Abstract
Cholangiopathies include a group of chronic progressive disorders, affecting the cholangiocytes, the epithelial cells that line the biliary tree, leading to liver parenchymal fibrosis and eventually end-stage liver disease necessitating transplantation. Experimental modeling of these multifactorial cholestatic diseases faces challenges due to the lack of adequate experimental in vitro and in vivo models. A novel approach employs three-dimensional organoid systems that offer several advantages for modeling disease and testing drug response in vitro. Organoids mimic intercellular communication, replicate the architecture of organs, and maintain the cell’s original phenotype. Cholangiocyte organoids provide an in vitro model to study the pathogenesis and pharmacotherapeutic treatment of cholangiopathies and show great promise for regenerative therapies. In particular, patient-derived organoids allow personalized medicine approaches and the study of individual disease characteristics. This review highlights the significance of cholangiocyte organoid models in advancing our understanding of cholangiopathies and driving advancements in regenerative medicine strategies.
1. Introduction
Cholangiopathies are chronic progressive diseases that affect the biliary epithelium and can cause fibrosis and damage to the liver parenchyma, culminating in end-stage liver disease, which requires liver transplantation (LT) [1]. Cholangiopathies, such as primary sclerosing cholangitis (PSC), biliary atresia, and cholangiocarcinoma, are highly heterogeneous, characterized by unclear pathogenesis, and lacking well-defined therapeutic approaches to date [2]. In particular, PSC is characterized by biliary obstruction and damage to the liver itself, as well as by progressive biliary inflammation and fibrosis. The pathogenesis of PSC is not yet fully understood and appears to be caused by genetic, viral, and environmental insults, as well as unknown stimuli that contribute to the damage of cholangiocytes [3]. Evidence suggests the cholangiocyte is not a target of exogenous or endogenous stimuli but also likely a dynamic actor that, by the interaction with immune cells, endothelial cells, and mesenchymal cells, plays a key role in the progression of the disease [4].
Because of a multifactorial etiology and multiple different features, cholestatic diseases have limited experimental models. In vitro cell culture models are key in liver research to supplement the lack of human samples or in vivo animal models [5,6]. However, conventional two-dimensional monolayer cell cultures lack the representation of intercellular cell-to-cell interaction, and primary human cholangiocytes are difficult to isolate and de-differentiate after a few passages [7]. On the other hand, three-dimensional cell culture systems, such as organoids, can effectively mimic cell-to-cell interaction, architecture, communication, and microenvironment among liver different cells. Organoids derived from primary human cholangiocytes have the advantage of preserving the original phenotypes of the cells; patient-derived cholangiocytes and cholangiocarcinoma organoids provide a tool for disease modeling purposes and offer an interesting platform for drug screening applications [8]. The utilization of healthy donor-derived human cholangiocyte organoids highlights their potential applications in tissue engineering and regenerative medicine and have shown the potential to restore damaged biliary epithelia in preclinical models [9].
This review synthesizes the insights into cholangiopathies and investigates cholangiocyte organoid models both as advanced tools for understanding cholangiopathies and as a cornerstone strategy in the field of regenerative medicine. A comprehensive analysis of the challenges that have to be overcome for organoids’ clinical application is also conducted.
2. Cholangiocytes and the Biliary Tree
The biliary tree is a complex network of tubular structures, or bile ducts, which begins with the Hering canals in the hepatic lobules and progressively merges into a system of interlobular, septal, and major ducts that all together form the extrahepatic bile ducts. The complex is divided into two compartments, intrahepatic and extrahepatic, which differ both anatomically and functionally [10] (Figure 1).
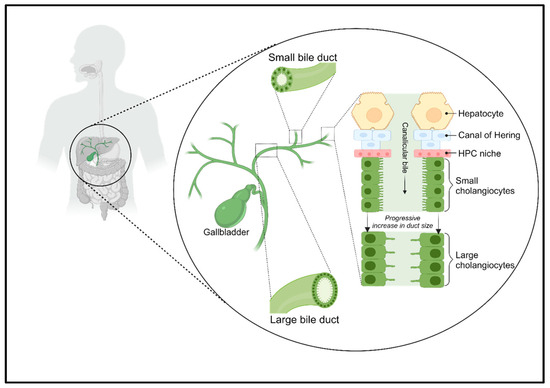
Figure 1.
