Left Atrium Volume Measured with Multislice Computed Tomography as a Prognostic Predictor for Atrial Fibrillation Catheter Ablation Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Multislice Computed Tomography
2.3. RFCA
2.4. Clinical Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
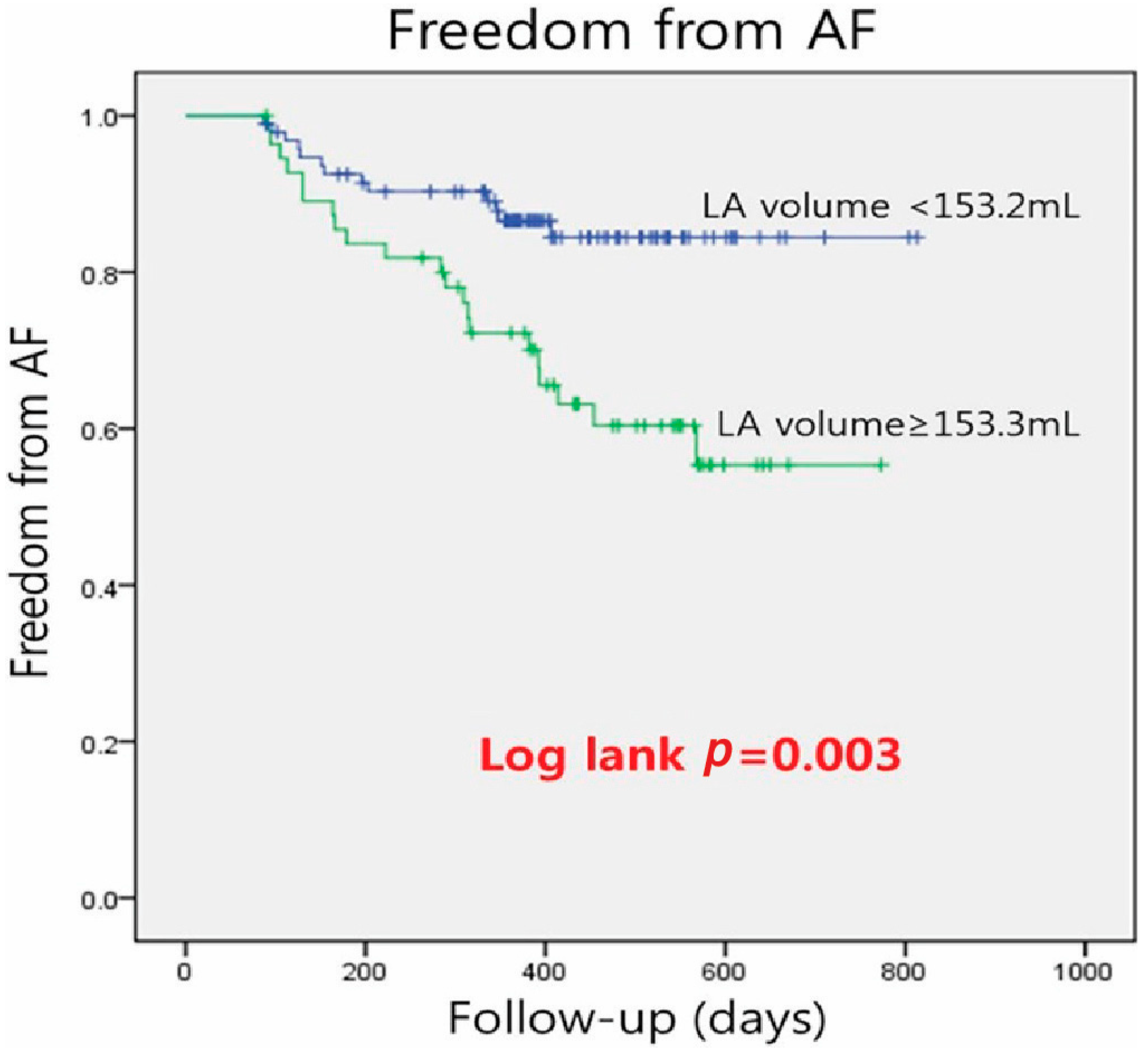
3.2. Clinical Outcomes
3.3. Univariate and Multivariate Analysis for Predicting AF Recurrence after RFCA
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.D.; Jaıs, P.; Hocini, M.; Sacher, F.; Klein, G.J.; Clementy, J.; Haïssaguerre, M. Catheter ablation for atrial fibrillation. Circulation 2007, 116, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Leventopoulos, G.; Koros, R.; Travlos, C.; Perperis, A.; Chronopoulos, P.; Tsoni, E.; Koufou, E.-E.; Papageorgiou, A.; Apostolos, A.; Kaouris, P.; et al. Mechanisms of Atrial Fibrillation: How Our Knowledge Affects Clinical Practice. Life 2023, 13, 1260. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Mörtsell, D.; Arbelo, E.; Dagres, N.; Brugada, J.; Laroche, C.; A Trines, S.; Malmborg, H.; Höglund, N.; Tavazzi, L.; Pokushalov, E.; et al. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: A study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace 2019, 21, 581–589. [Google Scholar] [CrossRef]
- Deng, H.; Bai, Y.; Shantsila, A.; Fauchier, L.; Potpara, T.S.; Lip, G.Y. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: A systematic review. Clin. Res. Cardiol. 2017, 106, 813–823. [Google Scholar] [CrossRef]
