An Increase in Mean Aortic Valve Gradients the Day After Transcatheter Aortic Valve Implantation: The Effects of Evolving Anesthesia Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Anesthesia and Hemodynamic Details
2.4. Echocardiographic Measurements
2.5. Endpoint Measurements
2.6. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Hemodynamics and Anesthesia
3.3. Echocardiographic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| ASE | American Society of Echocardiography |
| BIS | Bispectral index |
| f | Frequency (f) |
| GA | General anesthesia |
| IQR | Interquartile ranges |
| LVEF | Left ventricular ejection fraction |
| LVOT | Left ventricular outflow tract |
| MDN | Medians |
| NPO | Nil per os |
| SAVR | Surgical aortic valve replacement |
| SVR | Systemic vascular resistance |
| TAVI | Transcatheter aortic valve implantation |
| TTE | Transthoracic echocardiogram (TTE) |
References
- Kronzon, I.; Jelnin, V.; Ruiz, C.E.; Saric, M.; Williams, M.R.; Kasel, A.M.; Shivaraju, A.; Colombo, A.; Kastrati, A. Optimal imaging for guiding TAVR: Transesophageal or transthoracic echocardiography, or just fluoroscopy? JACC Cardiovasc. Imaging 2015, 8, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Saric, M.; Williams, M.R. Transthoracic echocardiography guidance for TAVR. J. Am. Coll. Cardiol. Imaging 2015, 8, 363–367. [Google Scholar]
- Didier, R.; Benic, C.; Nasr, B.; Le Ven, F.; Hannachi, S.; Eltchaninoff, H.; Koifman, E.; Donzeau-Gouge, P.; Fajadet, J.; Leprince, P.; et al. High Post-Procedural Transvalvular Gradient or Delayed Mean Gradient Increase after Transcatheter Aortic Valve Implantation: Incidence, Prognosis and Associated Variables. The FRANCE-2 Registry. J. Clin. Med. 2021, 10, 3221. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Pibarot, P.; Hahn, R.T.; Elmariah, S.; Pilgrim, T.; Bavry, A.A.; Maini, B.; Okuno, T.; Al-Azizi, K.; Waggoner, T.E.; et al. Transvalvular Pressure Gradients and All-Cause Mortality Following TAVR: A Multicenter Echocardiographic and Invasive Registry. JACC Cardiovasc. Interv. 2022, 15, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Ihlemann, N. Measuring Transvalvular Aortic Pressure Gradients: Answering Questions or Asking New Ones? JACC Cardiovasc. Interv. 2022, 15, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Stanova, V.; Rieu, R.; Zenses, A.-S.; Rodés-Cabau, J.; Pibarot, P. Doppler versus catheter transvalvular pressure gradients in self-expanding versus balloon expandable transcatheter aortic valves, an in vitro study. J. Am. Coll. Cardiol. 2020, 75, 1443. [Google Scholar] [CrossRef]
