Effectiveness of Atrial Natriuretic Peptide in the Treatment of Critically Ill Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
- Randomized trials or observational cohorts involving critically ill patients, who are defined as those admitted to intensive care units (ICUs), high-dependency units (HDUs), or presenting with life-threatening conditions such as acute kidney injury, cardiogenic shock, or postoperative complications requiring intensive monitoring and organ support.
- Studies comparing atrial natriuretic peptide to placebo.
- Studies that reported results which focused on our objectives.
- Studies that were available for full review.
- Studies published in English.
- Studies without a control group.
- Studies with patients overlapping populations.
- Case series or case reports.
- Previous systematic reviews and meta-analysis.
2.2. Search Strategy and Screening
2.3. Endpoints of the Systematic Review
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
2.7. Subgroup Analysis
- Hospital length of stay;
- ICU length of stay;
- Serum creatinine level at discharge.
3. Results
3.1. Demographics
- -
- Hypertension: reported in 10 studies, affecting 42.5% to 83.1% of participants.
- -
- Coronary artery disease (CAD): reported in 11 studies, with prevalence ranging from 17.5% to 100%.
- -
- Diabetes mellitus: reported in 10 studies, affecting 5% to 39.1% of participants.
- -
- Congestive heart failure (CHF): reported in five studies, with a prevalence ranging from 20.9% to 99.2%.
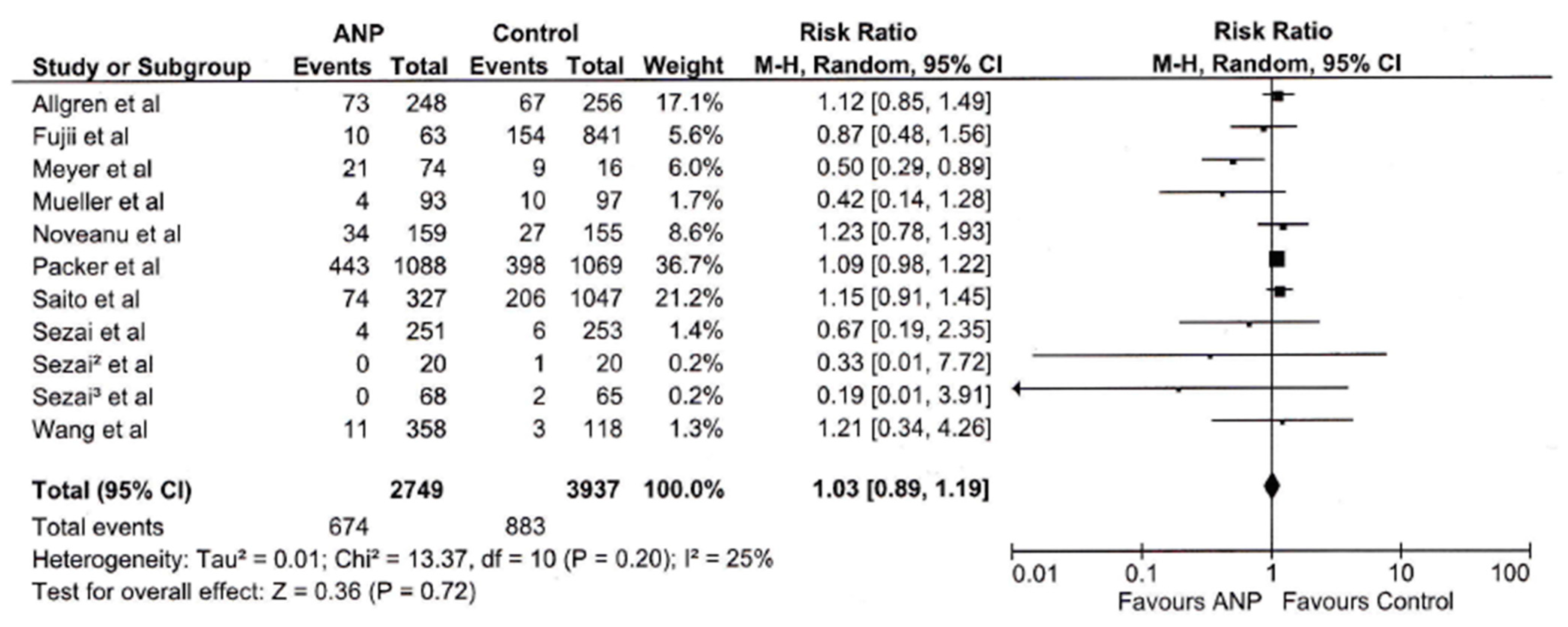
3.2. Mortality
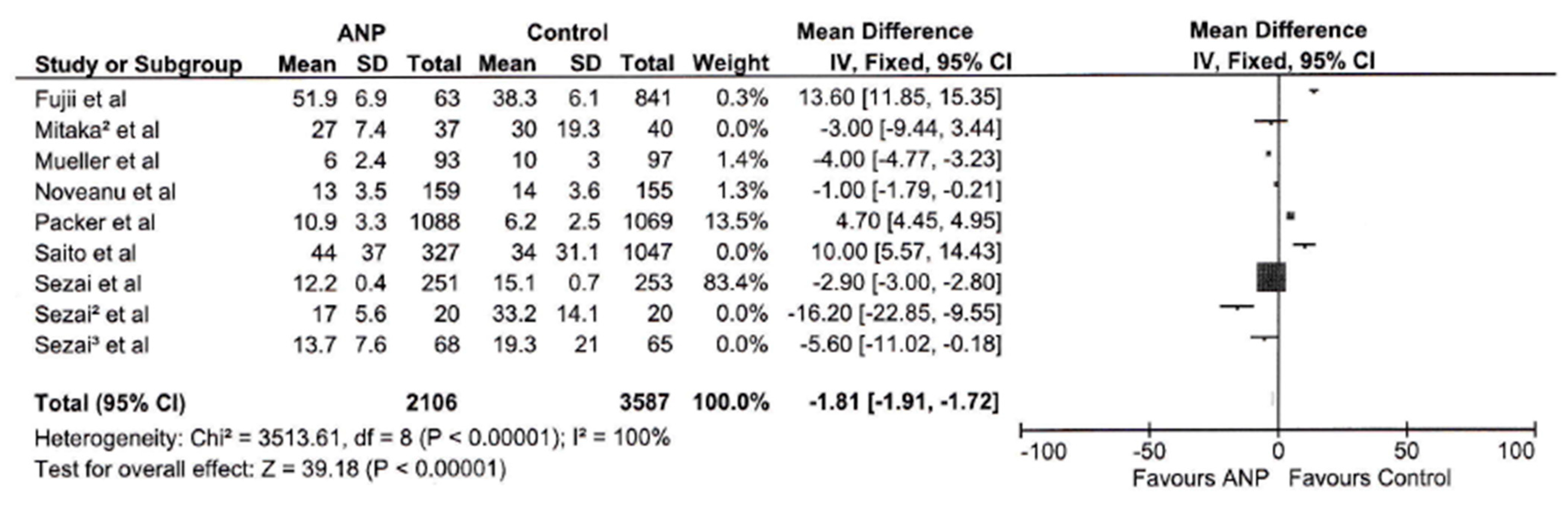
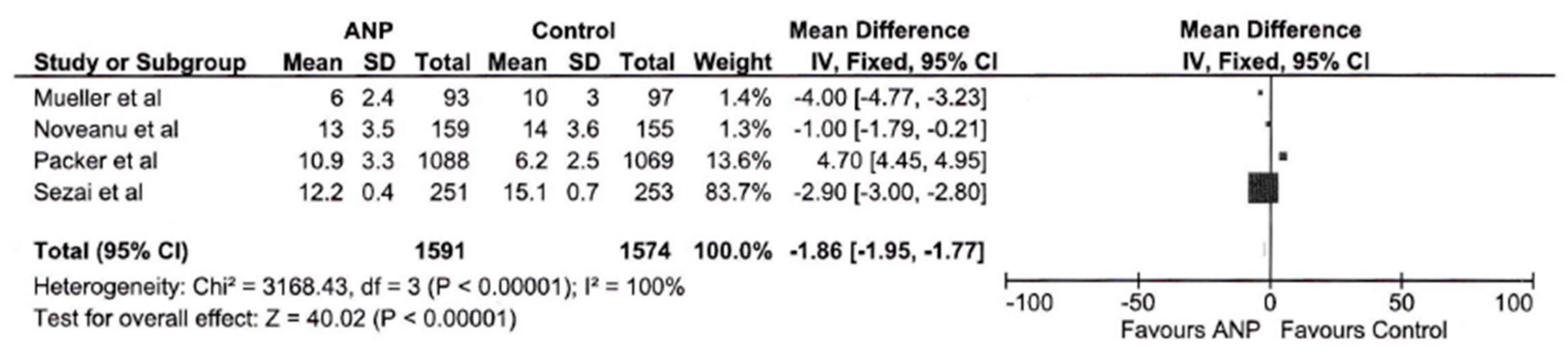
3.3. Hospital Length of Stay
3.4. ICU Length of Stay
3.5. Serum Creatinine Level
3.6. Blood Urea Nitrogen Level
4. Quality Assessment Result
5. Discussion
6. Study Limitations
- Large-scale, multicenter randomized controlled trials (RCTs) with standardized methodologies to more effectively delineate the most suitable patient populations for the administration of ANP therapy.