- Abecasis, J.; Dourado, R.; Ferreira, A.; Saraiva, C.; Cavaco, D.; Santos, K.R.; Morgado, F.B.; Adragão, P.; Silva, A. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 2009, 11, 1289–1294. [Google Scholar] [CrossRef]
- Hof, I.; Chilukuri, K.; Arbab-Zadeh, A.; Scherr, D.; Dalal, D.; Nazarian, S.; Henrikson, C.; Spragg, D.; Berger, R.; Marine, J.; et al. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J. Cardiovasc. Electrophysiol. 2009, 20, 1005–1010. [Google Scholar] [CrossRef]
- Oakes, R.S.; Badger, T.J.; Kholmovski, E.G.; Akoum, N.; Burgon, N.S.; Fish, E.N.; Blauer, J.J.; Rao, S.N.; DiBella, E.V.; Segerson, N.M.; et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009, 119, 1758–1767. [Google Scholar] [CrossRef]
- Helms, A.S.; West, J.J.; Patel, A.; Lipinski, M.J.; Mangrum, J.M.; Mounsey, J.P.; DiMarco, J.P.; Ferguson, J.D. Relation of Left Atrial Volume from Three-Dimensional Computed Tomography to Atrial Fibrillation Recurrence Following Ablation. Am. J. Cardiol. 2009, 103, 989–993. [Google Scholar] [CrossRef]
- Costa, F.M.; Ferreira, A.M.; Oliveira, S.; Santos, P.G.; Durazzo, A.; Carmo, P.; Santos, K.R.; Cavaco, D.; Parreira, L.; Morgado, F.; et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int. J. Cardiol. 2015, 184, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; A Chen, S.; Crijns, H.J.; Damiano, R.J.; Davies, D.W.; Dimarco, J.P.; et al. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012, 14, 528–606. [Google Scholar] [CrossRef] [PubMed]