- Naidu, S.; Chen, T.; Fiorilli, P.; Li, R.H.; Desai, N.; Szeto, W.Y.; Giri, J.; Kobayashi, T.; Atluri, P.; Herrmann, H.C. Measuring TAVR Prosthesis Gradient Immediately Post-Procedure May Underestimate its Significance. JACC Cardiovasc. Interv. 2022, 15, 120–121. [Google Scholar] [CrossRef]
- Sammour, Y.; Kerrigan, J.; Banerjee, K.; Gajulapalli, R.D.; Lak, H.; Chawla, S.; Andress, K.; Gupta, N.; Unai, S.; Svensson, L.G.; et al. Comparing outcomes of general anesthesia and monitored anesthesia care during transcatheter aortic valve replacement: The Cleveland Clinic Foundation experience. Catheter. Cardiovasc. Interv. 2021, 98, E436–E443. [Google Scholar] [CrossRef]
- Toppen, W.; Johansen, D.; Sareh, S.; Fernandez, J.; Satou, N.; Patel, K.D.; Kwon, M.; Suh, W.; Aksoy, O.; Shemin, R.J.; et al. Improved costs and outcomes with conscious sedation vs general anesthesia in TAVR patients: Time to wake up? PLoS ONE 2017, 12, e0173777. [Google Scholar] [CrossRef]
- Fadah, K.; Khalafi, S.; Corey, M.; Sotelo, J.; Farag, A.; Siddiqui, T.; Abolbashari, M. Optimizing Anesthetic Selection in Transcatheter Aortic Valve Replacement: Striking a Delicate Balance between Efficacy and Minimal Intervention. Cardiol. Res. Pr. 2024, 2024, 4217162. [Google Scholar] [CrossRef]
- Butala, N.M.; Chung, M.; Secemsky, E.A.; Manandhar, P.; Marquis-Gravel, G.; Kosinski, A.S.; Vemulapalli, S.; Yeh, R.W.; Cohen, D.J. Conscious Sedation Versus General Anesthesia for Transcatheter Aortic Valve Replacement: Variation in Practice and Outcomes. JACC Cardiovasc. Interv. 2020, 13, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Holmes, H.R.; Falasa, M.; Neal, D.; Choi, C.Y.; Park, K.; Bavry, A.A.; Freeman, K.A.; Manning, E.W.; Stinson, W.W.; Jeng, E.I. Monitored Anesthesia Care Versus General Anesthesia for Transcatheter Aortic Valve Replacement. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2022, 17, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kiramijyan, S.; Ben-Dor, I.; Koifman, E.; Didier, R.; Magalhaes, M.A.; Escarcega, R.O.; Negi, S.I.; Baker, N.C.; Gai, J.; Torguson, R.; et al. Comparison of clinical outcomes with the utilization of monitored anesthesia care vs. general anesthesia in patients undergoing transcatheter aortic valve replacement. Cardiovasc. Revasc. Med. 2016, 17, 384–390. [Google Scholar] [CrossRef]