- Studies investigating the cost-effectiveness of ANP treatment, particularly considering the implications of reduced lengths of hospital stay.
- Explorations into the potential determinants that contribute to the observed variability in treatment outcomes.
- Research concentrating on distinct patient subgroups to ascertain those individuals who are most likely to derive benefit from ANP therapy.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| ANP | Atrial Natriuretic Peptide |
| BNP | Brain Natriuretic Peptide |
| CAD | Coronary Artery Disease |
| CHF | Congestive Heart Failure |
| cGMP | Cyclic Guanosine Monophosphate |
| CI | Confidence Interval |
| DBP | Diastolic Blood Pressure |
| GFR | Glomerular Filtration Rate |
| HDU | High Dependency Unit |
| HR | Heart Rate |
| ICU | Intensive Care Unit |
| I2 | Higgins’ Heterogeneity Statistic |
| LVEF | Left Ventricular Ejection Fraction |
| MD | Mean Difference |
| NOS | Newcastle–Ottawa Scale |
| NT-proBNP | N-terminal pro–Brain Natriuretic Peptide |
| PMID | PubMed Identifier |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trial |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RR | Risk Ratio |
| SBP | Systolic Blood Pressure |
| SD | Standard Deviation |
| SOAP | Sepsis Occurrence in Acutely Ill Patients |
| RoB | Risk of Bias |
References
- Giovou, A.E.; Gladka, M.M.; Christoffels, V.M. The Impact of Natriuretic Peptides on Heart Development, Homeostasis, and Disease. Cells 2024, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, V.; Pacinella, G.; Todaro, F.; Pecoraro, R.; Tuttolomondo, A. The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects. Int. J. Mol. Sci. 2023, 24, 9642. [Google Scholar] [CrossRef]
- Umanath, K.; Emani, S. Getting to the Heart of the Matter: Review of Treatment of Cardiorenal Syndrome. Adv. Chronic Kidney Dis. 2017, 24, 261–266. [Google Scholar] [CrossRef]
- Goldstein, S.; Bagshaw, S.; Cecconi, M.; Okusa, M.; Wang, H.; Kellum, J.; Mythen, M.; Shaw, A.D. Pharmacological management of fluid overload. Br. J. Anaesth. 2014, 113, 756–763. [Google Scholar] [CrossRef]
- Adhikari, N.K.; Fowler, R.A.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet 2010, 376, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- de Bold, A.J.; Borenstein, H.B.; Veress, A.T.; Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981, 28, 89–94. [Google Scholar] [CrossRef]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [CrossRef]
- Mitaka, C.; Kudo, T.; Haraguchi, G.; Tomita, M. Cardiovascular and renal effects of carperitide and nesiritide in cardiovascular surgery patients: A systematic review and meta-analysis. Crit. Care 2011, 15, R258. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Coca, S.G.; Nigwekar, S.U.; Garg, A.X.; Garwood, S.; Parikh, C.R. Prevention and treatment of acute kidney injury in patients undergoing cardiac surgery: A systematic review. Am. J. Nephrol. 2010, 31, 408–418. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Navaneethan, S.D.; Parikh, C.R.; Hix, J.K. Atrial natriuretic peptide for preventing and treating acute kidney injury. Cochrane Database Syst. Rev. 2009, 7, CD006028. [Google Scholar] [CrossRef]
- Tamura, Y.; Nagata, H.; Sato, Y.; Nitta, H.; Wakabayashi, G. Usefulness of human atrial natriuretic peptide (hANP) on perioperative management for liver resection. Masui 2011, 60, 343–352. [Google Scholar] [PubMed]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.; et al. Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Allgren, R.L.; Marbury, T.C.; Rahman, S.N.; Weisberg, L.S.; Fenves, A.Z.; Lafayette, R.A.; Sweet, R.M.; Genter, F.C.; Kurnik, B.R.; Conger, J.D.; et al. Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N. Engl. J. Med. 1997, 336, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Laule-Kilian, K.; Scholer, A.; Frana, B.; Rodriguez, D.; Schindler, C.; Marsch, S.; Perruchoud, A.P. Use of B-type natriuretic peptide for the management of women with dyspnea. Am. J. Cardiol. 2004, 94, 1510–1514. [Google Scholar] [CrossRef]