- Hof, I.; Arbab-Zadeh, A.; Scherr, D.; Chilukuri, K.; Dalal, D.; Abraham, T.; Lima, J.; Calkins, H. Correlation of Left Atrial Diameter by Echocardiography and Left Atrial Volume by Computed Tomography. J. Cardiovasc. Electrophysiol. 2009, 20, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Ancona, R.; Pinto, S.C.; Caso, P.; D’andrea, A.; Di Salvo, G.; Arenga, F.; Coppola, M.G.; Sellitto, V.; Macrino, M.; Calabrò, R. Left Atrium by Echocardiography in Clinical Practice: From Conventional Methods to New Echocardiographic Techniques. Sci. World J. 2014, 2014, 451042. [Google Scholar] [CrossRef]
- Pinto, T.P.; Martins, O.M.; Ramos, R.; Rio, P.; Silva, C.P.; Delgado, A.S.; Pimenta, R.; Cruz Ferreira, R. Left atrial appendage volume as a new predictor of atrial fibrillation recurrence after catheter ablation. J. Interv. Card. Electrophysiol. 2017, 49, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Ryden, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006, 114, e257–e354. [Google Scholar] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Oliveira, M.M. How to follow atrial fibrillation ablation patients? J. Atr. Fibrillation 2014, 7, 1087. [Google Scholar]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Kowal, R.C. Predicting outcome from AF ablation: Size of the chamber, or is tissue the issue? J. Cardiovasc. Electrophysiol. 2009, 20, 1011–1013. [Google Scholar] [CrossRef]
- Kojodjojo, P.; Peters, N.S.; Davies, D.W.; Kanagaratnam, P. Characterization of the Electroanatomical Substrate in Human Atrial Fibrillation: The Relationship between Changes in Atrial Volume, Refractoriness, Wavefront Propagation Velocities, and AF Burden. J. Cardiovasc. Electrophysiol. 