Esophagus Dilation and Quality of Life in Adults with Scleroderma and Concomitant Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
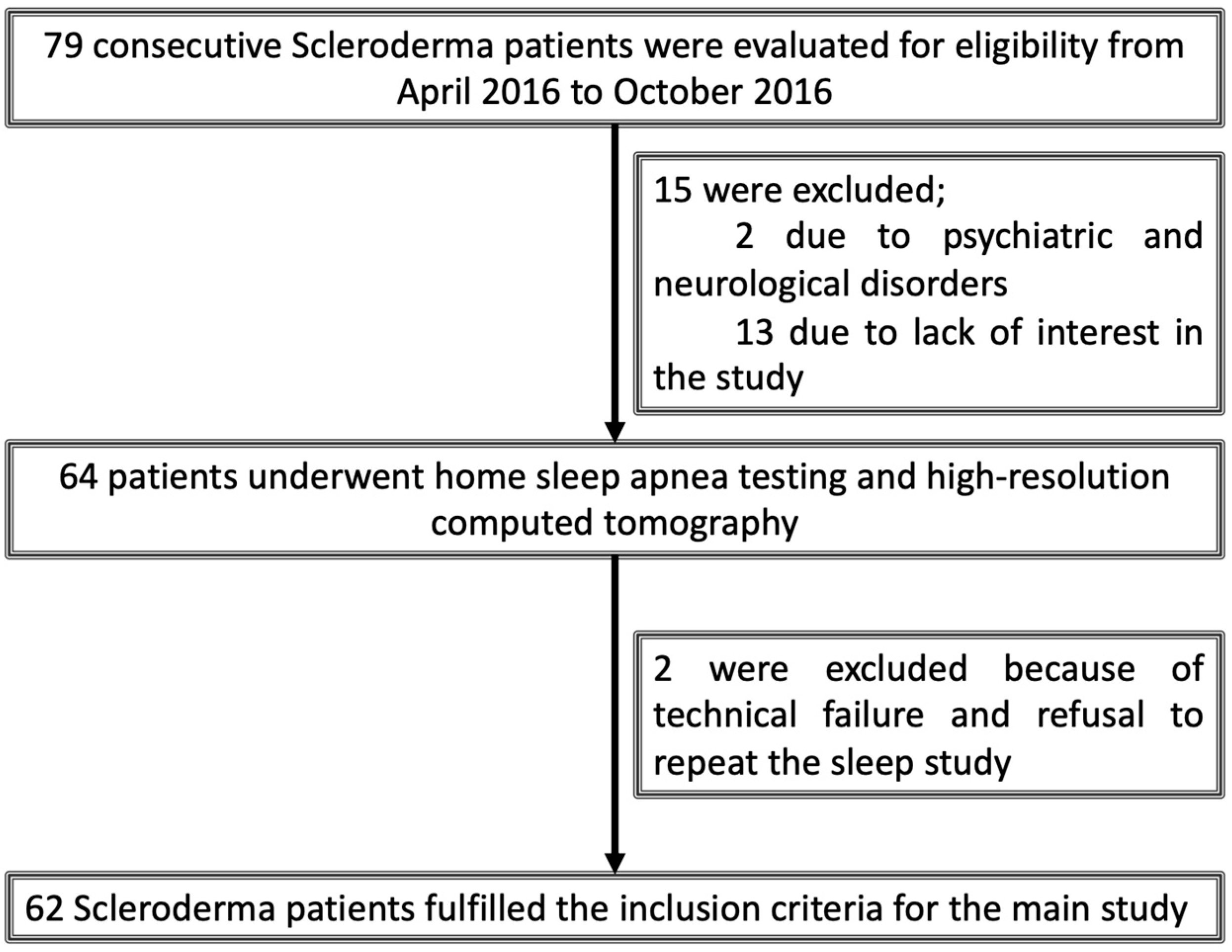
2.1. Study Design and Participant Selection
2.2. Basic Clinical Characteristics
2.3. Pulmonary Function Testing
2.4. High-Resolution Computed Tomography
2.5. Epworth Sleepiness Scale
2.6. The Short Form 36 Health Survey (SF-36)
2.7. Home Sleep Apnea Testing
2.8. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Wollheim, F.A. Classification of systemic sclerosis. Visions and reality. Rheumatology 2005, 44, 1212–1216. [Google Scholar] [CrossRef]
- Roman, S.; Hot, A.; Fabien, N.; Cordier, J.F.; Miossec, P.; Ninet, J.; Mion, F. Esophageal dysmotility associated with systemic sclerosis: A high-resolution manometry study. Dis. Esophagus 2011, 24, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Nietert, P.J.; Mitchell, H.C.; Bolster, M.B.; Curran, M.Y.; Tilley, B.C.; Silver, R.M. Correlates of depression, including overall and gastrointestinal functional status, among patients with systemic sclerosis. J. Rheumatol. 2005, 32, 51–57. [Google Scholar] [PubMed]
- Gottlieb, D.J. Sleep Apnea and Cardiovascular Disease. Curr. Diabetes Rep. 2021, 21, 64. [Google Scholar] [CrossRef]
- Chen, Y.H.; Keller, J.K.; Kang, J.H.; Hsieh, H.J.; Lin, H.C. Obstructive sleep apnea and the subsequent risk of depressive disorder: A population-based follow-up study. J. Clin. Sleep. Med. 2013, 9, 417–423. [Google Scholar] [CrossRef]
- Ing, A.J.; Ngu, M.C.; Breslin, A.B. Obstructive sleep apnea and gastroesophageal reflux. Am. J. Med. 2000, 108, 120S–125S. [Google Scholar] [CrossRef]
- Bjornsdottir, E.; Keenan, B.T.; Eysteinsdottir, B.; Arnardottir, E.S.; Janson, C.; Gislason, T.; Sigurdsson, J.F.; Kuna, S.T.; Pack, A.I.; Benediktsdottir, B. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J. Sleep Res. 2015, 24, 328–338. [Google Scholar] [CrossRef]
