Modern Conservative Management Strategies for Female Stress Urinary Incontinence: A Systematic Review
Abstract
1. Introduction
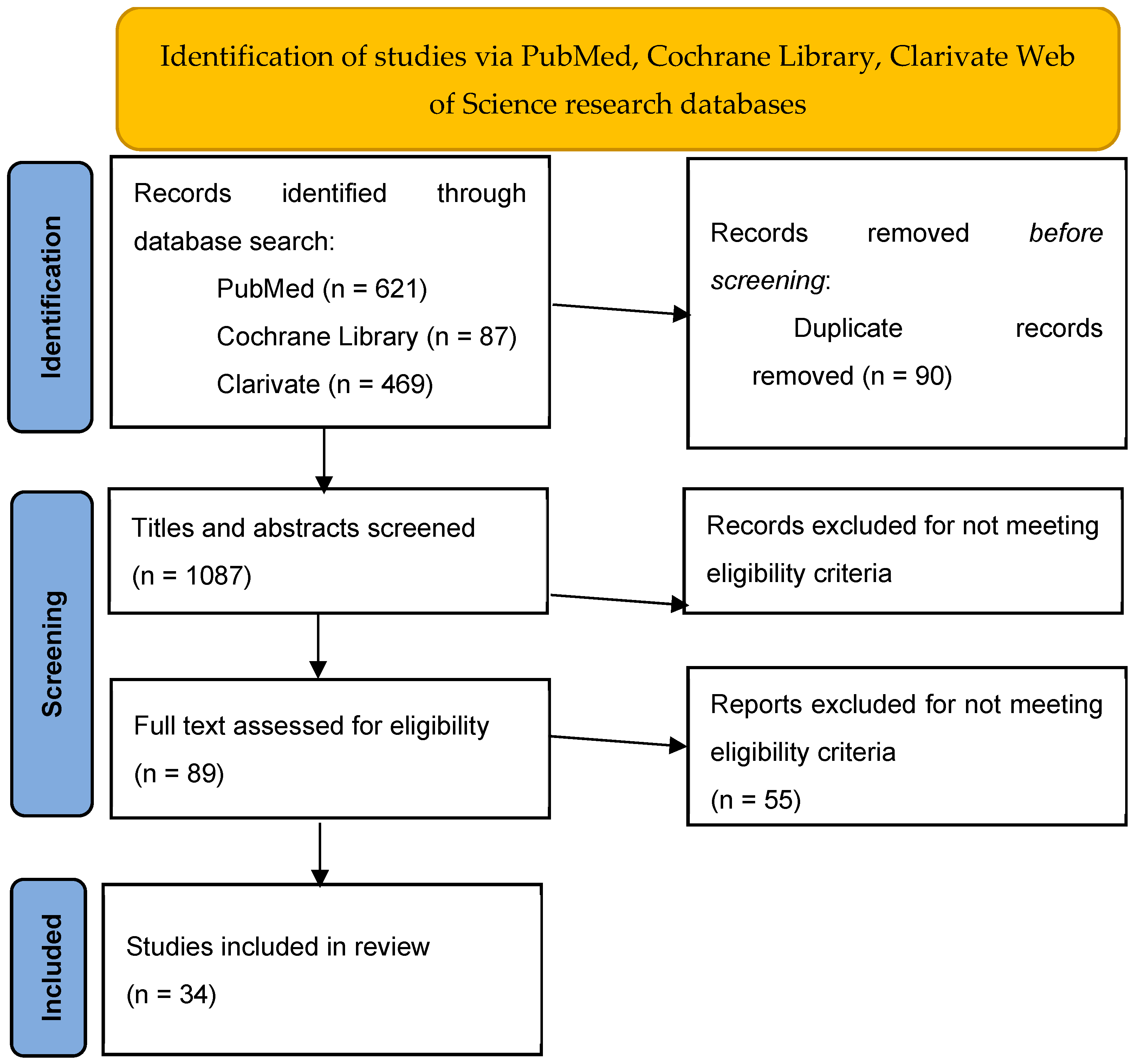
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Source
2.3. Search Strategy
2.4. Data Collection
2.5. Data Items
2.6. Selection Process
2.7. Data Collection Process
2.8. Risk of Bias
3. Results
3.1. Platelet-Rich Plasma Therapy
3.2. Laser, Infrared, and Radiofrequency
3.3. Bulking Agents
3.4. Stem Cell Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-PRP | Autologous platelet-rich plasma |
| APFQ | Australian Pelvic Floor Questionnaire |
| BMI | Body Mass Index |
| CO2 | Carbon Dioxide |
| Er:YAG | Erbium-doped yttrium aluminum garnet |
| FES | Functional electrical stimulation |
| ICIQ-FLUTS | International Consultation on Incontinence Questionnaire—Female Lower Urinary Tract Symptoms |
| ICIQ-SF | International Consultation on Incontinence Questionnaire—Urinary Incontinence Short Form |
| IIQ-7 | Incontinence Impact Questionnaire |
| IQOL-Q | Incontinence Quality of Life Questionnaire |
| KHQ | King’s Health Questionnaire |
| LS | Laser |
| N/A | Not available |
| OABSS | Overactive Bladder Symptom Score |
| PF | Pelvic floor |
| PFMT | Pelvic floor muscle training |
| PF-US | pelvic floor ultrasound |
| PGI-I | Patient Global Impression of Improvement |
| POPDI-6 | Pelvic Organ Prolapse Distress Inventory 6 |
| PTG | Physical Therapy Group |
| PWT | Pad weight test |
| RCT | Randomized controlled trial |
| RF | Radiofrequency |
| SUI | Stress urinary incontinence |
| UI | Urinary incontinence |
| UDI-6 | Urogenital Distress Inventory |
| UTI | Urinary tract infection |
| USA | United States of America |
| UUI | Urge Urinary Incontinence |
| VAS | Visual analog scale |
| VEL | Vaginal Erbium Laser |
| YAG | Yttrium-aluminum-garnet |
References
- Haylen, B.; de Ridder, D.; Freeman, R.; Swift, S.; Berghmans, B. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2009, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.; Chuang, Y.; Hsu, K.C.P.; Shen, Y.; Liu, S. Impact of female stress urinary incontinence on quality of life, mental health, work limitation, and healthcare seeking in China, Taiwan, and South Korea (LUTS Asia): Results from a cross-sectional, population-based study. Int. J. Women’s Health 2022, 14, 1871–1880. [Google Scholar] [CrossRef]
- Eurostat. Eurostat. 2021. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210224-1 (accessed on 31 January 2023).
- UNECE. UNECE. Available online: https://w3.unece.org/PXWeb/en/Table?IndicatorCode=34 (accessed on 19 December 2023).