- Sezai, A.; Hata, M.; Niino, T.; Yoshitake, I.; Unosawa, S.; Wakui, S.; Osaka, S.; Takayama, T.; Kasamaki, Y.; Hirayama, A.; et al. Influence of continuous infusion of low-dose human atrial natriuretic peptide on renal function during cardiac surgery: A randomized controlled study. J. Am. Coll. Cardiol. 2009, 54, 1058–1064. [Google Scholar] [CrossRef]
- Sezai, A.; Shiono, M.; Hata, M.; Iida, M.; Wakui, S.; Soeda, M.; Negishi, N.; Kasamaki, Y.; Saito, S.; Kato, J.; et al. Efficacy of continuous low-dose human atrial natriuretic peptide given from the beginning of cardiopulmonary bypass for thoracic aortic surgery. Surg. Today 2006, 36, 508–514. [Google Scholar] [CrossRef]
- Mitaka, C.; Kudo, T.; Jibiki, M.; Sugano, N.; Inoue, Y.; Makita, K.; Imai, T. Effects of human atrial natriuretic peptide on renal function in patients undergoing abdominal aortic aneurysm repair. Crit. Care Med. 2008, 36, 745–751. [Google Scholar] [CrossRef]
- Meyer, M.; Pfarr, E.; Schirmer, G.; Uberbacher, H.J.; Schöpe, K.; Böhm, E.; Flüge, T.; Mentz, P.; Scigalla, P.; Forssmann, W.G. Therapeutic use of the natriuretic peptide ularitide in acute renal failure. Ren. Fail. 1999, 21, 85–100. [Google Scholar] [CrossRef]
- Sezai, A.; Hata, M.; Niino, T.; Yoshitake, I.; Unosawa, S.; Wakui, S.; Fujita, K.; Takayama, T.; Kasamaki, Y.; Hirayama, A.; et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: The NU-HIT (Nihon University working group study of low-dose Human ANP Infusion Therapy during cardiac surgery) for left ventricular dysfunction. J. Am. Coll. Cardiol. 2010, 55, 1844–1851. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Mashiko, K.; Saito, N.; Matsumoto, H.; Hara, Y.; Kutsukata, N.; Yokota, H. Effectiveness of human atrial natriuretic peptide supplementation in pulmonary edema patients using the pulse contour cardiac output system. Yonsei Med. J. 2010, 51, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Noveanu, M.; Pargger, H.; Breidthardt, T.; Reichlin, T.; Schindler, C.; Heise, A.; Schoenenberger, R.; Manndorff, P.; Siegemund, M.; Mebazaa, A.; et al. Use of B-type natriuretic peptide in the management of hypoxaemic respiratory failure. Eur. J. Heart Fail. 2011, 13, 154–162. [Google Scholar] [CrossRef]
- Shibasaki, I.; Fukuda, H.; Yamada, Y.; Kuwata, T.; Hori, T.; Ogawa, H.; Tsuchiya, G. Effects of continuous infusion of low-dose human atrial natriuretic peptide (hANP) on the lungs during cardiac surgery. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, P.; Li, Y.; Liu, W.; Bai, S.; Zhen, Y.; Li, D.; Yang, P.; Chen, Y.; Hong, L.; et al. Efficacy and Safety of 1-Hour Infusion of Recombinant Human Atrial Natriuretic Peptide in Patients With Acute Decompensated Heart Failure: A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Medicine 2016, 95, e2947. [Google Scholar] [CrossRef]
- Packer, M.; O’Connor, C.; McMurray, J.J.V.; Wittes, J.; Abraham, W.T.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Holcomb, R.; Krum, H.; et al. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N. Engl. J. Med. 2017, 376, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, C.; Ohnuma, T.; Murayama, T.; Kunimoto, F.; Nagashima, M.; Takei, T.; Iguchi, N.; Tomita, M. Effects of low-dose atrial natriuretic peptide infusion on cardiac surgery-associated acute kidney injury: A multicenter randomized controlled trial. J. Crit. Care 2017, 38, 253–258. [Google Scholar] [CrossRef]
- Fujii, T.; Sato, T.; Uchino, S.; Doi, K.; Iwami, T.; Kawamura, T. Human atrial natriuretic peptide for acute kidney injury in adult critically ill patients: A multicenter prospective observational study. J. Crit. Care 2019, 51, 229–235. [Google Scholar] [CrossRef]
- Saito, K.; Uchino, S.; Fujii, T.; Saito, S.; Takinami, M.; Uezono, S. Effect of low-dose atrial natriuretic peptide in critically ill patients with acute kidney injury: A retrospective, single-center study with propensity-score matching. BMC Nephrol. 2020, 21, 31. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2009. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 15 March 2025).