The biliary tree complex. The biliary tree consists of small and large bile ducts, which are responsible for the active transport of electrolytes and solutes through cholangiocytes, which alter the flow of canalicular bile. The Hering canals connect hepatocytes to cholangiocytes and are lined by a niche of HPCs.
Hering canals connect the hepatocellular canalicular network that carries primary bile from the liver to the gallbladder, where it is stored, and which ends in the Vater Ampulla, from which the bile is poured into the small intestine where it contributes to the digestion of lipids [11,12]. Hering canals are the site of the hepatic stem cell niche [13]. Instead, the extrahepatic biliary network presents a niche of hepatic progenitor cells (HPCs), which differs from that present in the Hering canals, because the intra- and extrahepatic biliary trees have different embryonic origins [14].
Cholangiocytes are ciliated and highly specialized epithelial cells that line the intra- and extrahepatic bile ducts. They play a key role in liver repair, innate immunity, and progression of cholangiopathies [10]. They represent the first line of defense in the innate immunity of the liver; moreover, they can be both the target of immune-mediated aggression or the initiators of an inflammatory reaction that progresses to adaptive immune activation [4]. The contribution of biliary epithelial cells to liver immune responses was thought to be limited to the secretion of immunoglobulin (Ig) A in bile, but it is clear at present that the role of cholangiocytes in immune response is much more complex [15].
Cholangiocytes within various compartments of the biliary tree exhibit a distinct morphology. The small cholangiocytes line the Hering canals and distal branches of the biliary tree, featuring a cuboidal shape with a round basal nucleus. They exhibit rapid reactivity to liver and/or biliary damage [16]. On the other hand, large cholangiocytes line the larger-diameter ducts, displaying a cylindrical shape. These cells are involved in secretory functions and possess transport capacity, mediating processes such as alkalization, hydration, and the modification of bile [17].
The apical surface of cholangiocytes has a non-mobile primary cilium, which functions as a mechanosensor, chemosensor, and osmosensor. The cilia direct the bile flow and, by bending, activate the calcium ions (Ca2+) channel, allowing the influx of Ca2+ into the cell [11]. These structures can be involved in cell proliferation and senescence, in the activation of progenitor cell compartments, and in regeneration and development.
The extracellular vesicles present in the bile can bind to cilia and have been shown to inhibit the proliferation of bile duct cells, promoting a quiescent state of the biliary system under normal conditions [15]. Moreover, recent research has shown that exosomes (small extracellular vesicles ranging in size from 50 to 200 nm, containing genetic material, such as DNA, mRNA, and various types of non-coding RNAs) are promising diagnostic tools for cholangiocarcinoma and gallbladder carcinoma, as they are easily and rapidly accessible [18]. Circulating non-coding RNAs, found within exosomes, can play a significant role as effective biomarkers for the diagnosis of various diseases. Therefore, the analysis of exosomes and their genetic contents could represent an innovative approach to the early and precise diagnosis of various pathological conditions.
Biliary epithelial cells, thanks to their ability to secrete ions in a polarized way and their selective permeability to solutes and water, actively maintain hepatic homeostasis. In addition, the biliary epithelium acts as a barrier against the back diffusion of xenobiotics, toxic metabolites, and bile salts from the bile to the interstitial tissue [19].
The cells of the smaller branches of the bile ducts have different and specific biological properties, such as phenotypic plasticity, the ability to react to liver damage and behavior as the progenitors of hepatocytes, and they are awakened to varying degrees only after liver damage [10].
3. Biliary Injury
The damage to the biliary tree can be various in nature (Figure 2). Most of the disorders that cause biliary pain are due to calculi, that is, the formation of stones within the bile ducts and in the gallbladder causing choledocholithiasis and cholelithiasis [20]. These, in turn, can cause biliary colic and cholecystitis, scilicet inflammation of the gallbladder, which can be acute if it progresses in a few hours or chronic if it evolves for a longer time [21]. Furthermore, blockage of the bile ducts can also lead to inflammation of the bile ducts thus causing acute cholangitis. However, the blockage or slowing of the flow of the bile ducts, known as cholestasis, can also be caused by tumors or strictures following viral infections [20,22].