- Wallstrom, S.; Balcan, B.; Thunstrom, E.; Wolf, A.; Peker, Y. CPAP and Health-Related Quality of Life in Adults With Coronary Artery Disease and Nonsleepy Obstructive Sleep Apnea in the RICCADSA Trial. J. Clin. Sleep Med. 2019, 15, 1311–1320. [Google Scholar] [CrossRef]
- Gouda, W.; Mokhtar, M.; Elazab, S.A.; Alreefi, R.; Alomar, T.; Kushk, F.; Alahmadi, R.; Khalil, M.; Kamal, M. Sleep disorders in patients with rheumatoid arthritis: Association with quality of life, fatigue, depression levels, functional disability, disease duration, and activity: A multicentre cross-sectional study. J. Int. Med. Res. 2023, 51, 03000605231204477. [Google Scholar] [CrossRef]
- Yakut, T.; Balcan, B.; Karakurt, S.; Direskeneli, H.; Yalcinkaya, Y.; Peker, Y. Impact of concomitant obstructive sleep apnea on pulmonary involvement and main pulmonary artery diameter in adults with scleroderma. Sleep Breath. 2021, 25, 135–143. [Google Scholar] [CrossRef]
- Xerfan, E.M.; Facina, A.S.; Tomimori, J.; Xavier, S.D.; Tufik, S.; Andersen, M.L. Scleroderma and obstructive sleep apnea: A consideration of immunological aspects and the role of fibrosis. Sleep Breath. 2022, 26, 1–3. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Cotes, J.; Chinn, D.; Quanjer, P.H.; Roca, J.; Yernault, J.-C. Standardization of the measurement of transfer factor (diffusing capacity). Eur. Respir. J. 1993, 6 (Suppl. 16), 41–52. [Google Scholar] [CrossRef]
- Warrick, J.H.; Bhalla, M.; Schabel, S.I.; Silver, R.M. High resolution computed tomography in early scleroderma lung disease. J. Rheumatol. 1991, 18, 1520–1528. [Google Scholar]