- Marino, M.; Lilie, C.J.; Culp, W.C.; Schepel, S.R.; Tippett, J.C. The Evolution of Echocardiographic Type and Anesthetic Technique for Transcatheter Aortic Valve Replacement at a High-Volume Transcatheter Aortic Valve Replacement Center. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 29–35. [Google Scholar] [CrossRef]
- Villablanca, P.A.; Mohananey, D.; Nikolic, K.; Bangalore, S.; Slovut, D.P.; Mathew, V.; Thourani, V.H.; Rode’S-Cabau, J.; Núñez-Gil, I.J.; Shah, T.; et al. Comparison of local versus general anesthesia in patients undergoing transcatheter aortic valve replacement: A meta-analysis. Catheter. Cardiovasc. Interv. 2018, 91, 330–342. [Google Scholar] [CrossRef]
- Tchetche, D.; De Biase, C. Local Anesthesia-Conscious Sedation: The Contemporary Gold Standard for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Mathew, D.M.; Mathew, S.M.; Varghese, K.S.; Khaja, S.; Vega, E.; Pandey, R.; Thomas, J.J.; Mathew, C.S.; Ahmed, S.; et al. General Anesthesia Versus Local Anesthesia in Patients Undergoing Transcatheter Aortic Valve Replacement: An Updated Meta-Analysis and Systematic Review. J. Cardiothorac. Vasc. Anesthesia 2023, 37, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- İzgi, M.; Halis, A.; Şener, Y.Z.; Şahiner, L.; Kaya, E.B.; Aytemir, K.; Karagöz, A.H. Evaluation of Anaesthetic Approaches in Transcatheter Aortic Valv Implantation Procedures. Turk. J. Anaesthesiol. Reanim. 2023, 51, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Rex, S. Anesthesia for transcatheter aortic valve implantation: An update. Curr. Opin. Anaesthesiol. 2013, 26, 456–466. [Google Scholar] [CrossRef]
- Abbett, S.K.; Urman, R.D.; Resor, C.D.; Brovman, E.Y. The Effect of Anesthesia Type on Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, I.; Looser, P.M.; Maluenda, G.; Weddington, T.C.; Kambouris, N.G.; Barbash, I.M.; Hauville, C.; Okubagzi, P.; Corso, P.J.; Satler, L.F.; et al. Transcatheter aortic valve replacement under monitored anesthesia care versus general anesthesia with intubation. Cardiovasc. Revasc. Med. 2012, 13, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, G.; Jégou, A.; Dambrin, G.; Picard, F.; Anconina, J.; Pouzet, B.; Guesnier, L.; Khelifa, R.C.; Hilpert, L.; Doan, H.L.; et al. Comparison of TransFemoral Transcatheter Aortic Valve Replacement Performed With a Minimally Invasive Simplified Technique: "FAST" Versus a Standard Approach. J. Invasive Cardiol. 2019, 31, 300–306. [Google Scholar] [PubMed]
