Inconsistent Methods Used to Set Airway Pressure Release Ventilation in Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Regression Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Inclusion Criteria and Outcomes Measured
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
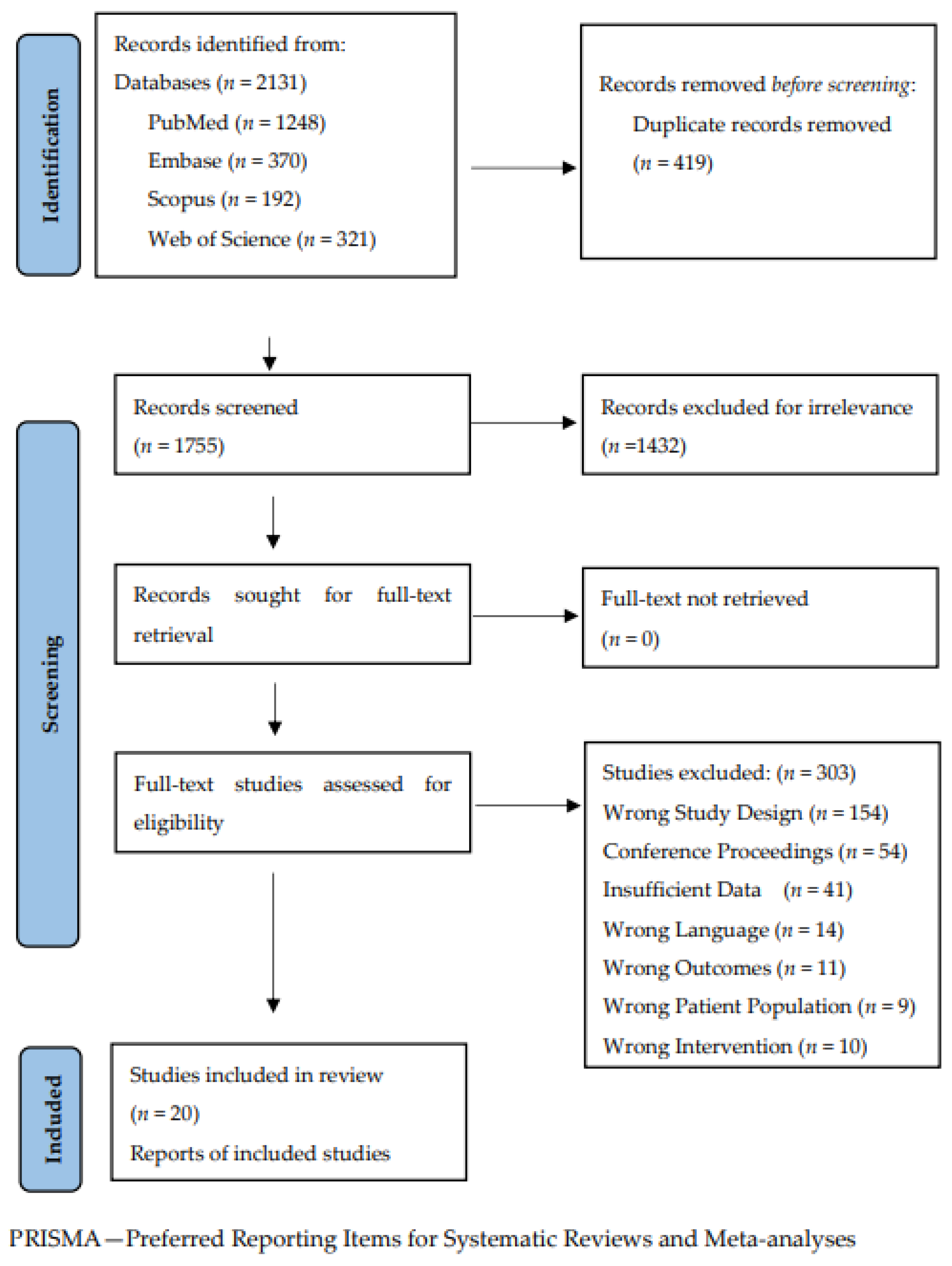
3.1. Study Selection and Characteristics
3.2. Bias and Study Quality Assessment
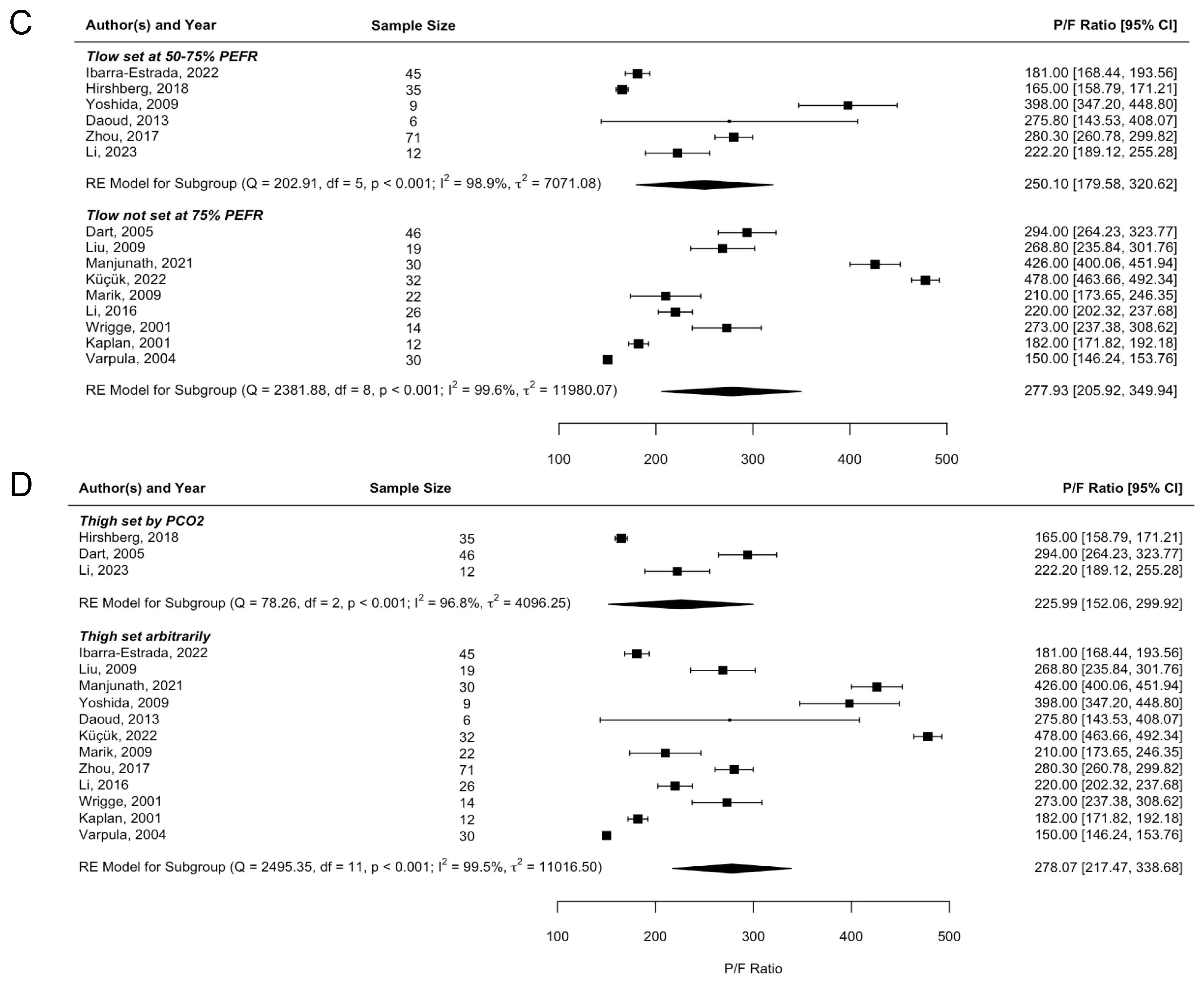
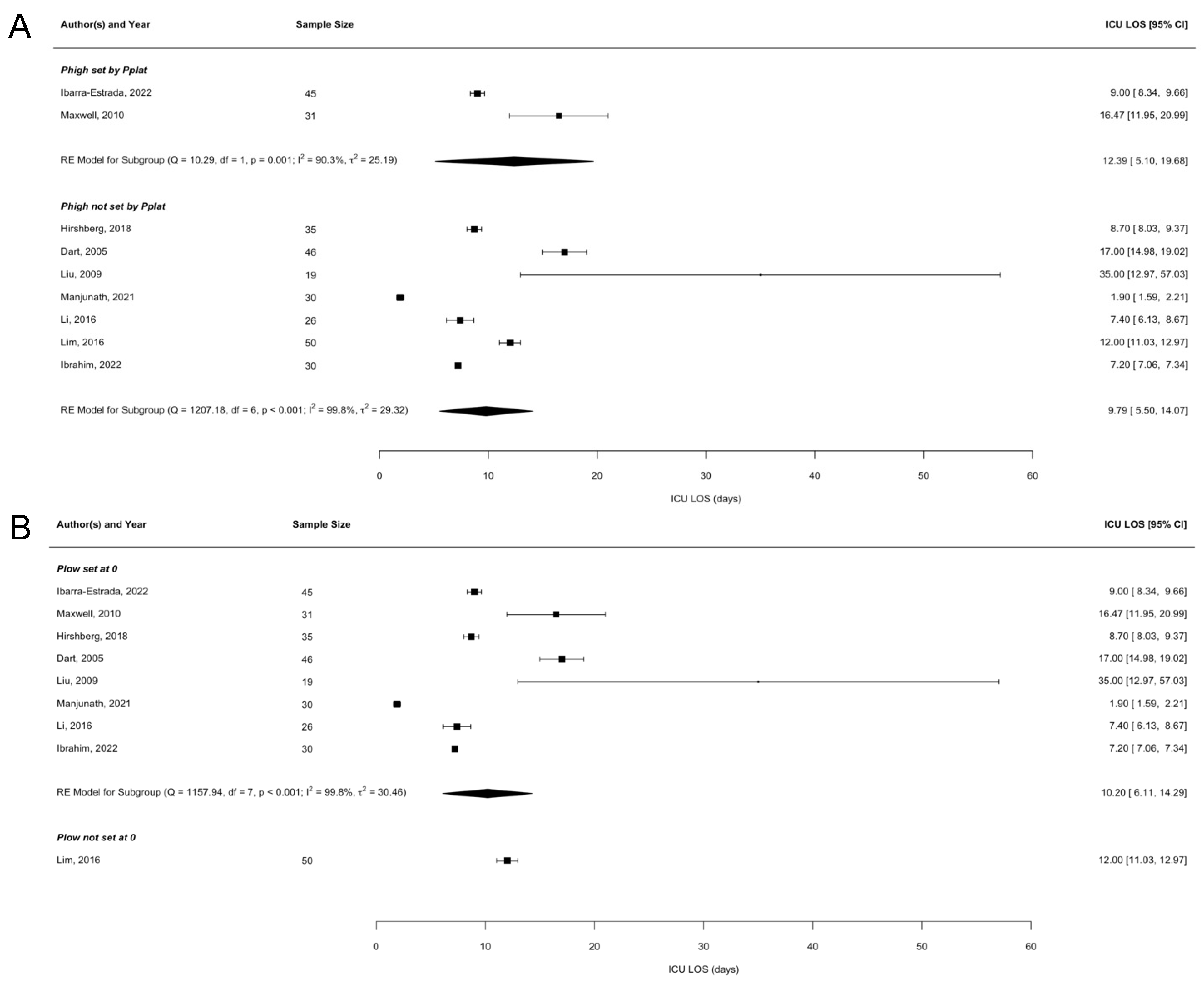
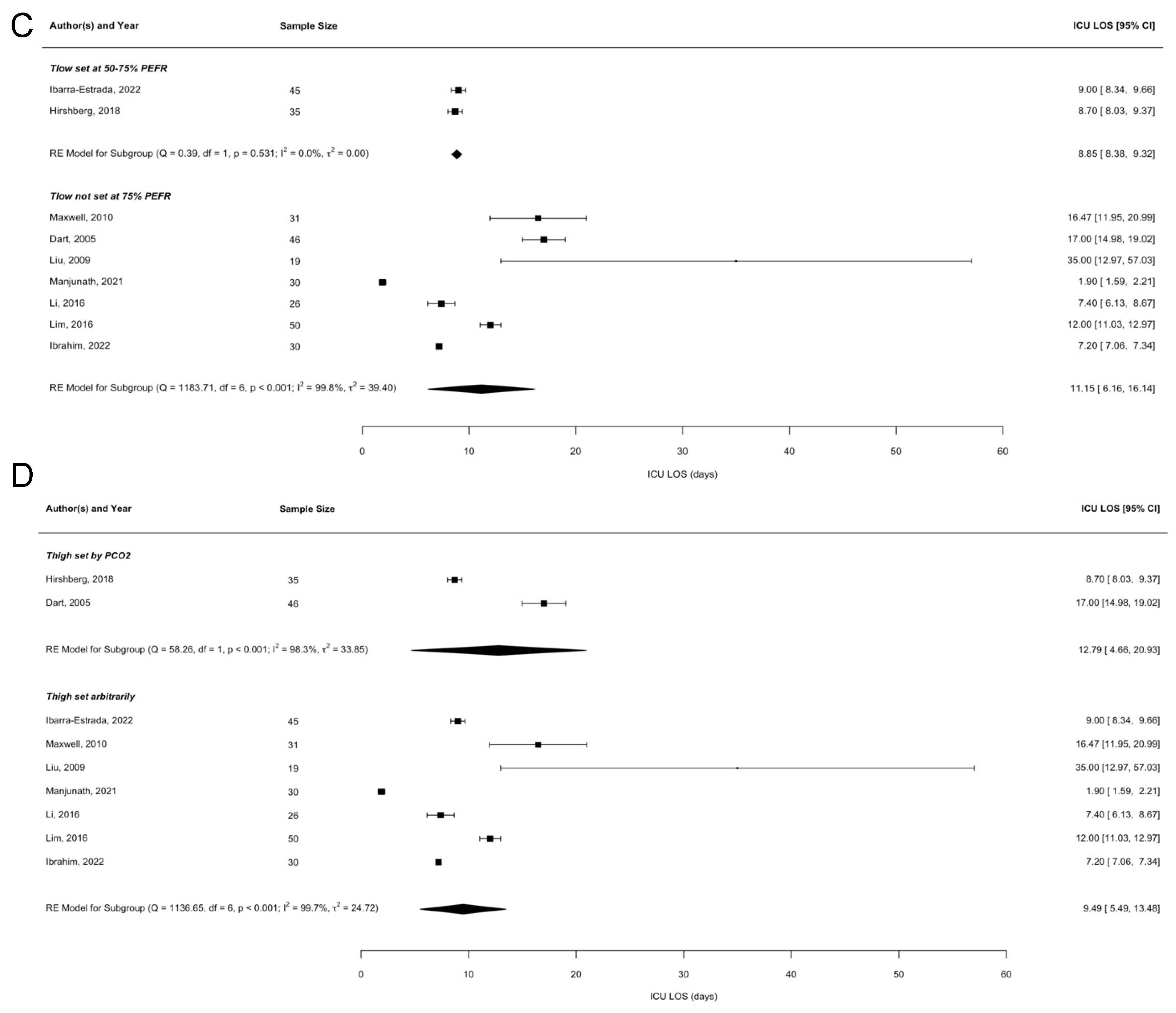
3.3. Meta-Regression Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Seah, A.S.; Grant, K.A.; Aliyeva, M.; Allen, G.B.; Bates, J.H. Quantifying the roles of tidal volume and PEEP in the pathogenesis of ventilator-induced lung injury. Ann. Biomed. Eng. 2011, 39, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Serpa Neto, A.; Pelosi, P.; Laffey, J.G.; De Haro, C.; Lorente, J.A.; Bellani, G.; Fan, E.; Brochard, L.J.; Pesenti, A.; et al. Outcomes of Patients Presenting with Mild Acute Respiratory Distress Syndrome: Insights from the LUNG SAFE Study. Anesthesiology 2019, 130, 263–283. [Google Scholar] [CrossRef]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial Investigators; Cavalcanti, A.B.; Suzumura, E.A.; Laranjeira, L.N.; Paisani, D.M.; Damiani, L.P.; Guimarães, H.P.; Romano, E.R.; de Moraes Regenga, M.; Taniguchi, L.N.; et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs. Low PEEP on Mortality in Patients with Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017, 318, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Jain, S.V.; Kollisch-Singule, M.; Sadowitz, B.; Dombert, L.; Satalin, J.; Andrews, P.; Gatto, L.A.; Nieman, G.F.; Habashi, N.M. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med. Exp. 2016, 4, 11. [Google Scholar] [CrossRef]
- Cressoni, M.; Chiurazzi, C.; Gotti, M.; Amini, M.; Brioni, M.; Algieri, I.; Cammaroto, A.; Rovati, C.; Massari, D.; di Castiglione, C.B.; et al. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology 2015, 123, 618–627. [Google Scholar] [CrossRef]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs Lower Positive End-Expiratory Pressure in Patients with Acute Lung Injury and Acute Respiratory Distress Syndrome. JAMA 2010, 303, 865. [Google Scholar] [CrossRef] [PubMed]