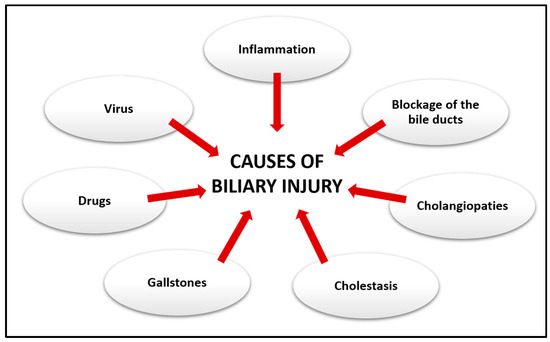
Figure 2.
Causes of biliary injury. Damage to the biliary system can result from a variety of different conditions.
Cholestasis in turn can progress into chronic liver disorders affecting cholangiocytes, known as cholangiopathies, which can result from proliferative, fibrotic, genetic, immune-mediated phenomena that can cause portal hypertension and progressive periportal fibrosis [23]. The different distributions of cholangiopathies along the biliary tree can be explained by the different types of damage that can affect cholangiocytes [24]. Cholangiopathies can generally be classified into immune-mediated diseases, infectious, genetic, inflammatory, and fibrosis, which lead to the development of primary biliary cholangitis (PBC), PSC, IgG4-related sclerosing cholangitis (ISC), and biliary atresia (BA) [19,25,26].
Cholangiocytes are the target of various stimuli of innate and adaptive immune responses, ischemia, cholestasis, and xenobiotics [27,28]. Their activation causes an increase in pro-inflammatory and pro-fibrotic mediators, and the recruitment of immune, vascular, and mesenchymal cells, which all together contribute to the development of biliary fibrosis, which can ultimately evolve into cholangiocarcinoma [4,29].
3.1. Cholangiopathies
PBC is a chronic and progressive disease mainly observed in females. Its incidence is 1–2 per 100,000 population per year, prevalence is 1 over 1000 in women older than 40 years, and is strongly associated with autoimmune syndromes, such as Hashimoto’s thyroiditis, Sjögren’s disease, celiac disease, or systemic sclerosis. PBC is characterized by anti-mitochondrial (AMA) or specific anti-nuclear antibody (ANA) positivity. Ninety per cent of PBC patients show AMA positivity. Histology of PBC shows the typical florid duct lesions and destruction of intralobular bile ducts. The pathogenesis is poorly understood; however, autoimmunity is likely involved [30].
Instead, PSC is mainly observed in men with a median age at diagnosis of 41 years; incidence is 0–1.3 cases for 100,000 persons per year. It is strongly associated with inflammatory bowel disease (IBD) and gallbladder and colorectal cancers. It represents the major risk factor for cholangiocarcinoma [31]. Magnetic Resonance Imaging and Endoscopic Retrograde Cholangiopancreatography (ERCP) show the characteristic strictures that confirm the diagnosis. The structures involve either the entire biliary tract (95%) or only the small ducts [32]. Pathogenesis is still unknown. The role of activated T cells has been proposed as a potential cause. Nevertheless, the presence in the liver parenchyma of microbial antigens could be involved in the early senescence of hepatocytes.
ISC is an uncommon variant of PSC. It has been associated with a worse prognosis, without IBD, even though the etiology is still not clear. Serum IgG4 is elevated both in ISC and in PSC, but ISC is associated with tissue IgG4 deposits and inflammatory disease of other glands, such as pancreatitis or sialadenitis. Lastly, ISC shows a good clinical response to glucocorticoid treatment, but the relapse percentage is frequent [33]. Table 1 shows the epidemiological data of the most widespread cholangiopathies.

Table 1.
The most common cholangiopathies.
