Does Radioactive Iodine Treatment Affect Thyroid Size and Tracheal Diameter?
Abstract
1. Introduction
2. Participants and Methods
2.1. Study Design and Data Collections
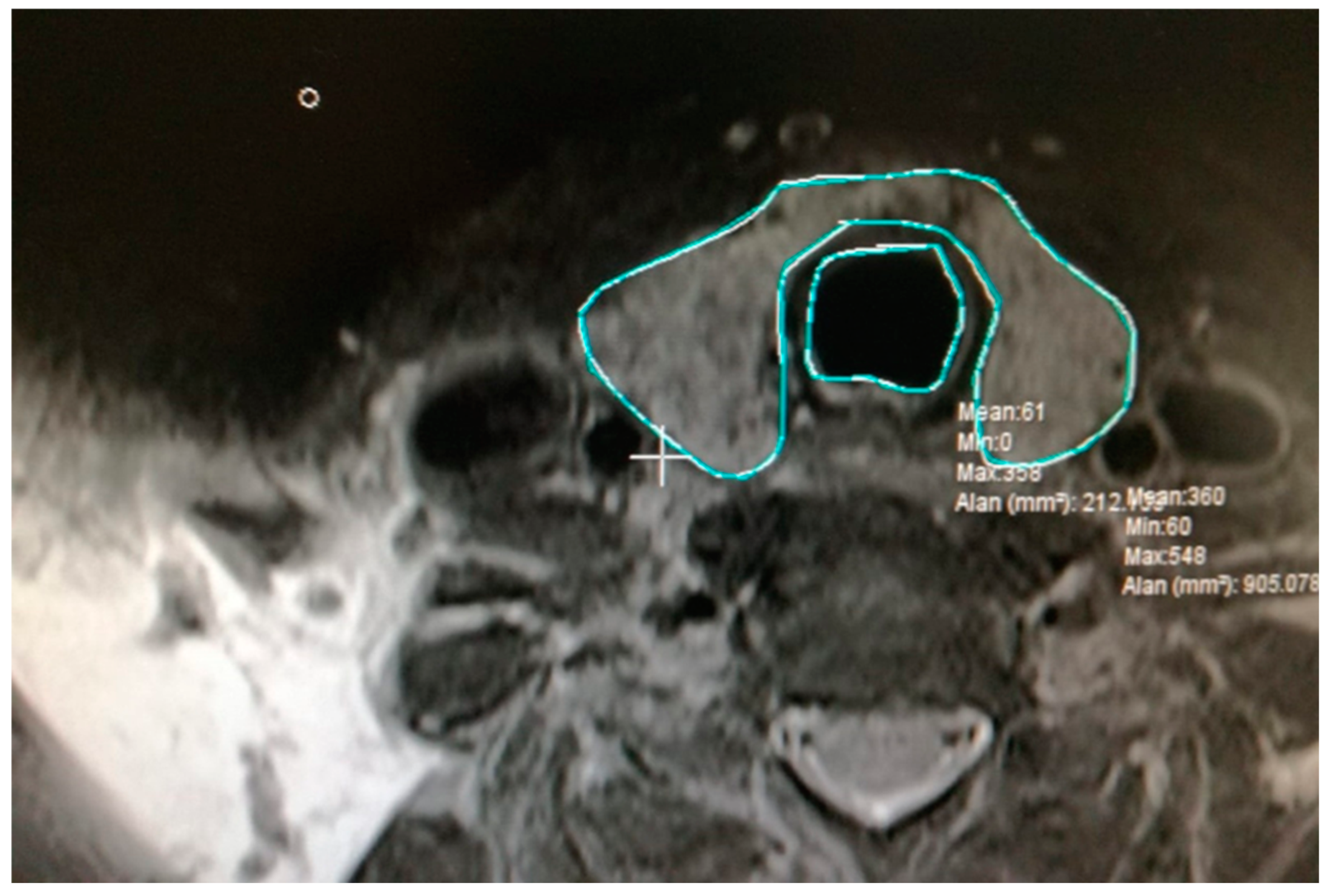
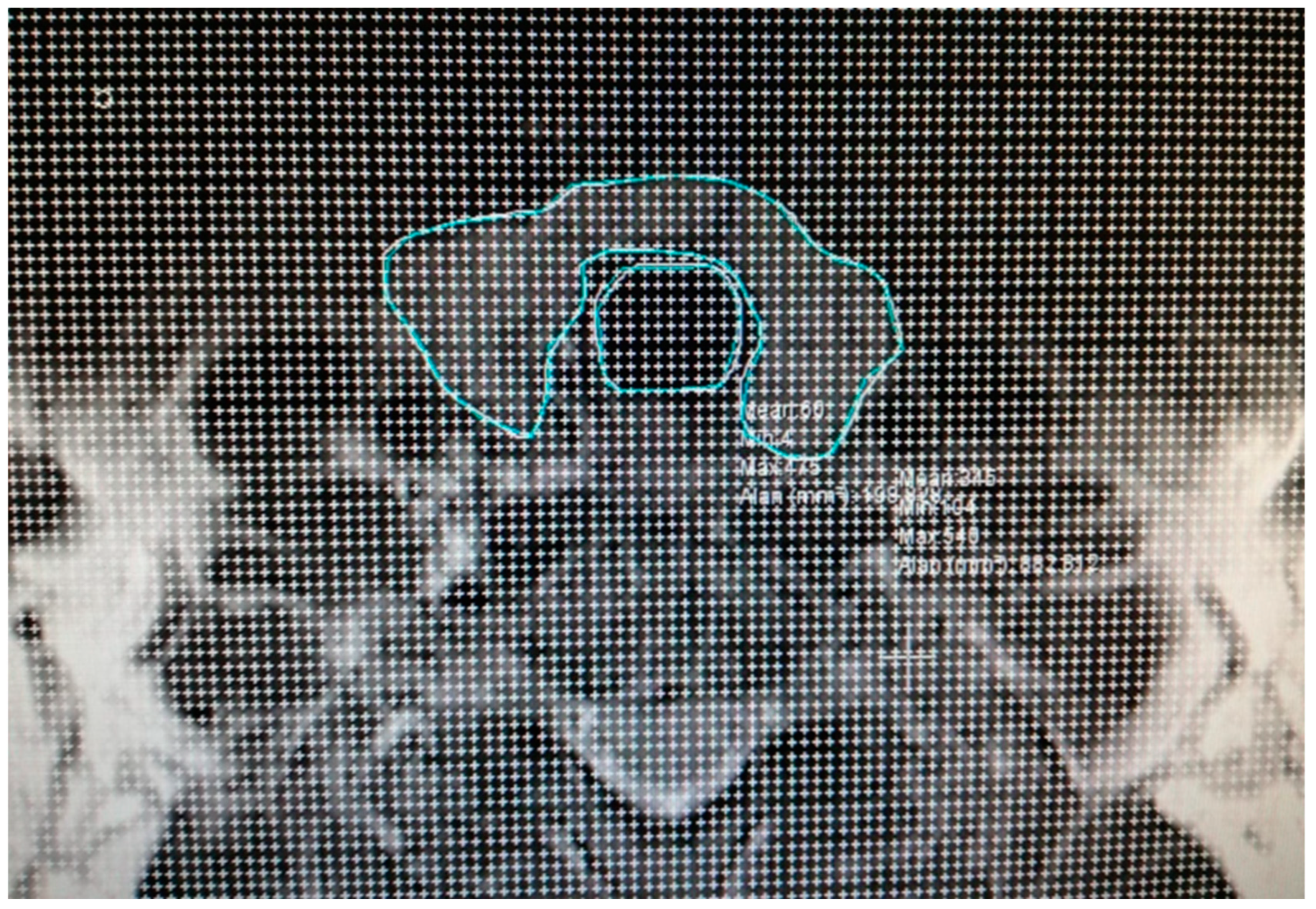
2.2. Calculation of Total Volume of Thyroid and Trachea
2.3. Statistical Analysis
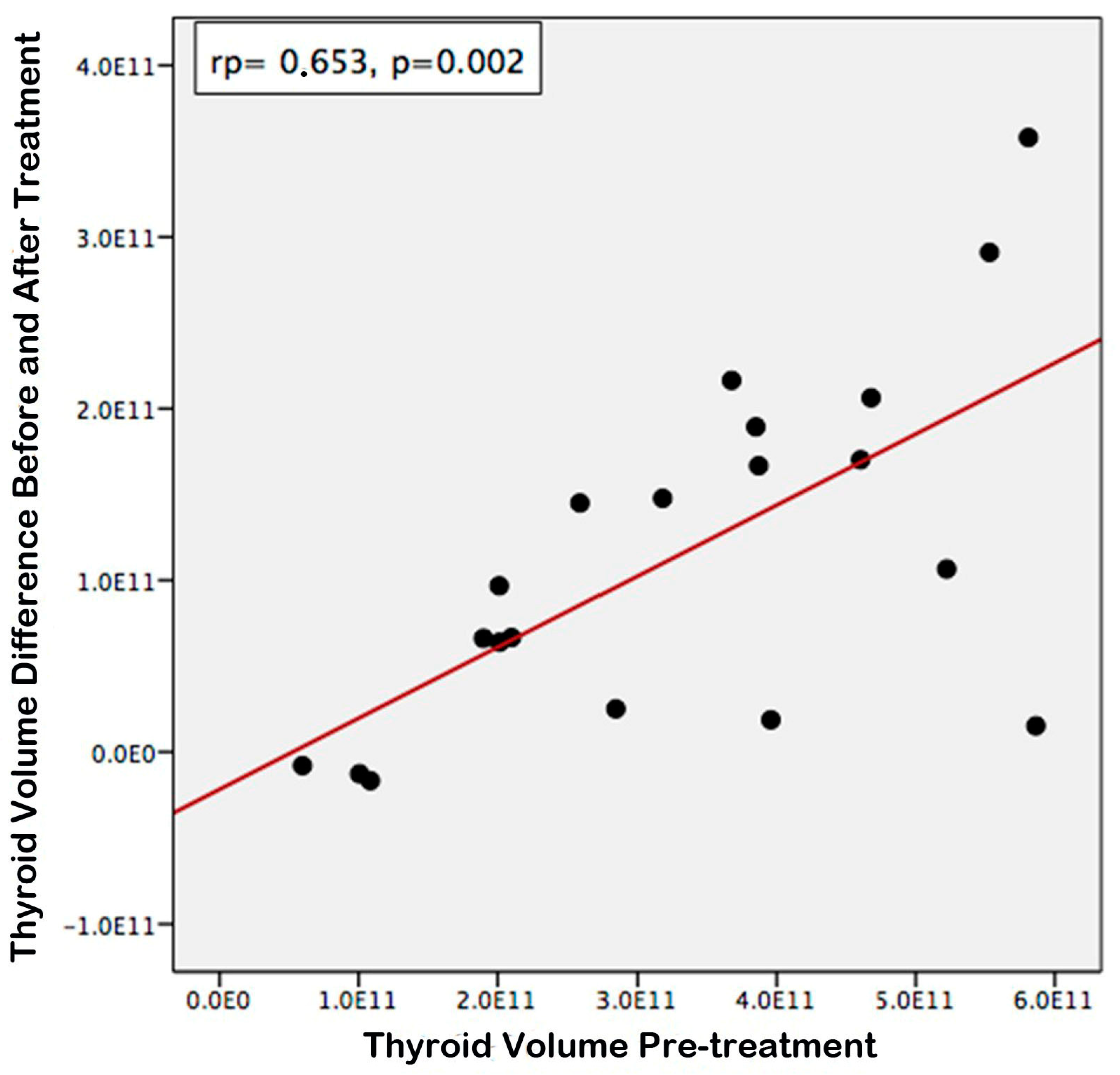
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Clinical practice. Graves’ disease. N. Engl. J. Med. 2008, 358, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Studer, H. Multinodular Goitre: “Much More to It than Simply Iodine Deficiency”. Best Pract. Res. Clin. Endocrinol. Metab. 2000, 14, 577–600. [Google Scholar] [CrossRef]
- Şakı, H.; Cengiz, A.; Yürekli, Y. Effectiveness of Radioiodine Treatment for Toxic Nodular Goiter. Mol. Imaging Radionucl. Ther. 2015, 24, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B.; Cooper, D.S.; Ross, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef]
- Kut, E.; Atmaca, H. Tirotoksikoz. J. Exp. Clin. Med. 2013, 29, 309–314. [Google Scholar] [CrossRef]
- Karaşahin, Ö.; İba-Yılmaz, S. Soft Tissue Infection after Antithyroid Drug-Associated Agranulocytosis. Klimik Derg. 2018, 31, 235–237. [Google Scholar] [CrossRef]
- Abdi, H.; Amouzegar, A.; Azizi, F. Antithyroid Drugs. Iran. J. Pharm. Res. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Romeo, A.N.; Običan, S.G. Teratogen Update: Antithyroid Medications. Birth Defects Res. 2020, 112, 1150–1170. [Google Scholar] [CrossRef]
- Dayanan, R.; Bilen, A.; Demirci, T.; Ciftel, S.; Ciftel, E.; Mercantepe, F.; Onalan, E.; Capoglu; Bilen, H. Investigation on the Prevalence of Thyroid Cancer in Graves’ Patients in Northeastern Part of Turkey: Is Surgery a Better Option for Patients with Graves’ Disease Who Develop Antithyroid Drug-Related Major Adverse Events? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3562–3569. [Google Scholar] [CrossRef]