- Richardson, C.; Agrawal, R.; Lee, J.; Almagor, O.; Nelson, R.; Varga, J.; Cuttica, M.J.; Jane, D.; Dematte, A.; Chang, R.W.; et al. (Eds.) Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. In Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Izci, B.; Ardic, S.; Firat, H.; Sahin, A.; Altinors, M.; Karacan, I. Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath. 2008, 12, 161–168. [Google Scholar] [CrossRef]
- Demiral, Y.; Ergor, G.; Unal, B.; Semin, S.; Akvardar, Y.; Kıvırcık, B.; Alptekin, K. Normative data and discriminative properties of short form 36 (SF-36) in Turkish urban population. BMC Public Health 2006, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Bell, S.C.; Branson, R.D.; Chalmers, J.D.; Marshall, R.; Maslove, D.M.; Ost, D.E.; Punjabi, N.M.; Schatz, M.; Smyth, A.R.; et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann. Am. Thorac. Soc. 2019, 16, 22–28. [Google Scholar] [CrossRef]
- Crowell, M.D.; Umar, S.B.; Griffing, W.L.; DiBaise, J.K.; Lacy, B.E.; Vela, M.F. Esophageal motor abnormalities in patients with scleroderma: Heterogeneity, risk factors, and effects on quality of life. Clin. Gastroenterol. Hepatol. 2017, 15, 207–213.e1. [Google Scholar] [CrossRef]
- Yang, H.; Xu, D.; Li, M.T.; Yao, Y.; Jin, M.; Zeng, X.F.; Qian, J.M. Gastrointestinal manifestations on impaired quality of life in systemic sclerosis. J. Dig. Dis. 2019, 20, 256–261. [Google Scholar] [CrossRef]
- Figueiredo, F.P.; Aires, G.D.; Nisihara, R.; Skare, T.L. Sleep disturbance in scleroderma. JCR J. Clin. Rheumatol. 2021, 27, S242–S245. [Google Scholar] [CrossRef]