- Dianti, J.; Tisminetzky, M.; Ferreyro, B.L.; Englesakis, M.; Del Sorbo, L.; Sud, S.; Talmor, D.; Ball, L.; Meade, M.; Hodgson, C.; et al. Association of Positive End-Expiratory Pressure and Lung Recruitment Selection Strategies with Mortality in Acute Respiratory Distress Syndrome: A Systematic Review and Network Meta-analysis. Am. J. Respir. Crit. Care Med. 2022, 205, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Qadir, N.; Sahetya, S.; Munshi, L.; Summers, C.; Abrams, D.; Beitler, J.; Bellani, G.; Brower, R.G.; Burry, L.; Chen, J.-T.; et al. An Update on Management of Adult Patients with Acute Respiratory Distress Syndrome: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2024, 209, 24–36. [Google Scholar] [CrossRef] [PubMed]
- ZZhong, X.; Wu, Q.; Yang, H.; Dong, W.; Wang, B.; Zhang, Z.; Liang, G. Airway pressure release ventilation versus low tidal volume ventilation for patients with acute respiratory distress syndrome/acute lung injury: A meta-analysis of randomized clinical trials. Ann. Transl. Med. 2020, 8, 1641. [Google Scholar] [CrossRef]
- Camporota, L.; Rose, L.; Andrews, P.L.; Nieman, G.F.; Habashi, N.M. Airway pressure release ventilation for lung protection in acute respiratory distress syndrome: An alternative way to recruit the lungs. Curr. Opin. Crit. Care 2024, 30, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Li, R.; Wu, Y.; Zhang, H.; Wang, A.; Zhao, X.; Yuan, S.; Yang, L.; Zou, X.; Shang, Y.; Zhao, Z. Effects of airway pressure release ventilation on lung physiology assessed by electrical impedance tomography in patients with early moderate-to-severe ARDS. Crit. Care 2023, 27, 178. [Google Scholar] [CrossRef] [PubMed]
- Varpula, T.; Valta, P.; Niemi, R.; Takkunen, O.; Hynynen, M.; Pettilä, V. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol. Scand. 2004, 48, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.J.; Bailey, H.; Formosa, V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit. Care 2001, 5, 221. [Google Scholar] [CrossRef]