2007, 18, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Makati, K.J.; Alsheikh-Ali, A.A.; Garlitski, A.C.; Link, M.S.; Homoud, M.; Weinstock, J.; Iii, N.A.M.E. Advances in mechanisms of atrial fibrillation: Structural remodeling, high-frequency fractionated electrograms, and reentrant AF drivers. J. Interv. Card. Electrophysiol. 2008, 23, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.H.; Klein, G.; Natale, A.; Packer, D.; et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005, 111, 1100–1105. [Google Scholar] [CrossRef]
- Calkins, H.; Brugada, J.; Packer, D.L.; Cappato, R.; Chen, S.-A.; Crijns, H.J.G.; Damiano, R.J.; Davies, D.W.; Haines, D.E.; Haissaguerre, M.; et al. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-UpA report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation Developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and Approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007, 9, 335–379. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, A.; Tamborero, D.; Mont, L.; Benito, B.; Tolosana, J.M.; Sitges, M.; Vidal, B.; Arriagada, G.; Méndez, F.; Matiello, M.; et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 2007, 28, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Watanabe, I.; Ohkubo, K.; Ashino, S.; Kofune, M.; Hashimoto, K.; Shindo, A.; Sugimura, H.; Nakai, T.; Kasamaki, Y.; et al. Prediction of the Efficacy of Pulmonary Vein Isolation for the Treatment of Atrial Fibrillation by the Signal-Averaged P-Wave Duration. Pacing Clin. Electrophysiol. 2007, 30, 304–313. [Google Scholar] [CrossRef]