- Alexandridis, V.; Drca, A.; Ek, M.; Soderberg, M.; Hamer, M.; Teleman, P. Retropubic slings are more efficient than transobturator at 10-year follow-up: A Swedish register-based study. Int. Urogynecol. J. 2023, 34, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Angioli, R.; Plotti, F.; Muzii, L.; Montera, R.; Panici, P.; Zullo, M. Tension-free vaginal tape versus transobturator suburethral tape: Five-year follow-up results of a prospective, randomised trial. Eur. Urol. 2010, 58, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Welk, B.; Dmochowski, R.; McCharty, K.; Keck, J.; Mourad, S.; Hashim, H. Complications associated with the use of mesh to treat female urinary incontinence and pelvic organ prolapse. Continence 2024, 12, 101713. [Google Scholar] [CrossRef]
- Zhu, L.; Schuster, P.; Klinge, U. Mesh implants: An overview of crucial mesh parameters. World J. Gastrointest. Surg. 2015, 7, 226–236. [Google Scholar] [CrossRef]
- Saraluck, A.; Chinthakanan, O.; Kijmanawat, A.; Aimjirakul, K.; Wattanayingcharoenchai, R.; Manonai, J. Autologous platelet rich plasma (A-PRP) combined with pelvic floor muscle training for the treatment of female stress urinary incontinence (SUI): A randomized control clinical trial. Neurourol. Urodyn. 2023, 43, 342–353. [Google Scholar] [CrossRef]
- Long, C.; Lin, K.; Shen, C.; Ker, C.; Liu, Y.; Loo, Z.; Hsiao, H.-H.; Lee, Y.-C. A pilot study: Effectiveness of local injection of autologous platelet-rich plasma in treating women with stress urinary incontinence. Sci. Rep. 2021, 11, 1584. [Google Scholar] [CrossRef]
- Athanasiou, S.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Pontikaki, A.; Grigoriadis, T. The Use of Platelet-rich Plasma as a Novel Nonsurgical Treatment of the Female Stress Urinary Incontinence: A Prospective Pilot Study. Female Pelvic Med. Reconstr. Surg. 2021, 27, e668–e672. [Google Scholar] [CrossRef]
- Grigoriadis, T.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Prodromidou, A.; Xadzilia, S.; Athanasiou, S. Platelet-Rich Plasma for the Treatment of Stress Urinary Incontinence—A Randomized Trial. Urogynecology 2024, 30, 42–49. [Google Scholar] [CrossRef]
- Behnia-Willison, F.; Nguyen, T.; Norbury, A.; Mohamadi, B.; Salvatore, S.; Lam, A. Promising impact of platelet rich plasma and carbon dioxide laser for stress urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 5, 100099. [Google Scholar] [CrossRef] [PubMed]
- Temtanakitpaisan, T.; Chongsomchai, C.; Buppasiri, P. Fractional CO2 laser treatment for women with stress predominant urinary incontinence: A randomized controlled trial. Int. Urogynecol. J. 2023, 34, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc, U.; Sencar, S.; Lenasi, H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg. Med. 2015, 47, 689–697. [Google Scholar] [CrossRef]
- Alexander, J.; Karjalainen, P.; Ow, L. CO2 surgical laser for treatment of stress urinary incontinence in women: A randomized controlled trial. Am. J. Obstet. Gynecol. 2022, 227, e1–e473. [Google Scholar] [CrossRef]