- Ibrahim, R.; Mohamed, Y.; Abd El-kader, M.; Azouz, A. Airway pressure release ventilation versus pressure-controlled ventilation in acute hypoxemic respiratory failure. Egypt. J. Chest Dis. Tuberc. 2022, 71, 74. [Google Scholar]
- Wrigge, H.; Zinserling, J.; Hering, R.; Schwalfenberg, N.; Stüber, F.; von Spiegel, T.; Schroeder, S.; Hedenstierna, G.; Putensen, C. Cardiorespiratory Effects of Automatic Tube Compensation during Airway Pressure Release Ventilation in Patients with Acute Lung Injury. Anesthesiology 2001, 95, 382–389. [Google Scholar] [CrossRef]
- Lim, J.; Litton, E.; Robinson, H.; Das Gupta, M. Characteristics and outcomes of patients treated with airway pressure release ventilation for acute respiratory distress syndrome: A retrospective observational study. J. Crit. Care 2016, 34, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Q.; Li, N.; Han, G.-J.; Pan, C.-G.; Zhang, Y.-H.; Shi, X.-Z.; Xu, J.-Y.; Lu, B.; Li, M.-Q. Clinical research about airway pressure release ventilation for moderate to severe acute respiratory distress syndrome. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2634–2641. [Google Scholar]
- Zhou, Y.; Jin, X.; Lv, Y.; Wang, P.; Yang, Y.; Liang, G.; Wang, B.; Kang, Y. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 1648–1659. [Google Scholar] [CrossRef]
- Marik, P.E.; Delgado, E.M.; Baram, M.; Gradwell, G.; Romeo, S.; Dutill, B. Effect of Airway Pressure Release Ventilation (APRV) with Pressure Support. (PS) on Indices of Oxygenation and Ventilation in Patients with Severe ARDS: A Cohort Study. Crit Care Shock 2009, 12, 43–48. [Google Scholar]
- Küçük, M.P.; Öztürk, Ç.E.; İlkaya, N.K.; Küçük, A.O.; Ergül, D.F.; Ülger, F. The effect of preemptive airway pressure release ventilation on patients with high risk for acute respiratory distress syndrome: A randomized controlled trial. Braz. J. Anesthesiol. (Engl. Ed.) 2022, 72, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Daoud, E.G.; Chatburn, R.L. Comparing surrogates of oxygenation and ventilation between airway pressure release ventilation and biphasic airway pressure in a mechanical model of adult respiratory distress syndrome. Respir. Investig. 