- Frech, T.; Hays, R.D.; Maranian, P.; Clements, P.J.; Furst, D.E.; Khanna, D. Prevalence and correlates of sleep disturbance in systemic sclerosis—Results from the UCLA scleroderma quality of life study. Rheumatology 2011, 50, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Di Carlo, M.; Carotti, M.; Fraticelli, P.; Gabrielli, A.; Giovagnoni, A. Relationship between interstitial lung disease and oesophageal dilatation on chest high-resolution computed tomography in patients with systemic sclerosis: A cross-sectional study. La Radiol. Medica 2018, 123, 655–663. [Google Scholar] [CrossRef]
- Raghu, G.; Montesi, S.B.; Silver, R.M.; Hossain, T.; Macrea, M.; Herman, D.; Barnes, H.; Adegunsoye, A.; Azuma, A.; Chung, L.; et al. Treatment of Systemic Sclerosis–associated Interstitial Lung Disease: Evidence-based Recommendations. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2023, 209, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Cairns, A.; Wickwire, E.; Schaefer, E.; Nyanjom, D. A pilot validation study for the NOX T3 TM portable monitor for the detection of OSA. Sleep Breath. 2014, 18, 609–614. [Google Scholar] [CrossRef] [PubMed]
| Lesions and Lung Segments | Score |
|---|---|
| Pulmonary Abnormalities | Disease Severity Score |
| Ground-glass opacity | 1 |
| Irregularities at the pleural | 2 |
| Septal/subpleural lines | 3 |
| Honeycombing | 4 |
| Subpleural cysts | 5 |
| Number of Affected Segments | Disease Extent Score |
| 1–3 | 1 |
| 4–9 | 2 |
| >9 | 3 |
| Esophageal Diameter <17.47 mm | Esophageal Diameter ≥17.47 mm | ||
|---|---|---|---|
| Variables | (n = 31) | (n = 31) | p Value |
| Age (years) | 47.5 ± 12.7 | 49.4 ± 9.8 | 0.486 |
| Female, n (%) | 29 (46.8) | 29 (46.8) | 1.000 |
| BMI (kg/m2) | 26.4 ± 5.2 | 27 ± 5 | 0.788 |
| Obesity, n (%) | 10 (32.3) | 11 (35.5) | 1.000 |
| Current smoker, n (%) | 3 (9.7) | 1 (3.2) | 0.612 |
| ESS score | 5.8 ± 4.3 | 5.7 ± 5.3 | 0.492 |
| Diffuse cutaneous SSc, n (%) | 4 (12.9) | 16 (51.6) | 0.002 |
| Hypertension, n (%) | 6 (19.4) | 5 (16.1) | 0.740 |