3.2. Biliary Complications Post-LT
Cholangiopathies are a frequent indication for LT. The European Liver Transplant Registry (ELTR) reported that 13,241 LTs were performed for cholestatic disease in the last 50 years (10% of the total): 44% due to PBC, 44% due to PSC, and the remaining due to secondary biliary cirrhosis. These percentages remained stable over the past 15 years, except for a slight increase in PSC [34]. About 40% of patients with PSC undergo an LT. However, a recurrence is observed in 10 to 40% of cases, leading to re-transplantations in up to 50% of cases. Acute or chronic rejection is frequent (39–71%) requiring a high immunosuppressive regimen. PSC remains a clinical and surgical challenge, with a 1-year survival rate of 85% and a 5-year survival rate of 72% [2,3,23,35].
Furthermore, the extension of the criteria for liver donation, with the inclusion of elderly donors and donors after cardiac death (DCD), caused an increased risk of developing complications that could lead to graft failure [36]. Among the most common complications are biliary complications, which are increasingly the cause of morbidity and mortality after transplantation, and the most relevant ones are: anastomotic (AS) and non-anastomotic stenosis (NAS).
NAS occurs due to irregularities of the biliary tree and represents the most common complication [37]. The origin is often multifactorial, and multiple causes may overlap with damage to the biliary system, such as bile duct injury and subsequent fibrosis and the gross narrowing of donor bile ducts. NAS can be further classified as an ischemic-type biliary lesion (ITBL), which is associated with arterial stenoses or thrombosis and ischemic cholangiopathy (IC), where normal vascular flows are present [38]. IC is characterized by an increase in cholestasis indices and bilirubin, often associated with fever and abdominal pain [39]. Strategies aimed at preventing IC include the implementation of dual (portal and arterial) perfusion at perfusion at procurement and ex-situ machine perfusion preservation, which are extremely relevant [36,40,41]. The current main explanations for a higher rate of NAS in DCD transplants are ischemia-reperfusion injury (IRI), immune processes, and bile salt toxicity that damage cholangiocytes [42]. DCD livers undergo a period of warm ischemia in the donor, which, combined with cold storage and other risk factors, makes the liver more susceptible to the development of ITBL [36,43]. IRI can be classified as a primary ischemia injury that affects the bile ducts during the transplantation stages, secondary ischemia that can occur after transplantation due to damage to the peribiliary vascular plexus, and insufficient regeneration of the biliary epithelium [36,44].
Primary injury can occur at various stages during the transplantation procedure. An extended warm ischemia time in the donor, particularly when combined with additional cold storage, represents a key risk factor for the development of biliary strictures [44,45,46]. Following reperfusion, the damage is aggravated as accumulated oxygen-containing reactive species (ROS) and damage-associated molecular patterns (DAMPs) proceed to activate the immune system causing necrosis and apoptosis. It has been shown that cholangiocytes are more susceptible to IRI than hepatocytes because of slower ATP regeneration, higher ROS production, and lower concentration of glutathione, which has an antioxidant action [47,48].
After that, DCDs undergo a second warm ischemia when the organ is harvested from ice and placed in the recipient’s abdomen, due to portal reperfusion, which is low in oxygen saturation, does not contribute sufficiently to biliary perfusion, and each additional minute of warm ischemia increases the risk of ITBL [49,50]. In addition, hepatic steatosis of the transplanted liver also contributes to secondary ischemia damage, as it causes the swelling of lipid-laden hepatocytes that causes the impairment of micro-circulation and increases the risk of developing biliary complications [44,51].
The IRI of the bile ducts has long been considered the main determinant of the development of ITBL. However, several clinical studies have shown that extensive injury and loss of the biliary epithelium can be found in more than 90% of transplanted livers, and only a minority of these develop post-transplant cholangiopathy [44,52]. This brings us back to the hypothesis that the insufficient regeneration of the biliary epithelium, rather than the initial amount of injury, determines whether a liver donor develops post-transplant cholangiopathy [52]. Currently, the treatment of post-transplant cholangiopathy consists of the use of antibiotics, endoscopies, resection of extrahepatic bile ducts, and liver re-transplantation, but these are often challenging and unsuccessful.