2014, 52, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Rinka, H.; Kaji, A.; Yoshimoto, A.; Arimoto, H.; Miyaichi, T.; Kan, M. The Impact of Spontaneous Ventilation on Distribution of Lung Aeration in Patients with Acute Respiratory Distress Syndrome: Airway Pressure Release Ventilation Versus Pressure Support Ventilation. Anesth. Analg. 2009, 109, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, V.; Reddy, B.; Prasad, S. Is airway pressure release ventilation, a better primary mode of post-operative ventilation for adult patients undergoing open heart surgery? A prospective randomised study. Ann. Card. Anaesth. 2021, 24, 288. [Google Scholar]
- Sydow, M.; Burchardi, H.; Ephraim, E.; Zielmann, S.; Crozier, T.A. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am. J. Respir. Crit. Care Med. 1994, 149, 1550–1556. [Google Scholar] [CrossRef]
- Liu, L.; Tanigawa, K.; Ota, K.; Tamura, T.; Yamaga, S.; Kida, Y.; Kondo, T.; Ishida, M.; Otani, T.; Sadamori, T.; et al. Practical use of airway pressure release ventilation for severe ARDS—A preliminary report in comparison with a conventional ventilatory support. Hiroshima J. Med. Sci. 2009, 58, 83–88. [Google Scholar] [PubMed]
- Dart, B.W.; Maxwell, R.A.; Richart, C.M.; Brooks, D.K.; Ciraulo, D.L.; Barker, D.E.; Burns, R.P. Preliminary Experience with Airway Pressure Release Ventilation in a Trauma/Surgical Intensive Care Unit. J. Trauma Inj. Infect. Crit. Care 2005, 59, 71–76. [Google Scholar] [CrossRef] [PubMed]
- MMaxwell, R.A.; Green, J.M.; Waldrop, J.; Dart, B.W.; Smith, P.W.; Brooks, D.; Lewis, P.L.; Barker, D.E. A Randomized Prospective Trial of Airway Pressure Release Ventilation and Low Tidal Volume Ventilation in Adult Trauma Patients with Acute Respiratory Failure. J. Trauma Inj. Infect. Crit. Care 2010, 69, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, E.L.; Lanspa, M.J.; Peterson, J.; Carpenter, L.; Wilson, E.L.; Brown, S.M.; Dean, N.C.; Orme, J.; Grissom, C.K. Randomized