- Darr, E.A.; Randolph, G.W. Management of Laryngeal Nerves and Parathyroid Glands at Thyroidectomy. Oral Oncol. 2013, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B.; Burman, K.D.; Cooper, D.S. A 2011 Survey of Clinical Practice Patterns in the Management of Graves’ Disease. J. Clin. Endocrinol. Metab. 2012, 97, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tian, R.; Peng, W.; He, Y.; Huang, R.; Kuang, A. Radiation Safety Precautions in 131I Therapy of Graves’ Disease Based on Actual Biokinetic Measurements. J. Clin. Endocrinol. Metab. 2015, 100, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Diehl, L.A.; Garcia, V.; Bonnema, S.J.; Hegedüs, L.; Albino, C.C.; Graf, H. Management of the Nontoxic Multinodular Goiter in Latin America: Comparison with North America and Europe, an Electronic Survey. J. Clin. Endocrinol. Metab. 2005, 90, 117–123. [Google Scholar] [CrossRef][Green Version]
- Paschke, R.; Hegedüs, L.; Alexander, E.; Valcavi, R.; Papini, E.; Gharib, H. Thyroid Nodule Guidelines: Agreement, Disagreement and Need for Future Research. Nat. Rev. Endocrinol. 2011, 7, 354–361. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Olsen, D.R. Magnetic Resonance Imaging (MRI) and Model-Free Estimates of Brain Volume Determined Using the Cavalieri Principle. J. Anat. 1991, 178, 133–144. [Google Scholar] [PubMed]
- Stehr, M.; Kiderlen, M.; Dorph-Petersen, K.A. Improving Cavalieri Volume Estimation Based on Non-Equidistant Planar Sections: The Trapezoidal Estimator. J. Microsc. 2022, 288, 40–53. [Google Scholar] [CrossRef]