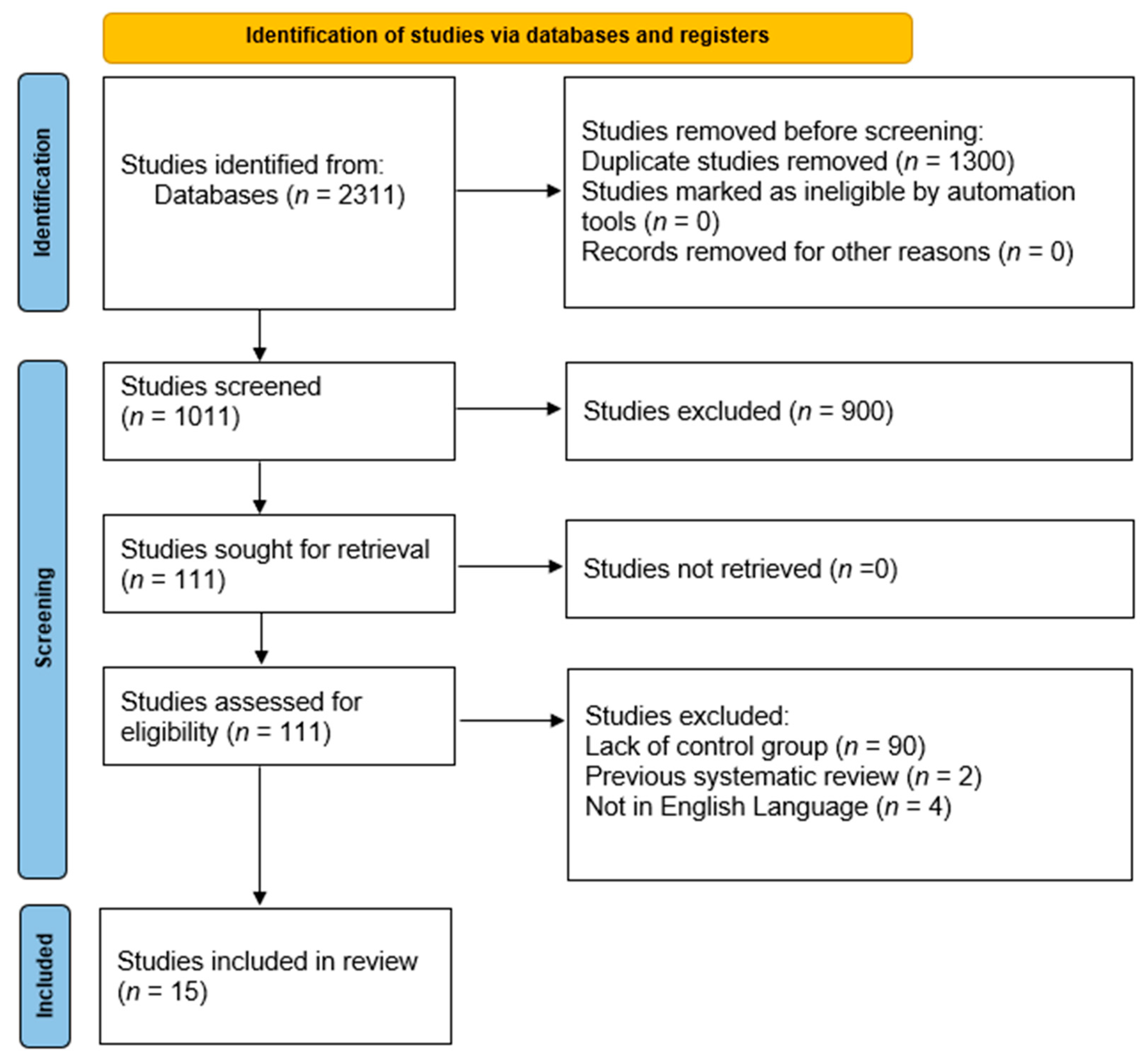
| Study | Country | Sample | Age * (yr) | Sex (%) | Admission Etiology | Hypertension (%) | ANP Regimen | CAD (%) | DM (%) | CHF (%) | LVEF (%) | HR/m | Serum Creatinine * (mmol/L) | SBP * (mmHg) | DBP * (mmHg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | |||||||||||||||
| Allgren et al., 1997 [14] | USA | 504 | 62 ± 1.0 | 50.2 | 49.8 | Acute tubular necrosis | 58 | 0.05 → 0.2 µg/kg/min IV over 90 min, then for 24 h | 47 | 29 | 28 | NA | NA | 4.6 ± 1.0 | 127.3 ± 12.3 | NA |
| Mueller et al., 2004 [15] | Switzerland | 452 | 70.8 ± 15.5 | 57.9 | 42.1 | Acute dyspnea | 52 | 2 µg/kg IV bolus, 0.01 µg/kg/min continuous infusion over 24–48 h | 50 | 23 | NA | NA | 98 ± 25.5 | NA | 146.5 ± 29 | 85.5 ± 19 |
| Sezai et al., 2006 [16] | Japan | 40 | 63.4 ± 12.8 | 50 | 50 | Cardiac surgery | NA | 0.025 µg/kg/min continuous IV infusion for 24 h postoperatively | 100 | NA | NA | 60.2 ± 1.3 | NA | NA | NA | NA |
| Sezai 2 et al., 2009 [17] | Japan | 504 | 65.9 ± 0.6 | 78.9 | 21.1 | Thoracic surgery | 70 | 0.02 μg/kg per minute until patient in ICU | 100 | 44 | NA | 60.2 ± 1.3 | NA | NA | NA | NA |
| Mitaka et al., 2008 [18] | Japan | 40 | 71.4 ± 8.2 | 87.5 | 12.5 | Abdominal aortic aneurysm repair | 43 | 0.02 μg/kg/min for 48 h | 75 | 5 | NA | NA | NA | NA | NA | NA |
| Meyer et al., 2009 [19] | Germany | 172 | 64.8 ± 10.8 | 63.9 | 36.1 | Acute renal failure | 40 | U5 (5 ng/kg/min), U20 (20 ng/kg/min), U40 (40 ng/kg/min), and U80 (80 ng/kg/min) for 5 days | 68 | 29 | 21 | NA | NA | NA | NA | NA |
| Sezai 3 et al., 2010 [20] | Japan | 133 | 65.9 ± 9.7 | 85.7 | 14.3 | Coronary artery bypass grafting | 74 | 0.02 μg/kg per minute for 12 h | 100 | 61 | NA | NA | NA | NA | NA | NA |
| Sakamoto et al., 2010 [21] | Japan | 10 | 63.9 ± 15.4 | 80 | 20 | Pulmonary edema | NA | 0.1 μg/kg/min | NA | NA | NA | NA | 95.7 ± 16.1 | 1.05 ± 0.4 | NA | NA |