- Gaspar, A.; Koron, N.; Silva, J.; Brandi, H. Vaginal erbium laser for treatment of stress urinary incontinence: Optimization of treatment regimen for a sustained long-term effect. Lasers Med. Sci. 2022, 37, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, R.; Aharoni, S.; Justman, N.; Farago, N.; Gruenwald, I.; Lowenstein, L. The efficacy and safety of a single maintenance laser treatment for stress urinary incontinence: A double-blinded randomized controlled trial. Int. Urogynecol. J. 2022, 33, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Brandi, H. Non-ablative erbium YAG laser for the treatment of type III stress urinary incontinence (intrinsic sphincter deficiency). Lasers Med. Sci. 2017, 32, 685–691. [Google Scholar] [CrossRef]
- Lin, Y.; Hsieh, W.; Huang, L.; Liang, C. Effect of non-ablative laser treatment on overactive bladder symptoms, urinary incontinence and sexual function in women with urodynamic stress incontinence. Taiwan. J. Obstet. Gynecol. 2017, 56, 815–820. [Google Scholar] [CrossRef]
- da Fonseca, L.; Giarreta, F.; Peterson, T.; Locali, P.; Baracat, E.; Ferreira, E.; Haddad, J.M. A randomized trial comparing vaginal laser therapy and pelvic floor physical therapy for treating women with stress urinary incontinence. Neurourol. Urodyn. 2023, 42, 1445–1454. [Google Scholar] [CrossRef]
- da Silva, A.; Lopes-Martins, R.; Oliveira, A.; Franca, P. Effect of photobiomodulation associated with strengthening pelvic floor muscles in volunteers with urinary incontinence: A randomized, double-blinded, and placebo-controlled clinical trial. Lasers Med. Sci. 2023, 38, 278. [Google Scholar] [CrossRef]
- Fistonic, N.; Fistonic, I.; Lukanovic, A.; Gustek Findri, S.; Sorta Bilajac Turina, I.; Franic, D. First assessment of short-term efficacy of Er:YAG laser treatment on stress urinary incontinence in women: Prospective cohort study. Climacteric 2015, 18 (Suppl. S1), 37–42. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Levancini, M.; Cervigni, M. Vaginal erbium laser: The second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric 2015, 18, 757–763. [Google Scholar] [CrossRef]
- Seki, A.; Bianchi-Ferraro, A.; Fonseca, E.; Sartori, M.; Girao, M.; Jarmy-Di Bella, Z. CO2 Laser and radiofrequency compared to a sham control group in treatment of stress urinary incontinence (LARF study arm 3). A randomized controlled trial. Int. Urogynecol. J. 2022, 33, 3535–3542. [Google Scholar] [CrossRef]
- Elser, D.; Mitchell, G.; Mijlos, J.; Nickell, K.; Cline, K.; Winkler, H.; Wells, W.G. Nonsurgical transurethral collagen denaturation for stress urinary incontinence in women: 18-month results from a prospective long-term study. Neurourol. Urodyn. 2010, 29, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.; Karram, M.; Dmochowski, R. Efficacy and safety of Bulkamid in the treatment of female stress incontinence: A randomized, prospective multicenter North-American study. J. Urol. 2014, 192, 843–849. [Google Scholar] [CrossRef]