- Mesquita, J.; Ferreira, A.M.; Cavaco, D.; Costa, F.M.; Carmo, P.; Marques, H.; Morgado, F.; Mendes, M.; Adragão, P. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure—ATLAS score. Europace 2018, 20, f428–f435. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Tang, K.; Li, X.; Peng, W.; Liang, C.; Xu, Y. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: A systematic review and meta-analysis of observational studies. Europace 2012, 14, 638–645. [Google Scholar] [CrossRef]
- D’Ambrosio, G.; Romano, S.; Alothman, O.; Frommhold, M.; Borisov, G.; El Garhy, M.; Issa, K.; Penco, M.; Raffa, S.; Geller, J.C. Computed tomography-derived left atrial volume index, sex, and age to predict the presence and the extent of left atrial low-voltage zones in patients with atrial fibrillation: The ZAQ score. J. Cardiovasc. Electrophysiol. 2020, 31, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Pan, J.; Wu, H.; Xu, D. Are left ventricular ejection fraction and left atrial diameter related to atrial fibrillation recurrence after catheter ablation? A meta-analysis. Medicine 2018, 97, e10822. [Google Scholar] [CrossRef]
- Wilton, S.B.; Fundytus, A.; Ghali, W.A.; Veenhuyzen, G.D.; Quinn, F.R.; Mitchell, L.B.; Hill, M.D.; Faris, P.; Exner, D.V. Meta-Analysis of the Effectiveness and Safety of Catheter Ablation of Atrial Fibrillation in Patients with Versus without Left Ventricular Systolic Dysfunction. Am. J. Cardiol. 2010, 106, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A.J.; Henzlova, M.; Rajagopalan, S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. J. Am. Med. Assoc. 