Feasibility Trial of a Low Tidal Volume-Airway Pressure Release Ventilation Protocol Compared with Traditional Airway Pressure Release Ventilation and Volume Control Ventilation Protocols. Crit. Care Med. 2018, 46, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Estrada, M.; García-Salas, Y.; Mireles-Cabodevila, E.; López-Pulgarín, J.A.; Chávez-Peña, Q.; García-Salcido, R.; Mijangos-Méndez, J.C.; Aguirre-Avalos, G. Use of Airway Pressure Release Ventilation in Patients with Acute Respiratory Failure Due to COVID-19: Results of a Single-Center Randomized Controlled Trial*. Crit. Care Med. 2022, 50, 586–594. [Google Scholar] [PubMed]
- Momtaz, O.M.; Ahmed El Hefeny, R.; Fawzy, R.M.; Fathy, A.; Khateeb, E. Study of Airway pressure release ventilation versus low tidal volume ventilation in hospital outcome of acute respiratory distress syndrome. Neuroquantology 2022, 20, 3436–3445. [Google Scholar]
- Nieman, G.F.; Kaczka, D.W.; Andrews, P.L.; Ghosh, A.; Al-Khalisy, H.; Camporota, L.; Satalin, J.; Herrmann, J.; Habashi, N.M. First Stabilize and then Gradually Recruit: A Paradigm Shift in Protective Mechanical Ventilation for Acute Lung Injury. J. Clin. Med. 2023, 12, 4633. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Time Course of Evolving Ventilator-Induced Lung Injury: The “Shrinking Baby Lung”. Crit. Care Med. 2020, 48, 1203–1209. [Google Scholar] [CrossRef]
- Habashi, N.M. Other approaches to open-lung ventilation: Airway pressure release ventilation. Crit. Care Med. 2005, 33, S228–S240. [Google Scholar] [CrossRef]
- Roy, S.; Habashi, N.; Sadowitz, B.; Andrews, P.; Ge, L.; Wang, G.; Roy, P.; Ghosh, A.; Kuhn, M.; Satalin, J.; et al. Early airway pressure release ventilation prevents ards—A novel preventive approach to lung injury. Shock 2013, 39, 28–38. [Google Scholar] [CrossRef]