| Diabetes mellitus, n (%) | 4 (12.9) | 1 (3.2) | 0.354 |
| Cardiac disease, n (%) | 2 (6.5) | 2 (6.5) | 1.000 |
| HSAT findings | |||
| AHI ≥ 15 | 9 (29) | 11 (35.5) | 0.587 |
| AHI (events/h) | 11.2 ± 10.2 | 14.5 ± 13.9 | 0.486 |
| ODI (events/h) | 9.2 ± 8.1 | 13 ± 12.8 | 0.410 |
| Minimum SpO2 (%) | 84.8 ± 6 | 83.5 ± 7.9 | 0.709 |
| Time below SpO2 < 90% (min) | 2.9 ± 5.7 | 6.9 ± 13.5 | 0.269 |
| Mean SpO2 drops (%) | 3.65 ± 0.8 | 3.8 ± 1.1 | 0.442 |
| HRCT, spirometry, and DLCO findings | |||
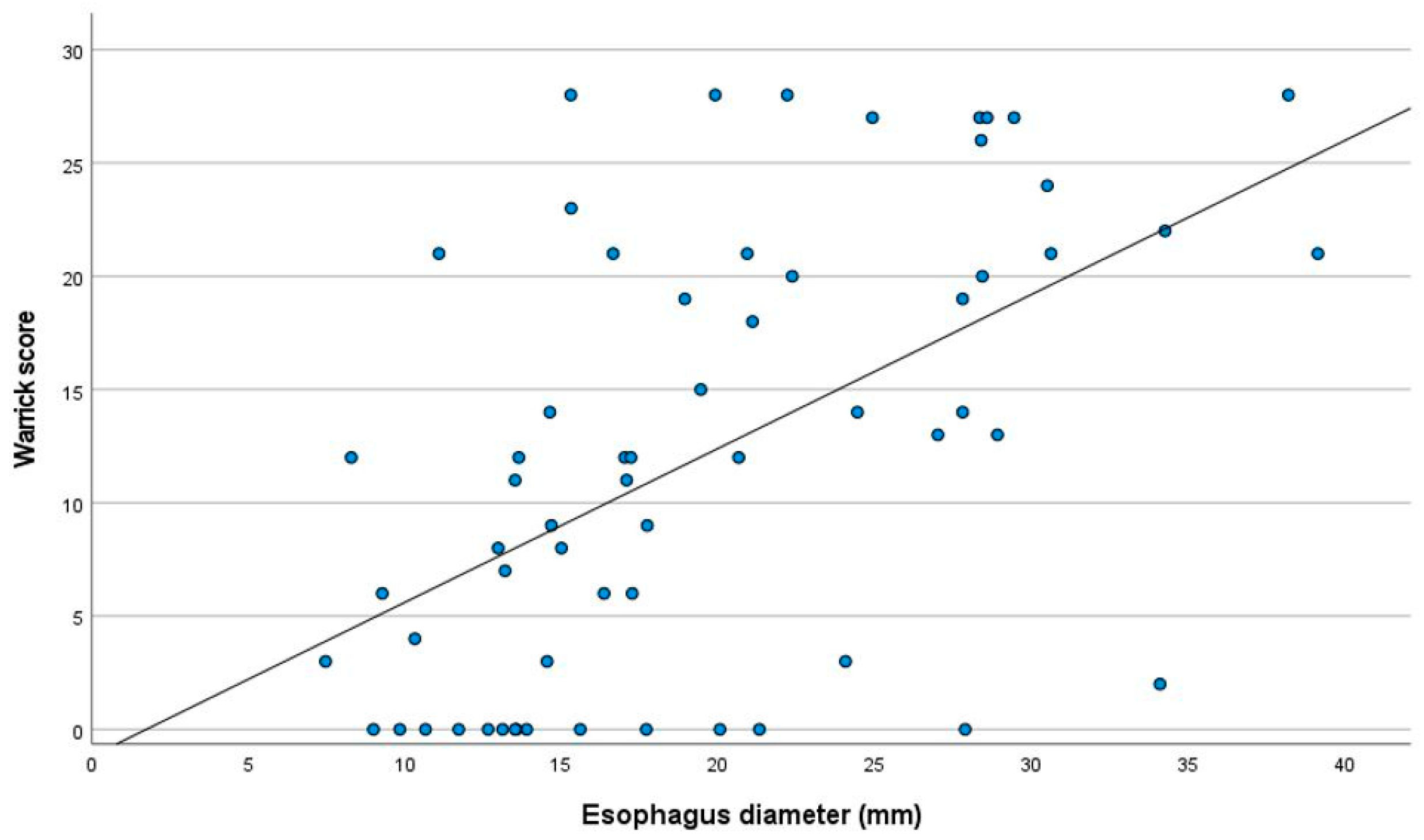
| Widest esophageal diameter | 13.4 ± 2.7 | 25.9 ± 5.7 | <0.001 |
| Warrick Score | 7.65 ± 7.7 | 16.7 ± 9.5 | <0.001 |
| DLCO (%) * | 72.2 ± 20.9 | 66.7 ± 19.7 | 0.389 |
| DLCO < 80% n (%) * | 20 (74.1) | 23 (73.3) | 0.643 |
| Medications | |||
| Corticosteroids, n (%) | 9 (29) | 13 (41.9) | 0.288 |
| Methotrexate, n (%) | 4 (12.9) | 5 (16.1) | 1.000 |
| Hydroxychloroquine, n (%) | 22 (71) | 17 (54.8) | 0.189 |
| Mycophenolate mofetil, n (%) | 4 (12.9) | 11 (35.5) | 0.073 |
| Azathiopyrin, n (%) | 4 (12.9) | 10 (32.3) | 0.127 |
| Leflunomide, n (%) | 2 (6.5) | 0 (0) | 0.492 |
| Standardized Coefficients Beta | 95% Confidence Interval for Beta | p Value | |
|---|---|---|---|
| Age | 0.023 | −0.138–0.170 | 0.955 |
| BMI | 0.118 | −0.208–0.584 | 0.839 |
| Sex | 0.016 | −5.913–6.871 | 0.881 |
| Disease duration (years) | 0.169 | −0.058–0.459 | 0.126 |
| Warrick score | 0.544 | 0.250–0.609 | <0.001 |
| DLCO (%) | 0.000 | −0.101–0.102 | 0.988 |
| Time below SpO2 < 90% (min) | 0.126 | −0.078–0.258 | 0.288 |
| Neither OSA nor EED (n:22) | EED Only (n:20) | p | OSA Only (n:9) | Both OSA and EED (n:11) | p | |
|---|---|---|---|---|---|---|
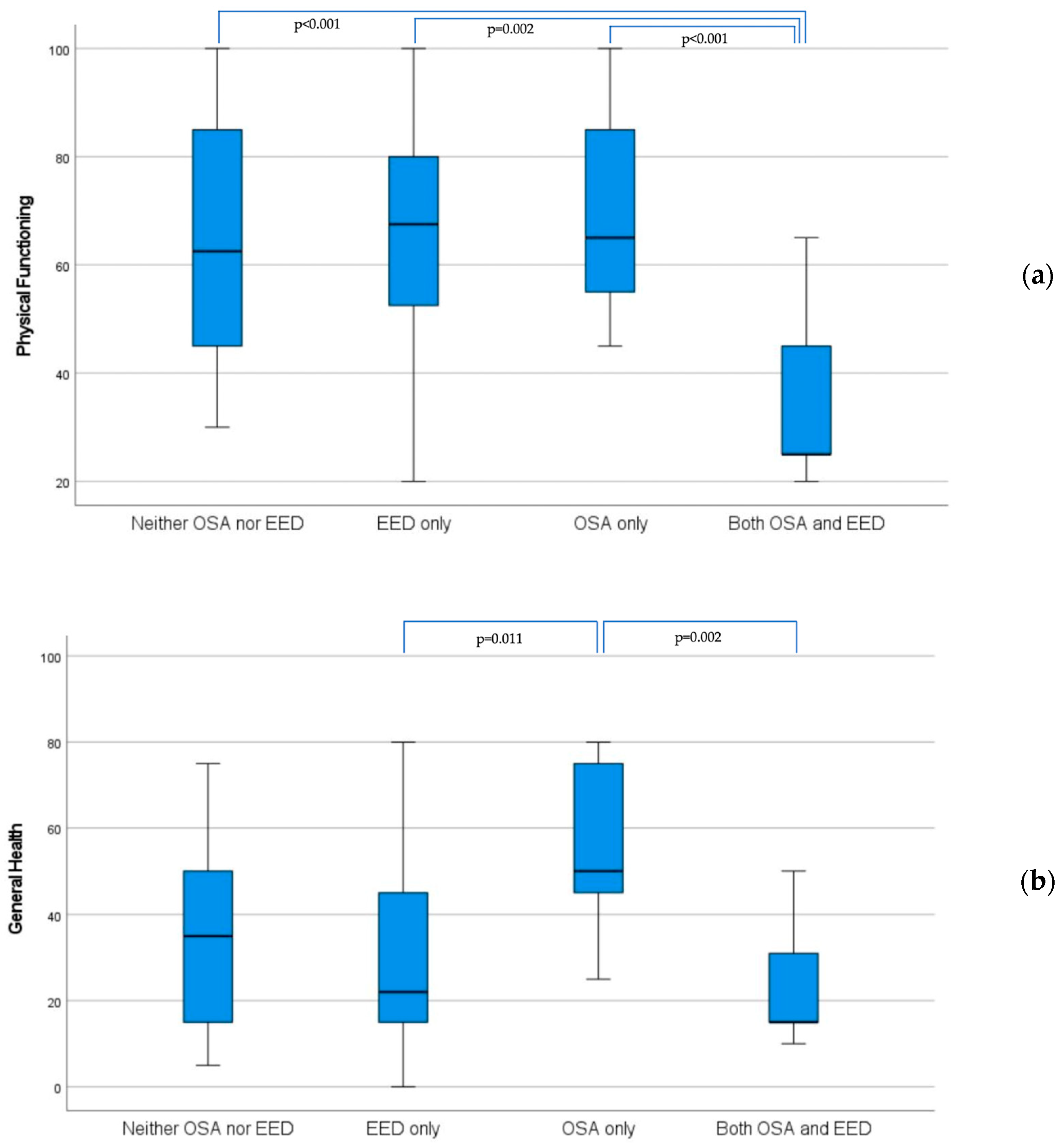
| Physical Functioning | 62.5 (45–86) | 67.5 (51–80) | 0.930 | 65 (53–85) | 25 (25–45) | <0.001 |