| Noveanu et al., 2010 [22] | Switzerland | 314 | 69 ± 13 | 57.6 | 42.4 | Hypoxemic respiratory failure | 51 | NA | 38 | NA | 27 | NA | 98.5 | NA | 127 | 67 |
| Shibasaki et al., 2015 [23] | Japan | 30 | 63.9 ± 7.9 | 80 | 20 | Cardiac surgery | 53 | 0.02 μg/kg per minute for 24 h | NA | 33 | NA | 60.4 ± 9.3 | NA | 0.8 ± 0.3 | NA | NA |
| Wang et al., 2016 [24] | China | 476 | 55.3 ± 13.3 | 75 | 25 | Acute decompensated heart failure | NA | 0.1 µg/kg/min adjusted half hour to 0.15 µg/kg/min for 1 h | 27 | NA | 99 | 29.4 ± 6.6 | 83.7 ± 16.9 | 0.1 ± 0.03 | 115.5 ± 18.9 | NA |
| Packer et al., 2017 [25] | USA | 2157 | 68.5 ± 11.4 | 65.8 | 34.2 | Acute heart failure | NA | 15 ng/kg/min for 48 h | 53 | 39 | 3 | 33.9 | 86 ± 18.9 | 1.24 ± 0.36 | 134.7 ± 17.9 | 79.2 ± 13.3 |
| Mitaka 2 et al., 2017 [26] | Japan | 77 | 73 | 71.4 | 28.6 | Acute kidney injury associated with cardiac surgery | 83 | 0.02 μg/kg/min until serum creatinine is back to normal | 9 | 24 | NA | NA | NA | 0.94 | NA | NA |
| Fujii et al., 2018 [27] | Japan | 904 | 66.9 | 69.5 | 30.5 | Acute kidney injury | 51 | 0.028 μg/kg/min for 2 days or longer | NA | 120 | NA | NA | NA | NA | NA | NA |
| Saito et al., 2020 [28] | Japan | 1374 | 68 | 66.9 | 33.1 | Acute kidney injury | NA | 0.019 μg/kg/min for 2 days | NA | NA | NA | NA | NA | 0.1 | NA | NA |
| Outcome | Participants | Higgins I2 | Z Score | p Value | Risk Ratio/Mean Difference 95% CI |
|---|---|---|---|---|---|
| Mortality | 6686 | 25% | 0.36 | 0.72 | 1.03 [0.89, 1.19] |
| Hospital Stay | 5693 | 100% | 39.18 | <0.00001 | −1.81 [−1.91, −1.72] |
| Hospital Stay Subgroup | 3165 | 100% | 40.02 | <0.00001 | −1.86 [−1.95, −1.77] |
| ICU Stay | 4999 | 96% | 2.00 | 0.05 | 0.13 [0.00, 0.25] |
| ICU Stay Subgroup | 3845 | 98% | 1.44 | 0.15 | 0.10 [−0.03, 0.23] |
| Creatinine | 3657 | 98% | 1.17 | 0.24 | −0.10 [−0.28, 0.07] |
| Creatinine Subgroup | 3375 | 99% | 86.21 | <0.00001 | −0.19 [−0.20, −0.19] |