- Rola, P.; Daxon, B. Airway Pressure Release Ventilation with Time-Controlled Adaptive Ventilation (TCAVTM) in COVID-19: A Community Hospital’s Experience. Front. Physiol. 2022, 13, 787231. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Meeder, J.H.; Seghers, L.; den Uil, C.A. Time controlled adaptive ventilationTM as conservative treatment of destroyed lung: An alternative to lung transplantation. BMC Pulm. Med. 2021, 21, 176. [Google Scholar] [CrossRef]
- Kollisch-Singule, M.; Jain, S.; Andrews, P.; Smith, B.J.; Hamlington-Smith, K.L.; Roy, S.; DiStefano, D.; Nuss, E.; Satalin, J.; Meng, Q.; et al. Effect of Airway Pressure Release Ventilation on Dynamic Alveolar Heterogeneity. JAMA Surg. 2016, 151, 64. [Google Scholar] [CrossRef] [PubMed]
- Kollisch-Singule, M.; Emr, B.; Jain, S.V.; Andrews, P.; Satalin, J.; Liu, J.; Porcellio, E.; Van Kenyon, V.; Wang, G.; Marx, W.; et al. The effects of airway pressure release ventilation on respiratory mechanics in extrapulmonary lung injury. Intensive Care Med. Exp. 2015, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Kollisch-Singule, M.; Emr, B.; Smith, B.; Ruiz, C.; Roy, S.; Meng, Q.; Jain, S.; Satalin, J.; Snyder, K.; Ghosh, A.; et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J. Am. Coll. Surg. 2014, 219, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Neyton, L.; Sarma, A.; Wu, N.; Jones, C.; Zhuo, H.; Liu, K.D.; Sanchez Guerrero, E.; Ghale, R.; Love, C.; et al. Molecular Phenotypes of Acute Respiratory Distress Syndrome in the ROSE Trial Have Differential Outcomes and Gene Expression Patterns That Differ at Baseline and Longitudinally over Time. Am. J. Respir. Crit. Care Med. 2024, 209, 816–828. [Google Scholar] [CrossRef]
- Juszczak, E.; Altman, D.G.; Hopewell, S.; Schulz, K. Reporting of Multi-Arm Parallel-Group Randomized Trials. JAMA 2019, 321, 1610. [Google Scholar] [CrossRef]

| Study | Design | Sample Size of APRV Group | Tlow (%) | Plow Set to 0 | Thigh Adjusted for PCO2 | Phigh Set Based on Pplat | Outcomes |
|---|---|---|---|---|---|---|---|
| Momtaz 2022 [36] | RCT | - | 75 | No | No | Yes | 1 |
| Li 2023 [17] | Cohort study | 12 | 50–75 | Yes | Yes | Yes | 1,2 |
| Varpula 2004 [18] | RCT | 30 | Arbitrary | No | No | No | 1, 2, 3 |
| Kaplan 2001 [19] | Cohort study | 12 | Arbitrary | - | No | - | 2 |
