patients with PPP (n = 33) | Ustekinumab 45/90 mg or placebo at week 0 4, and 16 | Not significant difference in PPPASI-50 at 16 week in ustekinumab vs. placebo (p = 1.000). |
| [52] | 52-week phase 3 RCT in Caucasian patients with moderate-to-severe PPP (n = 50) | Secukinumab 150 or 300 mg or placebo SC once weekly at week 1, 2 and 3, and per 4 weeks | At 16 weeks, PPPASI-75 with secukinumab 300 mg (26.6%, p = 0.041) vs. placebo At 16 weeks, PPPASI-75 with secukinumab 150 mg (17.5%, p = 0.572) vs. placebo At 52 week, PPPASI-75 41.8% for secukinumab 300 mg |
| [53] | 16-week phase 3 RCT in Japanese patients with moderate-to-severe PPP followed by a 52-week, open-label extension period (n = 112) | Brodalumab 210 mg or placebo SC at weeks 0, 1, 2, and per 2 weeks | At 16 weeks, PPPASI’s improvement was significantly higher with brodalumab vs. placebo (p = 0.0049). At 16 weeks, PPPASI-50/75/90: 54% vs. 24.2%/36.0% vs. 8.1%/16.0% vs. 0.0% (brodalumab vs. placebo) |
| [54] | 52-week phase 2 RCT in patients with moderate-to-severe PPP (n = 152) | Spesolimab (various) 1 or placebo SC per 4 weeks; thereafter, spesolimab per 4 weeks at week 16 | Mean differences for spesolimab vs. placebo: ranged from—14.6% to—5.3%; none reached significance. At 16 weeks, PGA 0/1: 21.1% and 4.7% of patients in the spesolimab and placebo groups. At 52 weeks, PGA 0/1: 54.1% and 27.9% of patients in the spesolimab and placebo-switch patients. |
| [55] | 8-week phase 4 RCT in patients with PPP requiring systemic therapy (n = 64) | Daily anakinra or placebo SC for 8 weeks | Mean PPPASI difference at week 8 for anakinra versus placebo: −1.65 (p = 0.300). Mean PPPASI-50/90 difference at week 8 for anakinra vs. placebo: 2.30 (p = 0.287)/3.80 (p = 0.285). |
| [58] | 32-week phase 2 RCT in Japanese patients with PPP requiring systemic therapy (n = 90) | Oral apremilast 30 mg twice daily or placebo for 16 weeks; thereafter, apremilast until 32 weeks | PPPASI-50 response at week 16 was significantly higher with apremilast vs. placebo (p = 0.0003). PPPASI improvement at week 16 was significantly higher with apremilast vs. placebo (p = 0.0013). Improvements were sustained through week 32 with apremilast. |
| [59] | 20-week phase 2 single-arm study in Japanese patients with moderate-to-severe PPP (n = 21) | Oral apremilast 30 mg twice daily until 20 weeks | PPPASI at week 20 showed a median reduction of 57.1% (p < 0.001) PPASI-50 response at week 20 was 61.9% |
| [63] | 52-week phase 3 RCT in Japanese patients with moderate-to-severe PPP (n = 176) | Oral apremilast 30 mg twice daily or placebo for 16 weeks; thereafter, apremilast until 52 weeks | PPPASI-50 response at week 16 was significantly higher with apremilast (68%) vs. placebo (35%) (p = 0.0003). PPPASI improvement at week 16 was significantly higher with apremilast vs. placebo (p < 0.0001). Improvements were sustained through week 52 with apremilast. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.-H.; Kim, Y.-S. Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors. J. Clin. Med. 2025, 14, 3273. https://doi.org/10.3390/jcm14103273
Nam K-H, Kim Y-S. Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors. Journal of Clinical Medicine. 2025; 14(10):3273. https://doi.org/10.3390/jcm14103273
Chicago/Turabian StyleNam, Kyung-Hwa, and Yoon-Seob Kim. 2025. "Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors" Journal of Clinical Medicine 14, no. 10: 3273. https://doi.org/10.3390/jcm14103273
APA StyleNam, K.-H., & Kim, Y.-S. (2025). Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors. Journal of Clinical Medicine, 14(10), 3273. https://doi.org/10.3390/jcm14103273