| Blood Urea Nitrogen | 756 | 97% | 9.22 | <0.00001 | 0.37 [0.29, 0.45] |
| Study | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias |
|---|---|---|---|---|---|
| Allgren et al. [14] | Low | Low | Low | Low | Low |
| Mueller et al. [15] | Low | Unclear | Low | Low | Low |
| Sezai et al. [16] | Unclear | Low | Low | Low | Low |
| Sezai2 et al. [17] | High | Unclear | Unclear | Low | Low |
| Mitaka et al. [18] | High | Low | Unclear | Low | Low |
| Meyer et al. [19] | High | High | High | Low | Low |
| Sezai3 et al. [20] | Low | Low | Unclear | Low | Low |
| Sakamoto et al. [21] | High | High | Unclear | Low | Low |
| Noveanu et al. [22] | Low | Low | Low | Low | Low |
| Shibasaki et al. [23] | Low | Low | Unclear | Low | Low |
| Wang et al. [24] | Low | Low | Low | Low | Low |
| Packer et al. [25] | Low | Low | Low | Low | Low |
| Mitaka2 et al. [26] | Low | Low | Unclear | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odutola, P.O.; Olarewaju, A.; Shah, P. Effectiveness of Atrial Natriuretic Peptide in the Treatment of Critically Ill Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3267. https://doi.org/10.3390/jcm14103267
Odutola PO, Olarewaju A, Shah P. Effectiveness of Atrial Natriuretic Peptide in the Treatment of Critically Ill Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(10):3267. https://doi.org/10.3390/jcm14103267
Chicago/Turabian StyleOdutola, Peter Olujimi, Ayodeji Olarewaju, and Priyank Shah. 2025. "Effectiveness of Atrial Natriuretic Peptide in the Treatment of Critically Ill Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 10: 3267. https://doi.org/10.3390/jcm14103267
APA StyleOdutola, P. O., Olarewaju, A., & Shah, P. (2025). Effectiveness of Atrial Natriuretic Peptide in the Treatment of Critically Ill Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(10), 3267. https://doi.org/10.3390/jcm14103267