- Luzzi, C.; Orlov, D.; Foley, K.; Horlick, E.; Osten, M.; Cusimano, R.J.; Djaiani, G. Choice of anesthesia technique is associated with earlier hospital discharge and reduced costs after transcatheter transfemoral aortic valve implantation. J. Thorac. Dis. 2024, 16, 1836–1842. [Google Scholar] [CrossRef]
- Tan, C.; Dean, L.S. Conscious sedation for TAVR: A wave of the future? Catheter. Cardiovasc. Interv. 2018, 91, 343–344. [Google Scholar] [CrossRef]
- Feistritzer, H.-J.; Kurz, T.; Stachel, G.; Hartung, P.; Lurz, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Vigelius-Rauch, U.; et al. Impact of Anesthesia Strategy and Valve Type on Clinical Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 77, 2204–2215. [Google Scholar] [CrossRef]
- Feistritzer, H.-J.; Kurz, T.; Vonthein, R.; Schröder, L.; Stachel, G.; Eitel, I.; Marquetand, C.; Saraei, R.; Kirchhof, E.; Heringlake, M.; et al. Effect of Valve Type and Anesthesia Strategy for TAVR: 5-Year Results of the SOLVE-TAVI Trial. J. Am. Coll. Cardiol. 2025, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.M.; Lansky, A.J.; Webb, J.; Roffi, M.; Toggweiler, S.; Reinthaler, M.; Wang, D.; Hutchinson, N.; Wendler, O.; Hildick-Smith, D.; et al. Local versus general anesthesia for transcatheter aortic valve implantation (TAVR)—systematic review and meta-analysis. BMC Med. 2014, 12, 41. [Google Scholar] [CrossRef]
- Mayr, N.P.; Michel, J.; Bleiziffer, S.; Tassani, P.; Martin, K. Sedation or general anesthesia for transcatheter aortic valve implantation (TAVI). J. Thorac. Dis. 2015, 7, 1518–1526. [Google Scholar] [CrossRef]
- Pani, S.; Cagino, J.; Feustel, P.; Musuku, S.R.; Raja, A.; Bruno, N.; Ursillo, C.; Arunakul, N.; Poulos, C.M.; Welljams-Dorof, M.; et al. Patient Selection and Outcomes of Transfemoral Transcatheter Aortic Valve Replacement Performed with Monitored Anesthesia Care Versus General Anesthesia. J. Cardiothorac. Vasc. Anesthesia 2017, 31, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Al-Agaty, A.; Asaad, O.; Mahmoud, M.; Omar, A.S.; Abdelrazik, A.; Mostafa, M. A comparative study between propofol and dexmedetomidine as sedative agents during performing transcatheter aortic valve implantation. J. Clin. Anesthesia 2016, 32, 242–247. [Google Scholar] [CrossRef]