| Ibrahim 2022 [20] | RCT | 30 | Arbitrary | Yes | No | No | 1, 4, 5 |
| Wrigge 2001 [21] | Cohort study | 14 | Arbitrary | No | No | No | 2 |
| Lim 2016 [22] | Cohort study | 50 | Arbitrary | No | No | No | 1, 2, 5 |
| Li 2016 [23] | RCT | 26 | Arbitrary | Yes | No | No | 1, 2, 3, 4, 5 |
| Zhou 2017 [24] | RCT | 71 | 50–75 | No | Yes | Yes | 1, 2, 3, 5 |
| Marik 2009 [25] | Cohort study | 22 | Arbitrary | No | No | Yes | 1, 2 |
| Kucuk 2022 [26] | RCT | 32 | Arbitrary | Yes | No | Yes | 1, 2, 3, 5 |
| Daoud 2013 [27] | Cohort study | 6 | 50–75 | Yes | No | Yes | 2 |
| Yoshida 2009 [28] | Cohort study | 9 | 50–75 | Yes | No | No | 2 |
| Manjunath 2021 [29] | RCT | 30 | Arbitrary | Yes | No | No | 2, 4, 5 |
| Sydow 1994 [30] | Cross-over study | 18 | Arbitrary | No | No | No | 1 |
| Liu 2009 [31] | Cohort study | 19 | Arbitrary | Yes | No | No | 1, 2, 5 |
| Dart 2005 [32] | Cohort study | 46 | Arbitrary | Yes | Yes | No | 1, 2, 3 |
| Hirshberg 2018 [34] | RCT | 35 | 50–75 | Yes | Yes | No | 1, 2, 3, 5 |
| Maxwell 2010 [33] | Cohort study | 31 | Arbitrary | Yes | No | Yes | 1, 5 |
| Ibarra-Estrada 2022 [35] | RCT | 45 | 50–75 | Yes | No | Yes | 1, 2, 3, 5 |
| Study ID | Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Sources of Bias |
|---|---|---|---|---|---|---|---|
| Momtaz 2022 [36] | Low | Low | Low | Low | Low | Low | Low |
| Li 2023 [17] | Low | Unsure | Low | Low | Low | Low | Low |
| Varpula 2004 [18] | Low | Low | High | Unsure | Low | Low | Low |
| Kaplan 2001 [19] | Unsure | High | High | High | High | Unsure | Low |
| Ibrahim 2022 [20] | Low | Low | High | High | Low | Low | Low |
| Wrigge 2001 [21] | Unsure | Unsure | High | High | High | Unsure | Low |
| Lim 2016 [22] | Low | Low | Unsure | Unsure | Low | High | Low |
| Li 2016 [23] | Low | Low | Unsure | Unsure | Unsure | Low | Low |
| Zhou 2017 [24] | Low | Low | High | Unsure | Low | Low | Low |
| Marik 2009 [25] | Unsure | High | High | High | High | High | Low |
| Küçük 2022 [26] | Low | Low | High | High | Low | Low | Low |
| Daoud 2013 [27] | High | High | Low | High | Low | Low | Low |