In recent years, attention has shifted to the use of mechanical perfusion as an emerging strategy to counteract IRIs [8,52]. Studies have indicated that the use of the normothermic machine perfusion (NMP) in cases of DCDs is associated with a reduced incidence of post-transplant biliary damage, due to the supply of oxygen and nutrients and consequent reduction in ischemic damage in the bile duct [36]. Using DCD livers from pigs, the positive effect of NMP on biliary damage and regeneration has been demonstrated [53,54]. But, it was also confirmed by the de Jong study [55], which found an increase in the proliferation of cholangiocytes during NMP and better preservation of the peribiliary glands (PBGs) containing progenitor cells that differentiate into mature cholangiocytes for biliary regeneration. Compared to NMP, hypothermic perfusion (HMP) also presents advantages. Multiple studies have demonstrated that HMP significantly reduces IRI. Furthermore, it appears to “resuscitate” mitochondrial function, thereby decreasing the formation of ROS and the activation of the immune system [42,44]. Additionally, studies on pigs regarding HMP showed the enhanced preservation and protection of the bile ducts, ultimately leading to an improved hepatobiliary function [56]. Moreover, MP can act as a platform for the direct release of therapeutic agents to organs before transplantation and for testing new therapeutic approaches and their effectiveness in repairing liver damage [36,56,57,58,59].
A new strategy would involve transplanting cholangiocyte organoids directly into intrahepatic ducts before organ transplantation, during machine perfusion (MP) [60]. Cholangiocytes play an important role in the etiopathogenesis of post-transplant cholangiopathies [42,49,61], and given the high incidence of biliary system disorders following transplantation, the use of cholangiocyte organoids has been proposed. The study by Sampaziotis et al. [8] highlights the high plasticity of cholangiocytes: cells taken from different regions of the biliary tree contain different transcriptional profiles, but cholangiocytes lose these differences by allowing cells from one region to repair a different region of the biliary tree.
4. Regeneration in Response to Biliary Damage
In response to biliary damage, cholangiocyte proliferation is activated to maintain normal homeostasis of the biliary tree. In physiological conditions, small cholangiocytes are quiescent, but in case of liver damage, they activate a marked proliferation as part of the hepatic reparative complex [62] (Figure 3). Moreover, cholangiocytes are involved in cell cycle phenomena that maintain tissue homeostasis in the biliary system through modulators of apoptosis and senescence, and damage to cholangiocytes can lead to cholangiopathies [19].
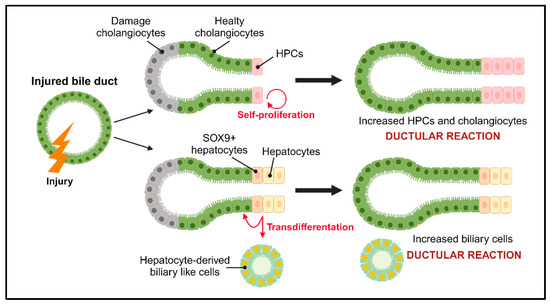
Figure 3.
Regeneration after biliary injury. After damage, cholangiocytes and HPCs are activated to compensate for the damaged cells through the ductal reaction. Depending on the type of damage, two mechanisms are implemented: self-proliferation in which the populations of HPCs and cholangiocytes increase, and the transdifferentiation of hepatocytes into biliary-like cells and/or cholangiocytes. HPCs: hepatic progenitor cells; SOX9: SRY-box transcription factor 9.
Based on the type of damage, a specific population of cholangiocytes is activated to start proliferating. The proliferation of cholangiocytes can be divided into three types: the “typical” type results in an increase in the number of intrahepatic bile ducts deriving from older pre-existing ducts [63]; the “atypical” type occurs in chronic liver injury as well as alcoholic liver disease and chronic extrahepatic biliary obstruction, and results from the transdifferentiation of hepatocytes into cholangiocytes [64]; and the last type is type III and it is the first step towards carcinogenesis in the liver, which leads to disorganized proliferation and distorted liver architecture [65].
Inflammation, caused by the activation of cholangiocytes following damage, is at the basis of the biliary repair process and biliary fibrosis known as the ductal reaction (DR), which involves inflammatory cells, HPCs, and activated cholangiocytes [66]. The DR is defined as a bile duct hyperplasia commonly observed in liver diseases and the cellular phenotypic profile that characterizes it is influenced by the location of the liver lesion and the etiology of the disease that causes the lesion [66,67]. Therefore, in case of damage to the biliary tree, the reaction is characterized by the proliferation of cells with a biliary profile, while damage to hepatocytes causes a proliferation of cells with a hepatocytic profile [67,68].