- Heidari, Z.; Mahmoudzadeh-Sagheb, H.; Shakiba, M.; Gorgich, E.A.C. Brain Structural Changes in Schizophrenia Patients Compared to the Control: A MRI-Based Cavalieri’s Method. Basic Clin. Neurosci. 2023, 14, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, W.; Ismail, N.; Zidan, M.; Elgyoum, A.; Hassan, H.; Abdelrahman, O. Estimation of Spleen Volume Using MRI Segmentation: Would One Slice Be Enough? Cureus 2022, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Karaca, O.; Buyukmert, A.; Tepe, N.; Ozcan, E.; Kus, I. Volume Estimation of Brain Ventricles Using Cavalieri’s Principle and Atlas-Based Methods in Alzheimer Disease: Consistency between Methods. J. Clin. Neurosci. 2020, 78, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Huysmans, D.A.K.C.; Hermus, A.R.M.M.; Corstens, F.H.M.; Barentsz, J.O.; Kloppenborg, P.W.C. Large, Compressive Goiters Treated with Radioiodine. Ann. Intern. Med. 1994, 121, 757–762. [Google Scholar] [CrossRef]
- Bonnema, S.J.; Bertelsen, H.; Mortensen, J.; Andersen, P.B.; Knudsen, D.U.; Bastholt, L.; Hegedüs, L. The Feasibility of High Dose Iodine 131 Treatment as an Alternative to Surgery in Patients with a Very Large Goiter: Effect on Thyroid Function and Size and Pulmonary Function. J. Clin. Endocrinol. Metab. 1999, 84, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, R.; Letizia, C.; Borghese, M.; De Toma, G.; Celi, M.; Izzo, L.; Cavallaro, A. Hyperthyroidism. Horm. Res. 2003, 60, 79–83. [Google Scholar] [CrossRef]
- Elbers, L.P.B.; Fliers, E.; Cannegieter, S.C. The Influence of Thyroid Function on the Coagulation System and Its Clinical Consequences. J. Thromb. Haemost. 2018, 16, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, C.; Liu, Z.; Wang, J.; Xu, Y.; Shi, J. Correlation between Thyroid Function Indices and Urine Iodine Levels in Patients with Nodular Goiter. Am. J. Transl. Res. 2023, 15, 4147–4154. [Google Scholar] [PubMed]