- Ghoniem, G.; Corcos, J.; Comiter, C.; Westnei, O.; Herschorn, S. Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J. Urol. 2010, 183, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Zullo, M.; Ruggiero, A.; Montera, R.; Plotti, F.; Muzii, L.; Angioli, R.; Panici, P.B. An ultra-miniinvasive treatment for stress urinary incontinence in complicated older patients. Maturitas 2010, 65, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Brosche, T.; Kuhn, A.; Lobodasch, K.; Sokol, E.R. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol. Urodyn. 2021, 40, 502–508. [Google Scholar] [CrossRef]
- Maggiore, U.; Alessandri, F.; Medic, M.; Gabelli, M.; Venturini, P.; Ferrero, S. Outpatient periurethral injections of polyacrylamide hydrogel for the treatment of female stress urinary incontinence: Effectiveness and safety. Arch. Gynecol. Obstet. 2013, 288, 131–137. [Google Scholar] [CrossRef]
- Carroll, T.; Christie, A.; Foreman, M.; Khatri, G.; Zimmern, P. Macroplastique for women with stress urinary incontinence secondary to intrinsic sphincter deficiency. Int. Braz. J. Urol. 2019, 45, 989–998. [Google Scholar] [CrossRef]
- Pai, A.; Al-Singary, W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid®) in the management of stress and mixed urinary incontinence: Three year follow up outcomes. Cent. Eur. J. Urol. 2015, 68, 428–433. [Google Scholar] [CrossRef]
- Plotti, F.; Montero, R.; Terranova, C.; Luvero, D.; Marrocco, F. Long-term follow-up of bulking agents for stress urinary incontinence in older patients. Menopause 2018, 25, 663–667. [Google Scholar] [CrossRef]
- Serati, M.; Soligo, M.; Braga, A.; Cantaluppi, S.; Coluccia, A.; Dedda, M.; Salvatore, S.; Cetin, I.; Ghezzi, F. Efficacy and safety of polydimethylsiloxane injection (Macroplastique®) for the treatment of female stress urinary incontinence: Results of a series of 85 patients with ≥3 years of follow-up. BJU Int. 2019, 123, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Safavi, M.; Heidari, R.; Aghayan, H. Concomitant Transurethral and Transvaginal-Periurethral Injection of Autologous Adipose Derived Stem Cells for Treatment of Female Stress Urinary Incontinence: A Phase One Clinical Trial. Acta Med. Iran. 2017, 55, 368–374. [Google Scholar]
- Mahboubeh, M.; Hamid, P.; Azar, D.; Ali, B.; Alireza, F.; Mohsen, B. Short and Medium-Term Results of the Autologous Adult Mucosa Stem Cell Therapy Compared with Mini-Sling Surgery in the Treatment of Women’s Stress Urinary Incontinence; A Randomized Clinical Trial. Curr. Stem Cell Res. Ther. 2023, 18, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arranz, M.; Alonso-Gregorio, S.; Fontana-Portella, P.; Bravo, E.; Sebastian, J.; Fernandez-Santos, M.; Garcia-Olmo, D. Two phase I/II clinical trials for the treatment of urinary incontinence with autologous mesenchymal stem cells. Stem Cells Transl. Med. 2020, 9, 1500–1508. [Google Scholar] [CrossRef]