- Mayr, N.P.; Wiesner, G.; van der Starre, P.; Hapfelmeier, A.; Goppel, G.; Kasel, A.M.; Hengstenberg, C.; Husser, O.; Schunkert, H.; Tassani-Prell, P. Dexmedetomidine versus propofol-opioid for sedation in transcatheter aortic valve implantation patients: A retrospective analysis of periprocedural gas exchange and hemodynamic support. Can. J. Anaesth. 2018, 65, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.A.; Hollinger, M.K.; Jain, H.B. Propofol-based versus dexmedetomidine-based sedation in cardiac surgery patients. J. Cardiothorac. Vasc. Anesthesia 2013, 27, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.A.; Dar, B.A.; Akhter, N.; Ahmad, N. A Comparative Study Evaluating Effects of Intravenous Sedation by Dexmedetomidine and Propofol on Patient He-modynamics and Postoperative Outcomes in Cardiac Surgery. Anesthesia Essays Res. 2018, 12, 555–560. [Google Scholar] [CrossRef]
- Abowali, H.A.; Paganini, M.; Enten, G.; Elbadawi, A.; Camporesi, E.M. Critical Review and Meta-Analysis of Postoperative Sedation after Adult Cardiac Surgery: Dexmedetomidine Versus Propofol. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-Q.; Chen, S.-Q.; Yao, X.; Xie, C.-B.; Wen, S.-H.; Liu, K.-X. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: A meta-analysis of randomized clinical trials. J. Surg. Res. 2013, 185, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Sanfilippo, F.; Arnemann, P.; Hessler, M.; Kampmeier, T.G.; D’egidio, A.; Orecchioni, A.; Santonocito, C.; Frati, G.; Greco, E.; et al. The Effect of Propofol and Dexmedetomidine Sedation on Norepinephrine Requirements in Septic Shock Patients: A Crossover Trial. Crit. Care Med. 2019, 47, e89–e95. [Google Scholar] [CrossRef] [PubMed]
- Georgia, N.; Ilias, S.; Panagiotis, D.; Mihalis, A.; Konstantina, R.; Anastasia, A.; Ioannis, A.; Nikolaos, S. Comparative study between sedation and general anesthesia as an anesthesiologic approach for patients treated with TAVR. Which is the best for hemodynamic stability? Hell. J. Cardiol. 2024, 79, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kronfli, A.P.; Lehman, E.; Yamane, K.; Roberts, S.M.; Cios, T.J. Dexmedetomidine Is an Equal Cost Alternative to Propofol in Transcatheter Aortic Valve Replacement, With Equivalent In-Hospital and 30-Day Outcomes. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 439–445. [Google Scholar] [CrossRef]