| Role Physical | 62.5 (0–100) | 37.5 (25–100) | 0.886 | 100 (50–100) | 25 (0–50) | 0.006 |
| Bodily Pain | 63.5 (21–86) | 52.5 (24–96) | 0.649 | 65 (31–85) | 45 (30–61) | 0.230 |
| General Health | 35 (14–52) | 22 (15–48) | 0.587 | 50 (40–78) | 15 (15–37) | 0.001 |
| Role Emotional | 66.6 (33–67) | 49.9 (8–67) | 0.487 | 67 (50–100) | 33.3 (0–67) | 0.025 |
| Vitality | 35 (19–65) | 42.5 (23–73) | 0.528 | 60 (45–70) | 35 (25–50) | 0.007 |
| Social Functioning | 50 (38–100) | 68.7 (48–100) | 0.635 | 62.5 (44–94) | 63 (25–75) | 0.295 |
| Mental Health | 44.5 (37–68) | 62 (37–84) | 0.283 | 60 (48–69) | 52 (36–72) | 0.370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakut, T.; Cinar, C.; Karakurt, S.; Direskeneli, H.; Yalcinkaya, Y.; Peker, Y. Esophagus Dilation and Quality of Life in Adults with Scleroderma and Concomitant Obstructive Sleep Apnea. J. Clin. Med. 2024, 13, 1884. https://doi.org/10.3390/jcm13071884
Yakut T, Cinar C, Karakurt S, Direskeneli H, Yalcinkaya Y, Peker Y. Esophagus Dilation and Quality of Life in Adults with Scleroderma and Concomitant Obstructive Sleep Apnea. Journal of Clinical Medicine. 2024; 13(7):1884. https://doi.org/10.3390/jcm13071884
Chicago/Turabian StyleYakut, Tugce, Caner Cinar, Sait Karakurt, Haner Direskeneli, Yasemin Yalcinkaya, and Yüksel Peker. 2024. "Esophagus Dilation and Quality of Life in Adults with Scleroderma and Concomitant Obstructive Sleep Apnea" Journal of Clinical Medicine 13, no. 7: 1884. https://doi.org/10.3390/jcm13071884
APA StyleYakut, T., Cinar, C., Karakurt, S., Direskeneli, H., Yalcinkaya, Y., & Peker, Y. (2024). Esophagus Dilation and Quality of Life in Adults with Scleroderma and Concomitant Obstructive Sleep Apnea. Journal of Clinical Medicine, 13(7), 1884. https://doi.org/10.3390/jcm13071884