2007, 298, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Mlčochová, H.; Tintěra, J.; Porod, V.; Peichl, P.; Čihák, R.; Kautzner, J. Magnetic Resonance Angiography of Pulmonary Veins: Implications for Catheter Ablation of Atrial Fibrillation. Pacing Clin. Electrophysiol. 2005, 28, 1073–1080. [Google Scholar] [CrossRef]
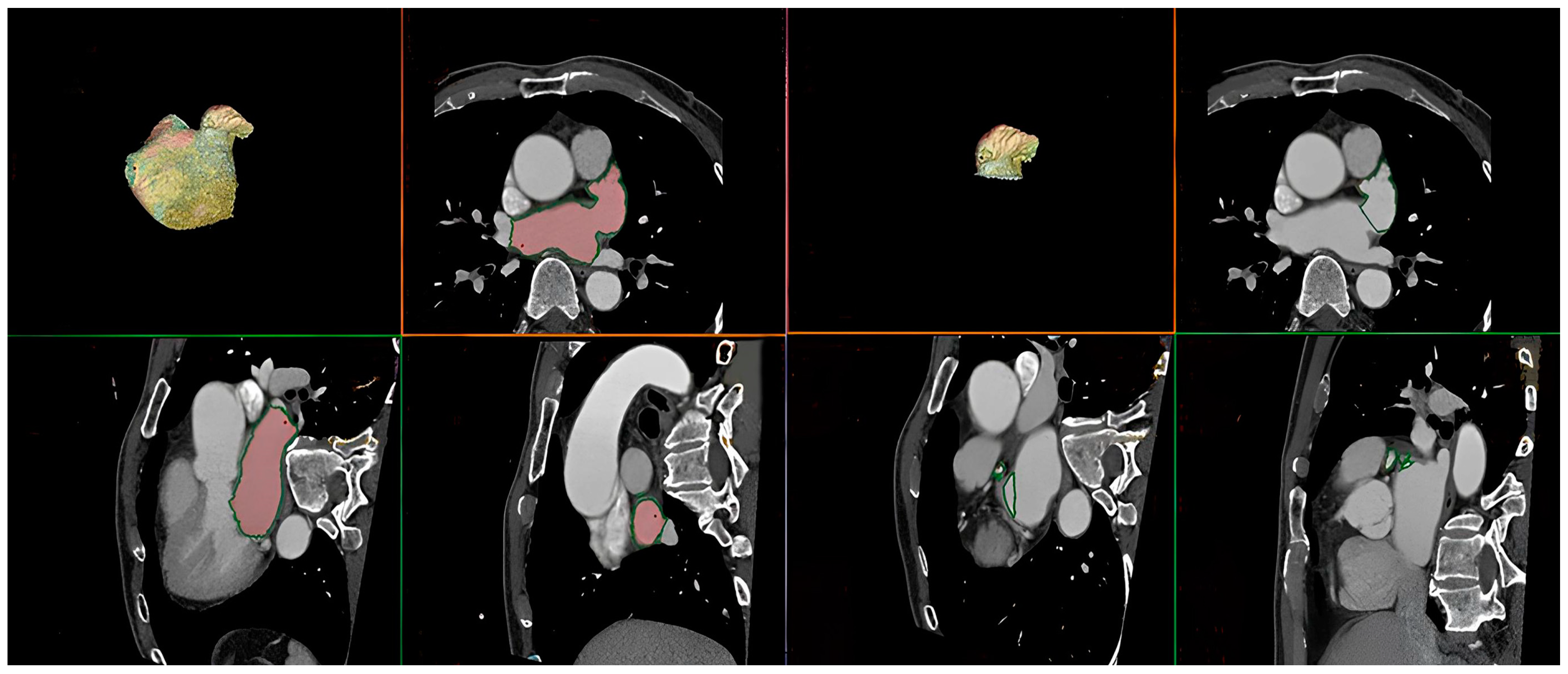
| All Patients (n = 152) | No Recurrence (n = 118) | Recurrence (n = 34) | p | |
|---|---|---|---|---|
| Age (years) | 55.8 ± 9.6 | 55.9 ± 9.5 | 55.1 ± 10.2 | 0.624 |
| Male, n (%) | 110 (72.4) | 85 (72.0) | 25 (73.5) | 1.000 |
| BMI (kg/m2) | 25.4 ± 3.3 | 25.2 ± 2.9 | 25.7 ± 4.3 | 0.428 |
| PAF, n (%) | 99 (65.1) | 87 (73.7) | 12 (35.3) | <0.0001 |
| Hypertension, n (%) | 53 (34.9) | 41 (34.7) | 12 (35.3) | 1.000 |
| Diabetes, n (%) | 11 (7.2) | 9 (7.6) | 2 (5.9) | 1.000 |
| Stroke, n (%) | 15 (9.2) | 8 (6.8) | 6 (17.6) | 0.086 |
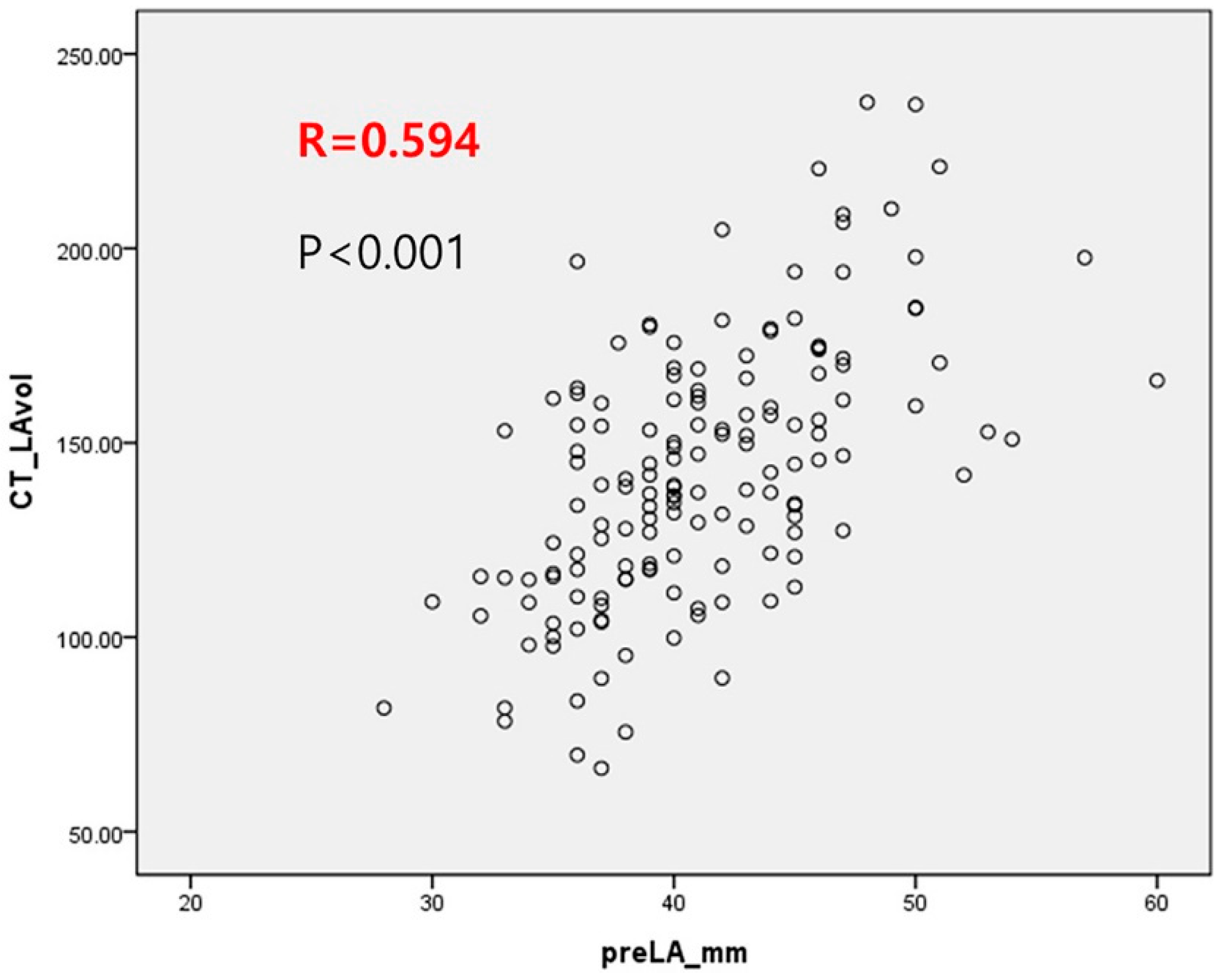
| LA volume (mL) | 142.5 ± 33.7 | 139.2 ± 34.1 | 153.8 ± 29.9 | 0.025 |
| LAA volume (mL) | 15.1 ± 6.5 | 14.7 ± 6.5 | 16.2 ± 6.3 | 0.235 |
| LVEF (%) | 58.9 ± 6.7 | 59.9 ± 5.1 | 55.5 ± 6.2 | 0.001 |
| Adjusted Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Age | 0.992 | (0.957, 1.029) | 0.674 |
| Sex | 1.135 | (0.529, 2.434) | 0.745 |
| BMI | 1.04 | (0.935, 1.158) | 0.469 |
| Hypertension | 1.029 | (0.509, 2.08) | 0.936 |
| Diabetes | 0.772 | (0.185, 3.225) | 0.723 |
| Stroke | 2.188 | (0.905, 5.291) | 0.082 |
| PeAF | 3.944 | (1.95, 7.975) | 0.001 |
| LA volume | 1.009 | (1, 1.018) | 0.063 |
| LAA volume | 1.021 | (0.976, 1.068) | 0.359 |
| LAA/LA ratio | 0.327 | (0, 560.421) | 0.769 |