- Horn, P.; Stern, D.; Veulemans, V.; Heiss, C.; Zeus, T.; Merx, M.W.; Kelm, M.; Westenfeld, R. Improved endothelial function and decreased levels of endothelium-derived microparticles after transcatheter aortic valve implantation. EuroIntervention 2015, 10, 1456–1463. [Google Scholar] [CrossRef]
- Comella, A.; Michail, M.; Chan, J.; Cameron, J.D.; Gooley, R.; Mathur, A.; Hughes, A.D.; Brown, A.J. Patients with aortic stenosis exhibit early improved endothelial function following transcatheter aortic valve replacement: The eFAST study. Int. J. Cardiol. 2021, 332, 143–147. [Google Scholar] [CrossRef]
- Pereyra, V.M.; Seitz, A.; Mahrholdt, H.; Bekeredjian, R.; Sechtem, U.; Ong, P. Coronary microvascular dysfunction in patients with mild-to-moderate aortic stenosis—Insights from intracoronary acetylcholine testing. IJC Hear. Vasc. 2020, 31, 100658. [Google Scholar] [CrossRef]
- Rajappan, K.; Rimoldi, O.E.; Dutka, D.P.; Ariff, B.; Pennell, D.J.; Sheridan, D.J.; Camici, P.G. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002, 105, 470–476. [Google Scholar] [CrossRef] [PubMed]
| General Anesthesia (n = 201) | Monitor Anesthesia Care (n = 543) | p-Value | |
|---|---|---|---|
| Age (years) | 78 [71–84] | 77 [71–83] | 0.335 |
| Gender (male) | 118 (58.7%) | 303 (55.8%) | 0.48 |
| Clinical history | |||
| Diabetes mellitus | 105 (52.2%) | 225 (41.3%) | 0.008 |
| Hypertension | 188 (93.5%) | 477 (87.5%) | 0.02 |
| Hyperlipidemia | 176 (87.6%) | 460 (84.4%) | 0.28 |
| Peripheral vascular disease | 93 (46.3%) | 99 (18.2%) | <0.001 |
| Stroke/TIA | 39 (19.4%) | 62 (11.4%) | 0.004 |
| COPD | 57 (28.4%) | 86 (15.8%) | <0.001 |
| Atrial fibrillation | 73 (36.3%) | 151 (27.7%) | 0.02 |
| Previous permanent pacemaker | 24 (11.9%) | 63 (11.6%) | 0.89 |
| CKD (any stage) | 121 (60.2%) | 208 (38.2%) | <0.001 |
| CKD stage 4 or ESRD | 28 (13.9%) | 39 (7.2%) | 0.004 |
| GFR | 54 [40–70] | 68 [50–85] | <0.001 |
| Coronary artery disease | 153 (76.1%) | 301 (55.2%) | <0.001 |
| Previous myocardial infarction | 67 (33.3%) | 59 (10.8%) | <0.001 |
| Previous CABG | 57 (28.4%) | 71 (13%) | <0.001 |
| Obstructive sleep apnea | 44 (21.9%) | 109 (20%) | 0.57 |