HPCs are stem cells that begin to proliferate and expand rapidly following severe liver damage and have bidirectional differentiation potential as they can differentiate into hepatocytes and/or cholangiocytes [69]. There are two distinct populations of progenitor cells: hepatic progenitor cells (HPCs) and biliary tree progenitor cells (BTPCs).
HPCs are found in the smallest branches of the biliary tree, in the Hering canals and bile ducts. Their activation is associated with the appearance of the ductular reaction and in the context of cholangiopathies support the renewal of cholangiocytes that are compromised in their proliferative abilities [70].
BTPCs, which are found in the PBGs of the large intrahepatic and extrahepatic bile ducts [71], proliferate in response to biliary damage to give rise to the progeny of cholangiocytes [72]. Furthermore, damage to PBGs at the time of transplantation is a risk factor for the development of biliary complications. Therefore, it is plausible to think that post-transplant cholangiopathies are determined by the effective regenerative capacity of the bile ducts, rather than the amount of epithelial damage [73].
In case of cholestatic liver diseases, such as PSC or PBC, an “atypical” proliferation occurs where hepatocytes transdifferentiate into cholangiocytes and/or cholangiocyte-like cells, contributing to functional repair and regeneration in liver damage. This transdifferentiation is a result of cellular reprogramming observed through the expression of biliary transcription factors and other specific markers [64,74].
5. “Old” Therapies for the Regeneration of the Biliary Tree
Over the years, several treatments have been designed to prevent the progression of cholangiopathies, but unfortunately, they have failed and almost always the only solution is LT (Table 2).
Ursodeoxycholic acid (UDCA) has been, for a long time, and is still the gold standard for the treatment of cholangiopathies [25,26]. It is a hydrophilic bile acid naturally present in bile, which is effective in preventing the progression of inflammation and fibrosis when taken early in the disease [75]. However, this treatment appears to be ineffective in 40% of patients [76]. UDCA efficacy in PSC is still debated, as it determines an improvement in blood chemistry without increasing survival. According to other studies, UDCA may even determine a worsening of the prognosis, with a higher incidence of cirrhosis and cholangiocarcinoma and the need for LT.
In general, even if UDCA is the main treatment for these pathologies, their effectiveness is limited, and also in PBC—where the evidence is stronger—there are no differences in symptoms, liver-related mortality, or transplant-free survival [77].
Obeticholic acid (OCA), 24-Norursodeoxycholic acid (norUDCA), and antibiotics are widely used, but they are still under study or have limitations/complications, like the risk that elevated serum fibroblast growth factor 19 (FGF19) levels lead to the development of hepatobiliary malignancy and the high dosage that is toxic [31,32,78,79].
It is not indicated in patients with decompensated cirrhosis or portal hypertension, but only in patients with Child–Pugh Class A. The dosage must be titrated progressively and is often not tolerated by patients due to the onset or worsening of itching, fatigue, nausea, and headache [77]. The data regarding the long-term impact on survival in patients treated with OCA are limited.
Other treatments are the application of immunosuppressants, glucocorticoids combined with UDCA, and B-cell depletion, but potential treatments, such as signal regulatory protein 1 and 4 (S1RP1, S1RP4) agonists and NADPH oxidase 1 and 4 (NOX1, NOX4) inhibitors, are under clinical evaluation [75]. Glucocorticoid and azathioprine represent the first-line therapy, however other antimetabolite drugs or calcineurin inhibitors can be administered. The response rate is high, but relapse is frequent. Transplantation may be necessary in cases of severe acute hepatitis [80].

Table 2.
Mechanisms of action of some treatments for cholangiopathies.
Table 2.