- Sharifiaghdas, F.; Zohrabi, F.; Moghdasali, R.; Shekarchian, S.; Jaroughi, N.; Bolurieh, T.; Baharvand, H.; Aghdami, N. Autologous Muscle-derived Cell Injection for Treatment of Female Stress Urinary Incontinence: A Single-Arm Clinical Trial with 24-months Follow-Up. Urol. J. 2019, 16, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Gräs, S.; Klarskov, N.; Lose, G. Intraurethral injection of autologous minced skeletal muscle: A simple surgical treatment for stress urinary incontinence. J. Urol. 2014, 192, 850–855. [Google Scholar] [CrossRef]
- Stangel-Wojcikiewicz, K.; Jarocha, D.; Piwowar, M.; Jach, R.; Uhl, T.; Basta, A.; Majka, M. Autologous Muscle-Derived Cells for the Treatment of Female Stress Urinary Incontinence: A 2-Year Follow-Up of a Polish Investigation. Neurourol. Urodyn. 2014, 33, 323–330. [Google Scholar] [CrossRef]
- Blaganje, M.; Lukanovic, A. Ultrasound-guided autologous myoblast injections into the extrinsic urethral sphincter: Tissue engineering for the treatment of stress urinary incontinence. Int. Urogynecol. J. 2012, 24, 533–535. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Rubio-Azpeitia, E.; Martin, J.; Abate, M. Current Concepts and Translational Uses of Platelet Rich Plasma Biotechnology. In Biotechnology; Ekinci, D., Ed.; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Lapii, G.; Yakovleva, A.; Neimark, A. Structural Reorganization of the Vaginal Mucosa in Stress Urinary Incontinence under Conditions of Er:YAG Laser Treatment. Bull. Exp. Biol. Med. 2017, 162, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A. Comparison of new minimally invasive Er: YAG laser treatment and hormonal replacement therapy in the treatment of vaginal atrophy. Climacteric 2014, 17, 48–108. [Google Scholar]
- Kobashi, K.; Vasavada, S.; Bloschichak, A. Updates to surgical treatment of female stress urinary incontinence (SUI): AUA/SUFU guideline (2023). J. Urol. 2023, 209, 1091–1098. [Google Scholar] [CrossRef]
- Hu, J.; Pierre, E. Urinary Incontinence in Women: Evaluation and Management. Am. Fam. Physician 2019, 100, 339–348. [Google Scholar]
- Nambiar, A.; Bosch, R.; Cruz, F.; Lombardo, R. EAU Guidelines on Assessment and Nonsurgical Management of Urinary Incontinence. Eur. Urol. 2018, 73, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Royal Australian and New Zealand College of Obstetricians and Gynecologists; Urological Society of Australia and New Zealand. Treatment Options for Stress Urinary Incontinence. 2018. Available online: https://usanz.org.au/publicassets/d163f1bf-0246-e911-a2c2-b75c2fd918c5/Patient-information---Treatment-options-for-stress-urinary-incontinence-SUI-Information-2018.pdf (accessed on 23 March 2024).