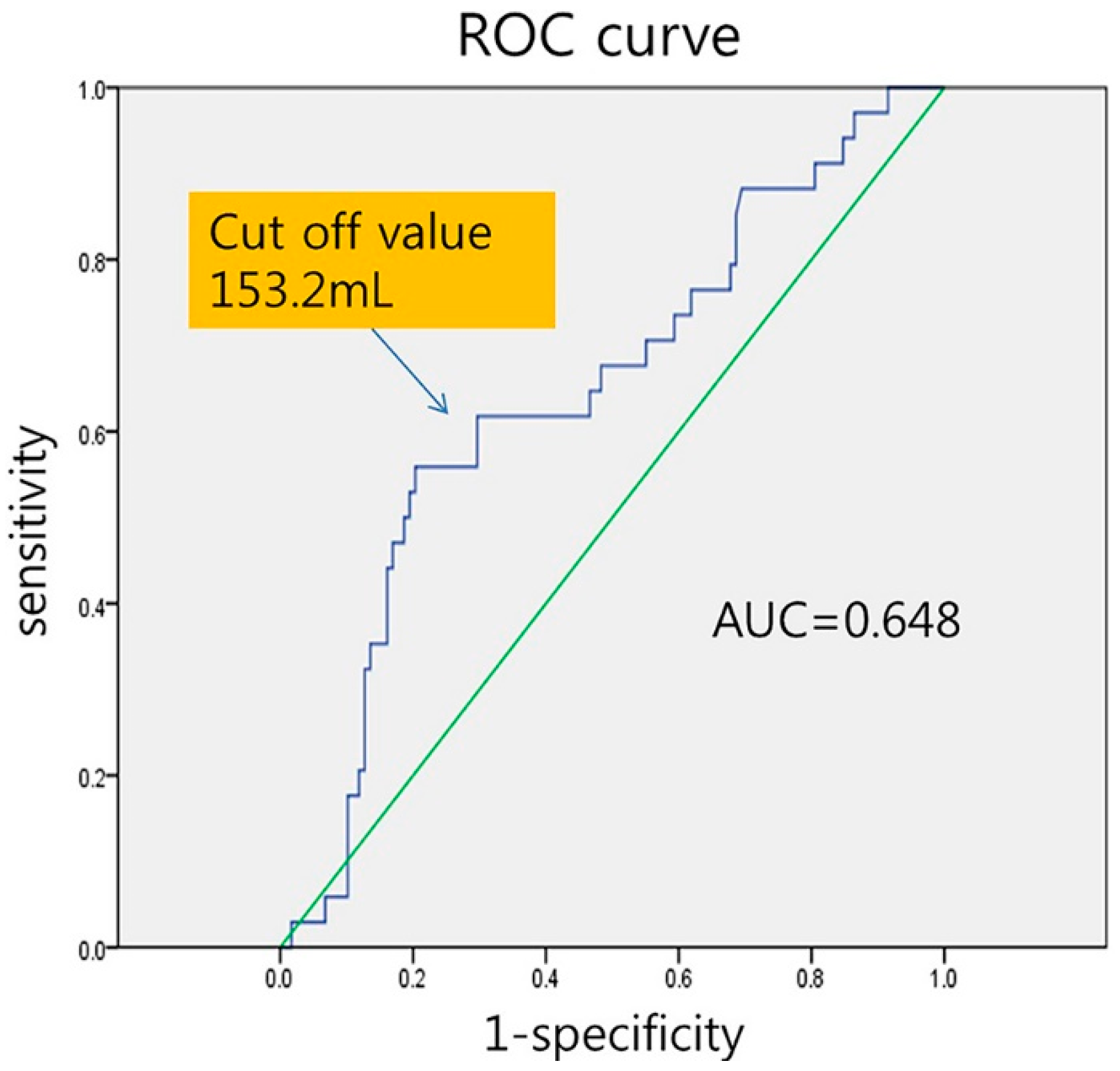
| LA volume ≥ 153 mL | 2.771 | (1.385, 5.541) | 0.004 |
| LVEF | 0.939 | (0.906, 0.974) | 0.001 |
| Number of AAD | 1.223 | (0.927, 1.614) | 0.155 |
| Adjusted | 95% | p | |
|---|---|---|---|
| Hazard Ratio | Confidence Interval | ||
| PeAF | 2.759 | (1.26, 6.041) | 0.011 |
| LVEF | 0.964 | (0.927, 1.002) | 0.065 |
| LA volume ≥ 153 mL | 1.616 | (0.752, 3.476) | 0.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Yang, D.-H.; Kim, J.-H.; Kim, Y.-R. Left Atrium Volume Measured with Multislice Computed Tomography as a Prognostic Predictor for Atrial Fibrillation Catheter Ablation Outcomes. J. Clin. Med. 2024, 13, 1859. https://doi.org/10.3390/jcm13071859
Park J-H, Yang D-H, Kim J-H, Kim Y-R. Left Atrium Volume Measured with Multislice Computed Tomography as a Prognostic Predictor for Atrial Fibrillation Catheter Ablation Outcomes. Journal of Clinical Medicine. 2024; 13(7):1859. https://doi.org/10.3390/jcm13071859
Chicago/Turabian StylePark, Jae-Hong, Dong-Hyun Yang, Ji-Hyun Kim, and Yoo-Ri Kim. 2024. "Left Atrium Volume Measured with Multislice Computed Tomography as a Prognostic Predictor for Atrial Fibrillation Catheter Ablation Outcomes" Journal of Clinical Medicine 13, no. 7: 1859. https://doi.org/10.3390/jcm13071859
APA StylePark, J.-H., Yang, D.-H., Kim, J.-H., & Kim, Y.-R. (2024). Left Atrium Volume Measured with Multislice Computed Tomography as a Prognostic Predictor for Atrial Fibrillation Catheter Ablation Outcomes. Journal of Clinical Medicine, 13(7), 1859. https://doi.org/10.3390/jcm13071859