| Yoshida 2009 [28] | High | High | High | High | High | Unsure | Low |
| Manjunath 2021 [29] | Unsure | Low | High | High | High | Low | Low |
| Sydow 1994 [30] | Low | Unsure | High | Unsure | Low | Unsure | Low |
| Liu 2009 [31] | Low | High | Unsure | Unsure | Low | Low | Low |
| Dart 2005 [32] | Unsure | Low | Low | Low | Low | Low | Low |
| Hirshberg 2018 [34] | Low | Low | Low | High | Low | Unsure | Low |
| Maxwell 2010 [33] | Low | Low | High | Unsure | Low | Unsure | Low |
| Ibarra-Estrada 2022 [35] | Low | Low | High | High | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, M.R.; Charlamb, J.; Kenna, J.R.; Smith, A.; Glatt, S.J.; Araos, J.D.; Andrews, P.L.; Habashi, N.M.; Nieman, G.F.; Ghosh, A.J. Inconsistent Methods Used to Set Airway Pressure Release Ventilation in Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Regression Analysis. J. Clin. Med. 2024, 13, 2690. https://doi.org/10.3390/jcm13092690
Lutz MR, Charlamb J, Kenna JR, Smith A, Glatt SJ, Araos JD, Andrews PL, Habashi NM, Nieman GF, Ghosh AJ. Inconsistent Methods Used to Set Airway Pressure Release Ventilation in Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Regression Analysis. Journal of Clinical Medicine. 2024; 13(9):2690. https://doi.org/10.3390/jcm13092690
Chicago/Turabian StyleLutz, Mark R., Jacob Charlamb, Joshua R. Kenna, Abigail Smith, Stephen J. Glatt, Joaquin D. Araos, Penny L. Andrews, Nader M. Habashi, Gary F. Nieman, and Auyon J. Ghosh. 2024. "Inconsistent Methods Used to Set Airway Pressure Release Ventilation in Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Regression Analysis" Journal of Clinical Medicine 13, no. 9: 2690. https://doi.org/10.3390/jcm13092690
APA StyleLutz, M. R., Charlamb, J., Kenna, J. R., Smith, A., Glatt, S. J., Araos, J. D., Andrews, P. L., Habashi, N. M., Nieman, G. F., & Ghosh, A. J. (2024). Inconsistent Methods Used to Set Airway Pressure Release Ventilation in Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Regression Analysis. Journal of Clinical Medicine, 13(9), 2690. https://doi.org/10.3390/jcm13092690