| BMI (kg/m2) | 29 [25–34.94] | 29 [25.1–34.4] | 0.987 |
| NYHA class III-IV symptoms | 184 (92%) | 442 (81.1%) | 0.004 |
| CHA2DS2-VASc score | 5 [4–6] | 4 [3–5] | <0.001 |
| Post-TAVI length of stay (days) | 3 [2–5] | 1 [1–2] | <0.001 |
| General Anesthesia (n = 201) | Monitor Anesthesia Care (n = 543) | p-Value | |
|---|---|---|---|
| Volume status | |||
| Pre-TAVI weight (kg) | 85.3 [71.1–99.8] | 83.9 [71.2–99.3] | 0.697 |
| Post-TAVI weight (kg) | 86.2 [71.6–100.7] | 83.2 [70.9–98.4] | 0.312 |
| Pre-TAVI systolic blood pressure (mmHg) | 149 [129–166] | 154 [133–174] | 0.1 |
| Pre-TAVI diastolic blood pressure (mmHg) | 65 [57–74] | 71 [63–82] | <0.001 |
| Immediate post-TAVI systolic blood pressure (mmHg) | 128 [114–144] | 124 [112–139] | 0.017 |
| Immediate post-TAVI diastolic blood pressure (mmHg) | 53 [46–64] | 59 [51–67] | <0.001 |
| The 24 h post-TAVI systolic blood pressure (mmHg) | 126 [117–139] | 131 [118–142] | <0.001 |
| The 24 h post-TAVI diastolic blood pressure (mmHg) | 57 [50–64] | 63 [56–70] | <0.001 |
| Peri-procedural fluid (mL) | 700 [300–950] | 500 [250–700] | <0.001 |
| The 24 h post-TAVI fluid balance (mL) | 742.1 [98.2–1624] | 494 [−64.3–1013] | <0.001 |
| Medications | |||
| Required vasopressors peri-procedurally | 159 (79.1%) | 194 (35.6%) | <0.001 |
| Total fentanyl dose (mg) | 250 [150–500] | 0 [0–75] | <0.001 |
| Total midazolam dose (mg) | 2 [0–3] | 0 [0–1] | <0.001 |
| Propofol bolus dose (mg) | 0 [0–50] | 0 [0–0] | <0.001 |
| Propofol infusion total (mg) | 0 [0–77.6] | 0 [0–0] | <0.001 |
| Total propofol received during TAVI (mg) | 50 [0–104] | 0 [0–0] | <0.001 |
| Dexmedetomidine bolus dose (mg) | 0 [0–0] | 70 [33.5–99.7] | <0.001 |
| Dexmedetomidine infusion total (mg) | 0 [0–0] | 51.9 [30.6–83.3] | <0.001 |
| Total dexmedetomidine received during TAVI (mg) | 0 [0–0] | 116.6 [79.9–160] | <0.001 |
| General Anesthesia (n = 201) | Monitor Anesthesia Care (n = 543) | p-Value | |
|---|---|---|---|
| Pre-TAVI | |||
| Left ventricular ejection fraction (%) | 55 [50–60] | 60 [55–65] | <0.001 |
| Aortic valve area (cm2) | 0.8 [0.68–0.90] | 0.79 [0.67–0.90] | 0.495 |
| Mean aortic valve gradient (mmHg) | 41 [30.1–48] | 39.2 [29.8–45] | 0.073 |
| Aortic regurgitation (moderate to severe) | 10 (5%) | 12 (2.2%) | 0.13 |
| Immediate Post-TAVI | |||
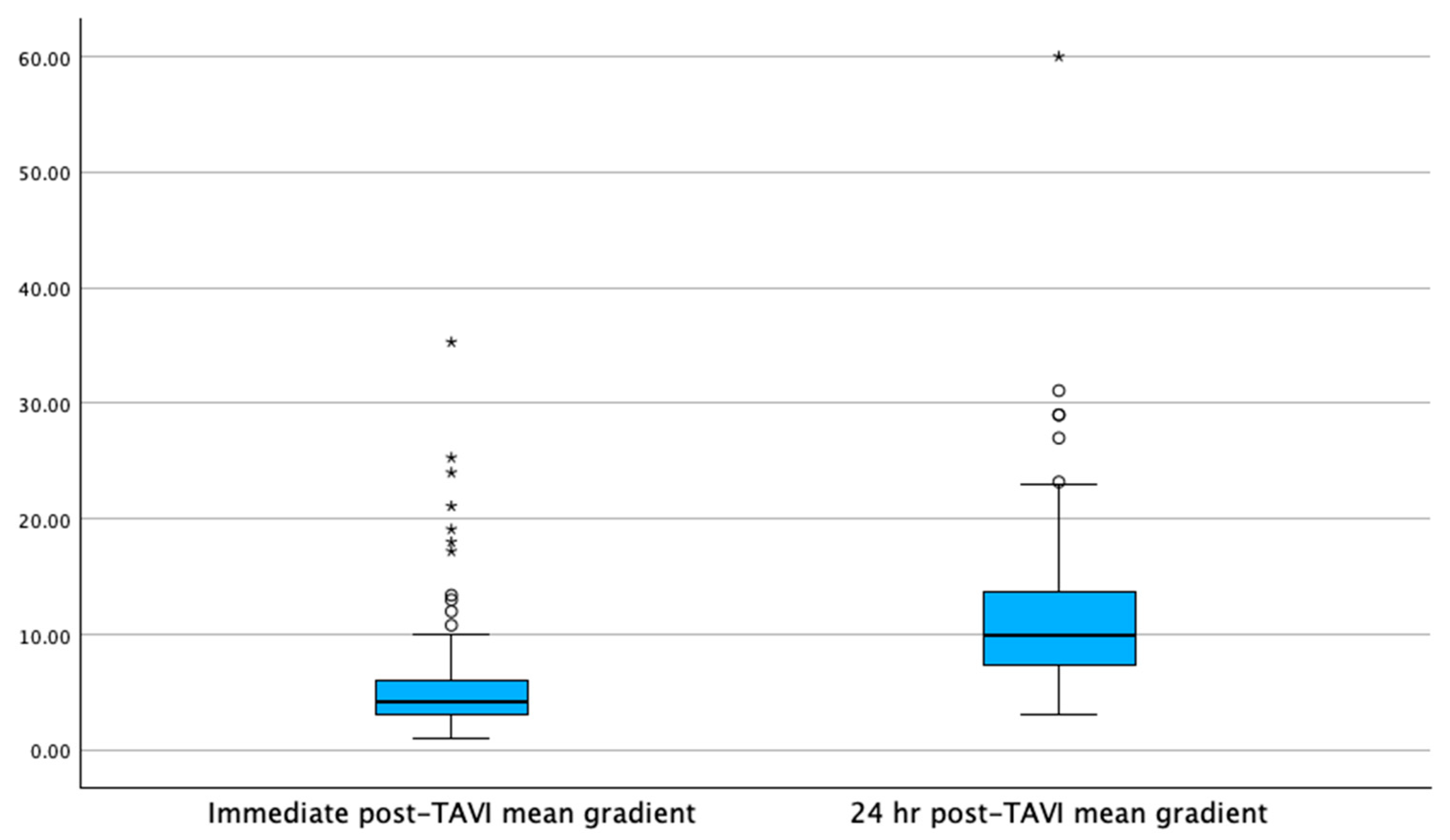
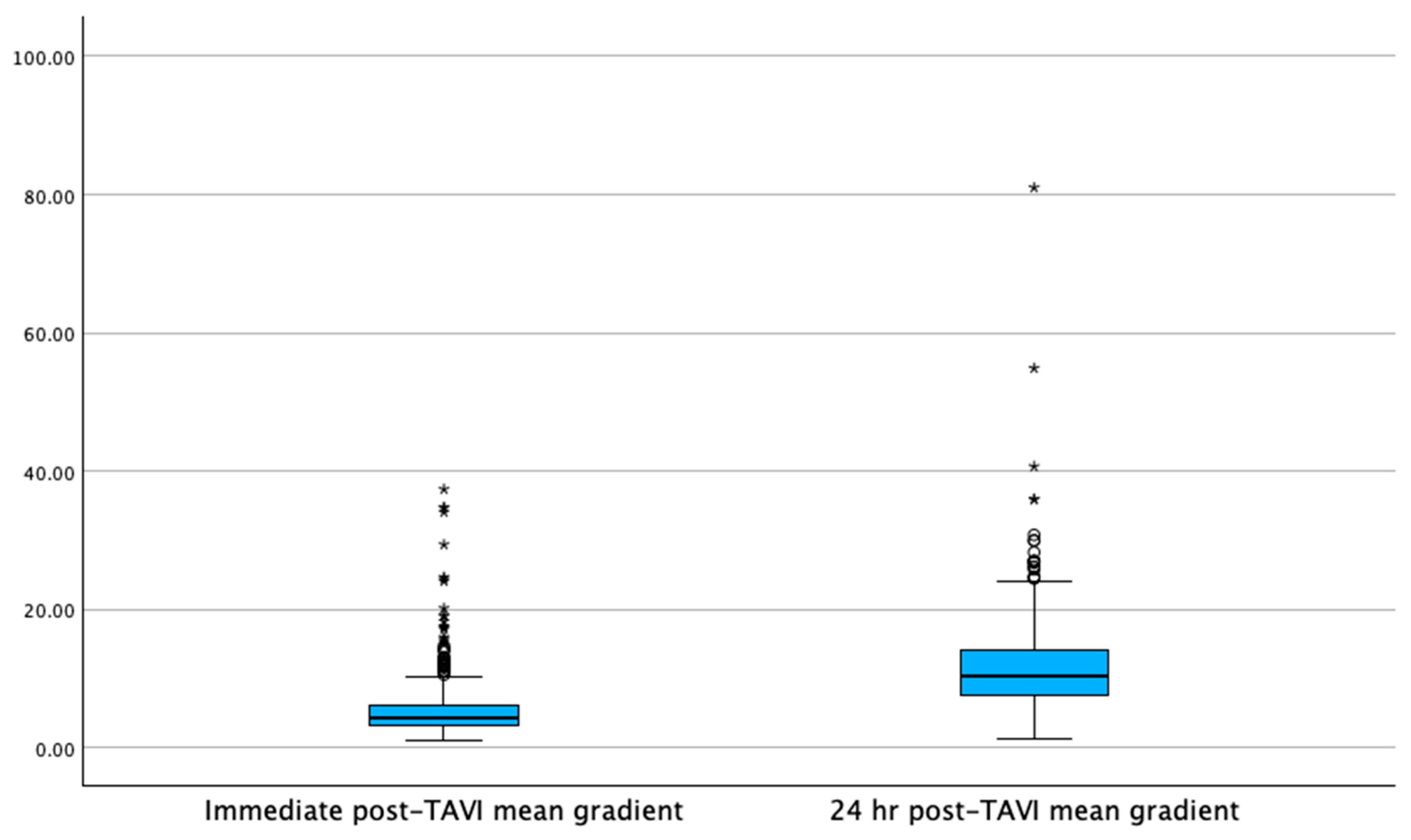