Mechanisms of action of some treatments for cholangiopathies.
| Treatment | Mechanism | References |
|---|---|---|
| UDCA | Protection of biliary epithelial cells and mitochondrial integrity, reduction in pro-inflammatory cytokines. | [76,79] |
| OCA | FXR agonist that suppresses bile acid synthesis, inflammation, and hepatic fibrosis, and induces the endogenous synthesis of FGF19. It is a promising potential therapy for PBC patients. | [81,82] |
| norUDCA | Increases resistance to biliary damage induced by bile acids and has a pleiotropic effect on inflammation, apoptosis, and fibrosis. | [83,84] |
| Antibiotics | Improvement in liver biochemistry observed with vancomycin, metronidazole, azithromycin, and minocycline. | [78] |
The ineffectiveness of the treatments used at present leads to the only therapeutic option of a transplant [85]. So, the lack of definitive therapies and the high cholangiocytic disease incidence have prompted the exploration of new alternatives, such as the utilization of organoids to repair biliary damage.
6. Cholangiocyte Organoids as a New Strategy for the Regeneration of the Biliary Tree
The failure of regeneration and limited treatments for advanced liver disease highlight the urgent need for new strategies in regenerative approaches that activate the body’s natural repair mechanisms and explore options such as cell-based therapies or bioengineered tissue for liver replacement.
The strategies of regenerative medicine, for the liver as well as for other organs, can be captured through the R3 paradigm: replacement, regeneration, and rejuvenation [6] The replacement strategy involves LT, the only clinically available regenerative medicine therapy in end-stage liver disease. However, this paradigm helps guide the development and implementation of complementary strategies, such as cell-based therapies (e.g., liver organoids) and bioengineered tissues. In contrast, regeneration involves the delivery and engraftment of stem cells or progenitor cells that then undergo growth and differentiation in vivo (e.g., stem cell transplant or stem cell-coated stents) [9,10,11]. Lastly, rejuvenation involves inducing tissue self-renewal through the activation of endogenous stem cells (e.g., gene therapy or exosome delivery) [14].
The hepatic, pancreatic, and biliary Organoid Consortium recently published a consensus document that defines an organoid as a “three-dimensional structure derived from (pluripotent) stem cells, progenitor, and/or differentiated cells that self-organize through cell–cell and cell–matrix interactions to recapitulate aspects of the native tissue architecture and function in vitro” [86]. The organoids are then classified according to single or multiple germ lines in epithelial, multi-tissue, and multi-organ organoids, and also subclassified according to cell type of origin.
6.1. Generation of Cholangiocyte Organoids
Cholangiocyte organoids can derive from the epithelial cells of different compartments of the biliary tree (intrahepatic, extrahepatic, and bile); therefore, a nomenclature has been proposed that allows us to identify the organoids based on their origin: intrahepatic cholangiocyte organoids (ICOs), gallbladder cholangiocyte organoids (GCOs), extrahepatic cholangiocyte organoids (ECOs), and cholangiocyte organoids derived from bile (BCOs) [60]. All of these structures share similar phenotypic characteristics when grown, but they have different identities based on the location and composition of the bile [87]. It has been observed that cholangiocytes have a plastic identity and lose some characteristics of the subpopulation of origin to assume a single common organoid identity, but if exposed to different bile concentrations and compositions, they can regain the identity of the original location [8,88]. Cholangiocyte organoids can be derived from both healthy and diseased individuals and can be generated from a variety of sources, including stem cells (iPSCs), organ-derived primary tissues, or body fluids, such as bile [86] (Figure 4).
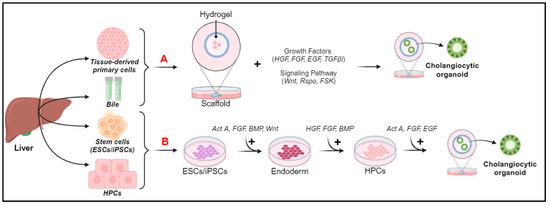
Figure 4.
Sources and generation of cholangiocyte organoids. (A) Organoid generation from tissue-derived primary cells and bile. (B) Organoid generation from ESCs/iPSCs and HPCs. Act A, Activin A; BMP, bone morphogenic protein; EGF, epidermal growth factor; ESCs, embryonic stem cells; FGF, fibroblast growth factor; FSK, forskolin; HGF, hepatocyte growth factor; HPCs, progenitor cells; iPSCs, pluripotent stem cells; OSM, oncostatin M; TGFβ1, transforming growth factor beta inhibitor; TNFα, tumor necrosis factor alpha.