- Kahaly, G.J. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J. Clin. Endocrinol. Metab. 2020, 105, 3704–3720. [Google Scholar] [CrossRef] [PubMed]
- Metso, S.; Jaatinen, P.; Huhtala, H.; Luukkaala, T.; Oksala, H.; Salmi, J. Long-Term Follow-up Study of Radioiodine Treatment of Hyperthyroidism. Clin. Endocrinol. (Oxf.) 2004, 61, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, L.; Molholm Hansen, B.; Knudsen, N.; Molholm Hansen, J. Reduction of Size of Thyroid with Radioactive Iodine in Multinodular Non-Toxic Goitre. Br. Med. J. 1988, 297, 661–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nygaard, B.; Hegedüs, L.; Ulriksen, P.; Nielsen, K.G.; Hansen, J.M. Radioiodine Therapy for Multinodular Toxic Goiter. Arch. Intern. Med. 1999, 159, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, B.; Hegedüs, L.; Nielsen, K.G.; Ulriksen, P.; Hansen, J.M. Long-Term Effect of Radioactive Iodine on Thyroid Function and Size in Patients with Solitary Autonomously Functioning Toxic Thyroid Nodules. Clin. Endocrinol. (Oxf.) 1999, 50, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, S.J.; Nielsen, V.E.; Hegedüs, L. Long-Term Effects of Radioiodine on Thyroid Function, Size and Patient Satisfaction in Non-Toxic Diffuse Goitre. Eur. J. Endocrinol. 2004, 150, 439–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sorensen, J.R.; Lauridsen, J.F.; Døssing, H.; Nguyen, N.; Hegedüs, L.; Bonnema, S.J.; Godballe, C. Thyroidectomy Improves Tracheal Anatomy and Airflow in Patients with Nodular Goiter: A Prospective Cohort Study. Eur. Thyroid J. 2017, 6, 307–314. [Google Scholar] [CrossRef]