- Weber, A.; Walters, M. Cost-effectiveness of urodynamic testing before surgery for women with pelvic organ prolapse and stress urinary incontinence. Am. J. Obstet. Gynecol. 2000, 183, 1338–1346. [Google Scholar] [CrossRef]
- Demaagd, G.; Davenport, T. Management of Urinary incontinence. Pharm. Ther. 2012, 37, 345–361H. [Google Scholar]
- Dmchowski, R.; Appell, R. Injectable agents in the treatment of stress urinary incontinence in women: Where are we now? Urology 2000, 56 (Suppl. S1), 32–40. [Google Scholar] [CrossRef]
- Mamut, A.; Carlson, K. Periurethral bulking agents for female stress urinary incontinence in Canada. Can. Urol. Assoc. J. 2017, 11 (Suppl. S2), S152–S154. [Google Scholar] [CrossRef]
- Pourebrahimi, A.; Khalili, A.; Behzadi, S.; Eftekhari, B.; Reyhani, H.; Larijani, A.; Norouzi, N.; Madani, A.H. Platelet-rich plasma for treatment of female stress urinary incontinence. Int. Urol. Nephrol. 2024, 57, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Dankova, I.; Pyrgidis, N.; Tishukov, M.; Georgiadou, E. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Female Sexual Dysfunction and Stress Urinary Incontinence: A Systematic Review. Biomedicines 2023, 11, 2919. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Mehrnoush, V.; Darsareh, F. Vaginal Laser Therapy for Stress Urinary Incontinence: A Systematic Review of Prospective Randomized Clinical Trials. J. Menopausal Med. 2022, 28, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Phillips, C. What women want now! Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 286, 118–120. [Google Scholar] [CrossRef]
- Robinson, D.; Anders, K.; Cardozo, L. What Do Women Want?: Interpretation of the Concept of Cure. Female Pelvic Med. Reconstr. Surg. 2003, 9, 273–277. [Google Scholar] [CrossRef]
| Keywords Used | PubMed | Cochrane Library | Clarivate | ||||
|---|---|---|---|---|---|---|---|
| Total | Clinical Trials Included | Total | Clinical Trials Included | Total | Clinical Trials Included | ||
| 1 | ((platelet rich plasma) OR (PRP)) AND (stress urinary incontinence) AND (female) | 15 | 5 | 14 | 4 | 12 | 5 |
| 2 | ((laser) OR (radiofrequency)) AND (stress urinary incontinence) AND (female) | 172 | 13 | 14 | 5 | 113 | 10 |
| 3 | (bulking agents) AND (stress urinary incontinence) AND (female) | 260 | 9 | 41 | 8 | 192 | 9 |
| 4 | (stem cell) AND (stress urinary incontinence) AND (female) | 174 | 7 | 18 | 4 | 152 | 6 |
| Author | Year | Location | Time Frame | Study Design | Population | Mean Age | Therapy Used | Subjective | Objective | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Saraluck et al. [9] | 2023 | Thailand | 2022–2023 | randomized, parallel two-group trial | 60 | 30–68 | A-PRP + PFMT vs. PFMT alone | ICIQ-FLUTS, IQOL-Q, PGI, subjective improvement | 1 h PWT |
| 2 | Long et al. [10] | 2021 | Taiwan | 2018 | prospective interventional trial | 20 | 44.5 ± 9.1 | A-PRP | ICIQ-SF, UDI6, IIQ-7, OABSS, POPDI-6 | 1 h PWT |
| 3 | Athanasiou et al. [11] | 2021 | Greece | prospective observational trial | 20 | N/A | A-PRP | ICIQ-FLUTS, KHQ, PGI-I, VAS | 1 h PWT | |