| Mean transvalvular gradient (mmHg) | 4.2 [3–6] | 4.4 [3.2–6.1] | 0.21 |
| Changes 24 h Post-TAVI | |||
| Mean transvalvular gradient (mmHg) | 10 [7.4–13.7] | 10.4 [7–13] | 0.192 |
| Effective orifice area (cm2) | 1.7 [1.5–2.0] | 1.62 [1.39–2] | 0.02 |
| Left ventricular ejection fraction (%) | 56 [53.4–60] | 61.4 [56–66] | <0.001 |
| Presence of paravalvular leak (trace to mild) | 37 (18.4%) | 26 (4.8%) | <0.001 |
| Presence of paravalvular leak (moderate to severe) | 1 (0.5%) | 1 (0.2%) | 0.47 |
| Gradient Changes in 24 h | |||
| Mean transvalvular gradient after 24 hr increase (mmHg) | 5.8 | <0.001 | |
| Mean transvalvular gradient after 24 hr increase (mmHg) | 6 | <0.001 | |
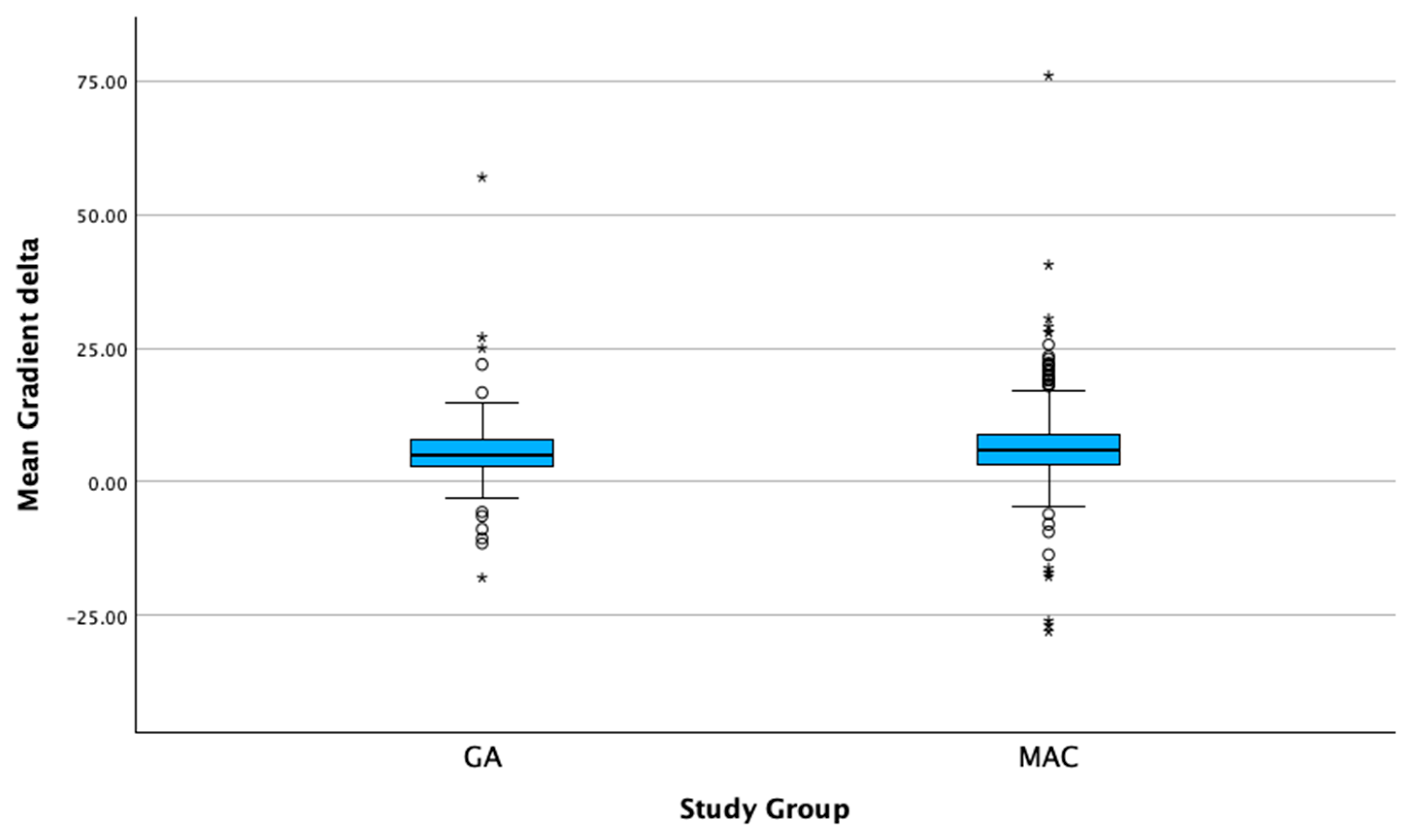
| Meant transvalvular gradient delta (mmHg) | 5.1 [3–8.1] | 5.8 [3.2–9] | 0.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogelson, B.; Baljepally, R.; Morvant, B.; Nowell, T.C.; Heidel, R.E.; Ferlita, S.; Weston, S.; Amro, A.; Spires, Z.; Ferraro, K.; et al. An Increase in Mean Aortic Valve Gradients the Day After Transcatheter Aortic Valve Implantation: The Effects of Evolving Anesthesia Techniques. J. Clin. Med. 2025, 14, 3272. https://doi.org/10.3390/jcm14103272
Fogelson B, Baljepally R, Morvant B, Nowell TC, Heidel RE, Ferlita S, Weston S, Amro A, Spires Z, Ferraro K, et al. An Increase in Mean Aortic Valve Gradients the Day After Transcatheter Aortic Valve Implantation: The Effects of Evolving Anesthesia Techniques. Journal of Clinical Medicine. 2025; 14(10):3272. https://doi.org/10.3390/jcm14103272
Chicago/Turabian StyleFogelson, Benjamin, Raj Baljepally, Billy Morvant, Terrance C. Nowell, Robert Eric Heidel, Steve Ferlita, Stefan Weston, Aladen Amro, Zachary Spires, Kirsten Ferraro, and et al. 2025. "An Increase in Mean Aortic Valve Gradients the Day After Transcatheter Aortic Valve Implantation: The Effects of Evolving Anesthesia Techniques" Journal of Clinical Medicine 14, no. 10: 3272. https://doi.org/10.3390/jcm14103272
APA StyleFogelson, B., Baljepally, R., Morvant, B., Nowell, T. C., Heidel, R. E., Ferlita, S., Weston, S., Amro, A., Spires, Z., Ferraro, K., & Patel, P. (2025). An Increase in Mean Aortic Valve Gradients the Day After Transcatheter Aortic Valve Implantation: The Effects of Evolving Anesthesia Techniques. Journal of Clinical Medicine, 14(10), 3272. https://doi.org/10.3390/jcm14103272