- Weetman, A.P. Radioiodine Treatment for Benign Thyroid Diseases. Clin. Endocrinol. (Oxf.) 2007, 66, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Tasche, K.K.; Dorneden, A.M.; Swift, W.M.; Boyd, N.H.; Shonka, D.C.; Pagedar, N.A. Airway Management in Substernal Goiter Surgery. Ann. Otol. Rhinol. Laryngol. 2022, 131, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Kobe, C.; Bor, S.; Rahlff, I.; Dietlein, M.; Schicha, H.; Schmidt, M. Radioiodine Therapy for Thyroid Volume Reduction of Large Goitres. Nucl. Med. Commun. 2009, 30, 466–471. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Bonelo-Perdomo, A.; Sierra-Torres, C.H.; Meza-Cabrera, I. Autoimmune Thyroid Disease (Flajani-Parry-Graves-von Basedow Disease): An Overview of Treatment BT—Thyroid Disorders: Basic Science and Clinical Practice. In Thyroid Disorders; Imam, S.K., Ahmad, S.I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 169–184. ISBN 978-3-319-25871-3. [Google Scholar]
- Kaniuka-Jakubowska, S.; Lewczuk, A.; Mizan-Gross, K.; Piskunowicz, M.; Zapaśnik, A.; Lass, P.; Sworczak, K. Large Multinodular Goitre—Outpatient Radioiodine Treatment. Endokrynol. Pol. 2015, 66, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.H.; Charkes, N.D. Radioiodine treatment for nontoxic multinodular goiter. J. Nucl. Med. 1999, 40, 1313–1316. [Google Scholar] [PubMed]
- Nygaard, B.; Hegedus, L.; Gervil, M.; Hjalgrim, H.; Soe-Jensen, P.; Molholm Hansen, J. Radioiodine Treatment of Multinodular Non-Toxic Goitre. Br. Med. J. 1993, 307, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, L.; Bonnema, S.J. Approach to Management of the Patient with Primary or Secondary Intrathoracic Goiter. J. Clin. Endocrinol. Metab. 2010, 95, 5155–5162. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.; Vera, L.; Schiavo, M.; Lagasio, C.; Caputo, M.; Bagnasco, M.; Minuto, F.; Giusti, M. Ultrasonography Thyroid Volume Estimation in Hyperthyroid Patients Treated with Individual Radioiodine Dose. J. Endocrinol. Investig. 2007, 30, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, S.J.; Hegedüs, L. A 30-Year Perspective on Radioiodine Therapy of Benign Nontoxic Multinodular Goiter. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 379–384. [Google Scholar] [CrossRef]
- Nielsen, V.E.; Bonnema, S.J.; Boel-Jørgensen, H.; Veje, A.; Hegedüs, L. Recombinant Human Thyrotropin Markedly Changes the 131I Kinetics during 131I Therapy of Patients with Nodular Goiter: An Evaluation by a Randomized Double-Blinded Trial. J. Clin. Endocrinol. Metab. 2005, 90, 79–83. [Google Scholar] [CrossRef][Green Version]
- Fast, S.; Nielsen, V.E.; Bonnema, S.J.; Hegegüs, L. Time to Reconsider Nonsurgical Therapy of Benign Non-Toxic Multinodular Goitre: Focus on Recombinant Human TSH Augmented Radioiodine Therapy. Eur. J. Endocrinol. 2009, 160, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Braverman, L.; Kloos, R.T.; Law, B.; Kipnes, M.; Dionne, M.; Magner, J. Evaluation of Various Doses of Recombinant Human Thyrotropin in Patients with Multinodular Goiters. Endocr. Pract. 2008, 14, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Albino, C.C.; Mesa, C.O.; Olandoski, M.; Ueda, C.E.; Woellner, L.C.; Goedert, C.A.; Souza, A.M.; Graf, H. Recombinant Human Thyrotropin as Adjuvant in the Treatment of Multinodular Goiters with Radioiodine. J. Clin. Endocrinol. Metab. 2005, 90, 2775–2780. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albino, C.C.; Graf, H.; Paz-Filho, G.; Diehl, L.A.; Olandoski, M.; Sabbag, A.; Buchpiguel, C. Radioiodine plus Recombinant Human Thyrotropin Do Not Cause Acute Airway Compression and Are Effective in Reducing Multinodular Goiter. Braz. J. Med. Biol. Res. 2010, 43, 303–309. [Google Scholar] [CrossRef]