| 4 | Grigoriadis et al. [12] | 2024 | Greece | double blind, randomized controlled trial | 50 | N/A | PRP vs. sham control group (sodium chloride 0.9%) | ICIQ-FLUTS, KHQ, PGI-I, | 1 h PWT | |
| 5 | Behnia-Willison et al. [13] | 2020 | Australia | prospective observational trial | 62 | 55.98 ± 11.27 | CO2 laser + PRP | APFQ | N/A | |
| 6 | Temtanakitpaisan et al. [14] | 2023 | Thailand | 2019–2021 | randomized controlled trial | 59 | 51.25 ± 11.36 | CO2 vs. sham | ICIQ-SF | PF ultrasonography |
| 7 | Ogrinc et al. [15] | 2015 | Slovenia | 2012–2013 | prospective, non-randomized | 175 | 49.7 (±10) | Er:YAG | ICIQ, ISI, VAS, satisfaction | N/A |
| 8 | Alexander et al. [16] | 2022 | Australia | 2017–2020 | participant-blinded, sham-controlled, parallel group | 97 | 53 (34–79) | CO2 vs. sham | N/A | cough stress test, urodynamic stress incontinence, 24 h PWT |
| 9 | Gaspar et al. [17] | 2022 | Argentina | prospective noncontrolled | 43 | 56 (33–64) | Er:YAG | ICIQ-SF | 1 h PWT, 24 h PWT, 3 day voiding diary | |
| 10 | Lauterbach et al. [18] | 2022 | Israel | 2019–2020 | double-blinded, prospective RCT | 131 | 52 (±3.7) | CO2 vs. sham | UDI-6, ICIQ-UI | cough test, 1 h PWT |
| 11 | Gaspar et al. [19] | 2017 | Argentina | 22 | 57.9 (33–66) | Er:YAG | ICIQUI-SF | 1 h PWT | ||
| 12 | Lin et al. [20] | 2017 | Taiwan | 2015 | retrospective | 30 | 52.6 ± 8.8 | Er:YAG | ICIQ-SF, OABSS, UDI6, IIQ-7, POPDI-6, VAS | 1 h PWT, urodynamic studies |
| 13 | da Fonseca et al. [21] | 2023 | Brazil | RCT | 32 | 60.3 ± 8 | Er:YAG vs. PFMT | KHQ, IQOL | 1 h PWT | |
| 14 | da Silva [22] | 2023 | Brazil | RCT | 22 | N/A | infrared + PFMT vs. placebo + PFMT | ICIQ-SF | biofeedback | |
| 15 | Fistonic et al. [23] | 2015 | Croatia | prospective cohort | 73 | 47 (41–54) | Er:YAG | ICIQ-SF, VAS | N/A | |
| 16 | Gambacciani et al. [24] | 2015 | Italy | pilot prospective longitudinal | 19/62 | N/A | ER:YAG vs. standard vaginal gel with estriol | ICIQ-SF | N/A | |
| 17 | Seki et al. [25] | 2022 | Brazil | 3-arm double-blind RCT | 114 | 50 ± 8.9 | RF vs. LS vs. SHAM | IQOL, ICIQ-SF, Likert subjective scale, VAS | cough stress test, 1 h PWT, 7 day voiding diary | |
| 18 | Elser et al. [26] | 2010 | USA | prospective open label | 136 | 47 (26–87) | RF | IQOL, UDI-6, PGI-I | % of patients with a >50% reduction in SUI episodes, stress pad test | |
| 19 | Sokol et al. [27] | 2014 | USA and Canada | 2008–2011 | single-masked, randomized, prospective, 2-arm, parallel | 303 | 57.8 | Bulkamid vs. Contigen (collagen) | ICIQ-UI, IQOL, Likert | % of patients with a >50% reduction in SUI episodes, stress pad test; at least 50% reduction from baseline in self-reported daily number if UI episodes; 24 h PWT, diary, responder rate |
| 20 | Ghoniem et al. [28] | 2010 | USA | 2001–2004 | extension of a previously published 12mo ITT study | 67 | 62.4 ± 11.6 | Macroplastique | IQOL, PGI-I | 3 day voiding diary, cystoscopy, urodynamics, 1 h PWT, Stamey |
| 21 | Zullo et al. [29] | 2010 | USA | 2005–2008 | prospective cohort | 27 | 77 (75–85) | Macroplastique | VAS | 3 day voiding diary, stress test |
| 22 | Brosche et al. [30] | 2021 | Germany | 2005- | retrospective | 388 | 65.7 (±10.4) | Bulkamid | 4 point scale (cured, improved, unchanged, worse), ICIQUI-SF, VAS | number of UI pads used, % of subjects requiring reinjection |
| 23 | Maggiore et al. [31] | 2013 | Italy | 2008–2010 | retrospective | 82 | 54.3 ± 7.9 | Bulkamid | ICIQSF, IIQ7, PGI-I | # of UI episodes in 24 h, 24 h PWT |
| 24 | Carroll et al. [32] | 2019 | USA | 2011–2017 | prospective | 28/106 | 65.4 ± 8.3 | Macroplastique | self-report, UDI-6, VAS QOL | 3D ultrasound |
| 25 | Pai et al. [33] | 2015 | United Kingdom | 2006–2011 | 256 | N/A | Bulkamid | ICIQ, VAS | # of UI episodes in 24 h | |
| 26 | Plotti et al. [34] | 2018 | Italy | 1999–2013 | retrospective | 63 | 76 ± 8.2 | UBAs | ICIQUI-SF, PGI-I, IIQ-7 | N/A |
| 27 | Serati et al. [35] | 2019 | Italy | 2008–2014 | observational prospective | 85 | 64 (40–76) | Macroplastique | ICIQ-SF, PGI-I, patient satisfaction scale, UDI | voiding diary, stress test |
| 28 | Arjmand et al. [36] | 2017 | Iran | 2012 | prospective | 10 | 45.8 ± 8.7 | abdominal subcutaneous adipose tissue | ICIQ | 24 h voiding diary, 24 h PWT, urodynamic studies |
| 29 | Mahboubeh et al. [37] | 2023 | Iran | 2016–2018 | noninferiority randomized clinical trial | 30 | 52 | mucosa-derived SC vs. mini-sling | IIQ | Marshal |
| 30 | Garcia-Arranz et al. [38] | 2020 | Spain | 2012–2014 | prospective | 10 | 56.8 ± 9 | adipose-derived mesenchymal stem cells | SF36, ICIQUI-SF | urodynamic studies |
| 31 | Sharifiaghdas et al. [39] | 2019 | Iran | 2013–2016 | prospective | 20 | 51.5 (30–70) | muscle-derived stem cells | IIQ-7, UDI-6 | cough stress, 1 h PWT, urodynamic studies |
| 32 | Gräs et al. [40] | 2014 | Denmark | 2010–2013 | prospective | 45 | 52 (34–80) | minced autologous skeletal muscle tissue | ICIQUI-SF | 3 day voiding diary |
| 33 | Stangel-Wojcikiewicz et al. [41] | 2014 | Poland | 2009–2011 | prospective | 16 | 56.75 ± 7.63 | muscle-derived stem cells | Gaudenz | stress test, urodyn, PF-US |
| 34 | Blaganje et al. [42] | 2012 | Slovenia | 2010 | explorative clinical trial | 38 | 52 (18–75) | autologous myoblast + functional electrical stimulation for 5 weeks | VAS, PGI-I, IQOL | 3 day voiding diary, stress test, pad test, amount of leaked urine quantitatively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petca, A.; Fotă, A.; Petca, R.-C.; Rotar, I.C. Modern Conservative Management Strategies for Female Stress Urinary Incontinence: A Systematic Review. J. Clin. Med. 2025, 14, 3268. https://doi.org/10.3390/jcm14103268
Petca A, Fotă A, Petca R-C, Rotar IC. Modern Conservative Management Strategies for Female Stress Urinary Incontinence: A Systematic Review. Journal of Clinical Medicine. 2025; 14(10):3268. https://doi.org/10.3390/jcm14103268
Chicago/Turabian StylePetca, Aida, Andreea Fotă, Răzvan-Cosmin Petca, and Ioana Cristina Rotar. 2025. "Modern Conservative Management Strategies for Female Stress Urinary Incontinence: A Systematic Review" Journal of Clinical Medicine 14, no. 10: 3268. https://doi.org/10.3390/jcm14103268
APA StylePetca, A., Fotă, A., Petca, R.-C., & Rotar, I. C. (2025). Modern Conservative Management Strategies for Female Stress Urinary Incontinence: A Systematic Review. Journal of Clinical Medicine, 14(10), 3268. https://doi.org/10.3390/jcm14103268