| Variables | N = 20 | |
| Age, years (min-max) | 61 (36–84) | |
| Gender | Male, n (%) | 6 (30) |
| Female, n (%) | 14 (70) | |
| Volume | Minimum Volume (µm3) | Maximum Volume (µm3) | Mean Volume (µm3) |
|---|---|---|---|
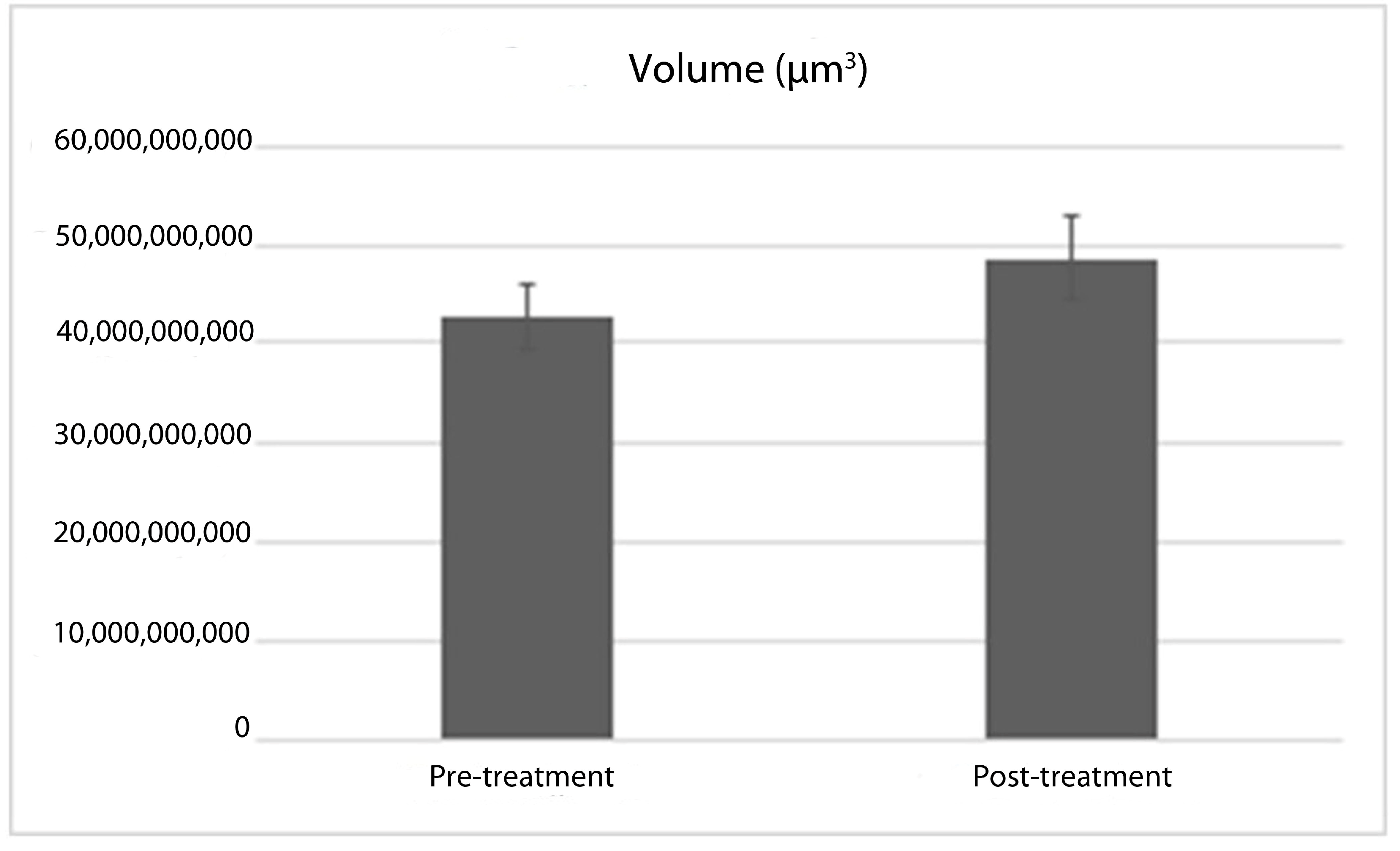
| Pre-RAI Thyroid volume | 59,625,000,000 | 586,440,000,000 | 331,940,725,000 |
| Post-RAI Thyroid volume | 67,612,500,000 | 571,275,000,000 | 216,330,725,000 |
| Pre-RAI Trachea volume | 16,920,000,000 | 71,662,500,000 | 42,768,375,000 |
| Post-RAI Trachea volume | 17,010,000,000 | 99,450,000,000 | 48,594,875,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazici Demir, K.; Kaya, Z.; Dayanan, R.; Mercantepe, T.; Mercantepe, F. Does Radioactive Iodine Treatment Affect Thyroid Size and Tracheal Diameter? J. Clin. Med. 2025, 14, 115. https://doi.org/10.3390/jcm14010115
Yazici Demir K, Kaya Z, Dayanan R, Mercantepe T, Mercantepe F. Does Radioactive Iodine Treatment Affect Thyroid Size and Tracheal Diameter? Journal of Clinical Medicine. 2025; 14(1):115. https://doi.org/10.3390/jcm14010115
Chicago/Turabian StyleYazici Demir, Kadriye, Zulkuf Kaya, Ramazan Dayanan, Tolga Mercantepe, and Filiz Mercantepe. 2025. "Does Radioactive Iodine Treatment Affect Thyroid Size and Tracheal Diameter?" Journal of Clinical Medicine 14, no. 1: 115. https://doi.org/10.3390/jcm14010115
APA StyleYazici Demir, K., Kaya, Z., Dayanan, R., Mercantepe, T., & Mercantepe, F. (2025). Does Radioactive Iodine Treatment Affect Thyroid Size and Tracheal Diameter? Journal of Clinical Medicine, 14(1), 115. https://doi.org/10.3390/jcm14010115