Organoids derived from tissue-derived primary cells have greater stability and ease of propagation than organoids derived from iPSCs, but require access to the primary tissue, and this is not always possible [89]. For organoid generation from tissue-derived primary cells or bile (a schematic representation is shown in Figure 4A), the Scaffold technique that exploits the extracellular matrix (ECM)-based hydrogel or Matrigel is used [90,91]. It is commercially available, and, through different growth factors in the culture medium and signaling pathways, recreates a bioactive micro-environment in which the cells differentiate and generate spherical structures and finally mature into cholangiocytes or hepatocyte organoids [60,90].
Pluripotent stem cells (iPSCs), do not require access to primary tissue, as they allow us to easily obtain cholangiocytic organoids with minimally invasive procedures from different materials, such as the blood, urine, or skin [92]. The resulting cells differentiate into hepatoblasts, which can give rise to the monolayer of HPCs [93] (Figure 4B). Then, single-cell or multicellular approaches can be used to obtain cells that exhibit the key functions of mature cholangiocytes [92]. The multicellular approach involves the interaction of HPCs with OP9 cells, a stromal cell line that expresses the Notch ligand (of the bile duct regeneration pathway), which form cells that express primary biliary and cilia functions [70]. The single-cell approach is less variable, as it relies only on key factors of biliary regeneration pathways (such as Wnt, Notch, and TGFβ), which lead to the formation of cells expressing markers (cytokeratin19, SOX9, and CFTR), primary cilia, and stimuli secretors [94]. Despite the presence of many features of mature cholangiocytes, iPSC-derived cholangiocyte organoids are characterized by incomplete maturation, some fetal characteristics, and the genetic instability associated with iPSC [48]. To overcome this challenge, Ogawa et al. [70] devised a monolayer-based differentiation strategy, enabling the generation of a significant number of mature and ciliated cholangiocytes from various pluripotent stem cell lines, thus paving the way for exciting opportunities to develop targeted cellular therapies for regenerating compromised and/or diseased bile ducts in patients with cholangiopathies [95].
Cholangiocyte organoids that are derived from HPCs are an exciting area of research in the fields of regenerative medicine and modeling liver diseases and their use to produce cholangiocyte organoids has several advantages: these cells have significant proliferative potential, which means they can multiply quickly in vitro and provide a generous source of material for experiments; the genomic stability is crucial in ensuring the consistency of results in organoid cultures; and the intrinsic re-differentiation capacity of HPCs within organoids guarantees the flexibility to transform into various cell types, including cholangiocytes, and this feature enhances the organoids’ ability to reflect the complexity and heterogeneity of liver tissue [96,97].
Primary cholangiocytes can be grown using two main complementary platforms, based on canonical or noncanonical Wnt signaling, resulting in a different cell phenotype. Wnt seems to be a master regulator of a mature versus stem cell phenotype [98,99]. Cells grown in the canonical Wnt signaling condition have a stem cell-like phenotype and can differentiate toward both the hepatic and biliary lineage, but do not fully recapitulate the functions of mature cholangiocytes or hepatocytes in vitro. Primary cholangiocytes grown in conditions based on noncanonical Wnt signaling give rise to mature primary cholangiocyte organoids in the long term while maintaining genetic stability, expression of key mature biliary markers, and cholangiocyte functions in vitro, maintaining their plasticity.
6.2. Applications of Cholangiocyte Organoids
The use of cholangiocyte-derived organoids in the context of cholangiopathies and bile system disorders holds great promise. This is attributed to the regenerative potential of bile epithelia distributed throughout the liver and the unique plasticity of cholangiocytes, endowing these cells with a unique potential for tissue repair while retaining the functions and characteristics of the original tissue [8]. Fundamentally, organoids are well-suited for basic research on liver pathophysiology, disease modeling, pharmacological treatment assessment, and the development of personalized treatments, as well as applications in regenerative medicine for repairing deficits in the bile epithelium [100]. Additionally, the three-dimensional structure of organoids offers the opportunity to develop multi-organ systems and evaluate the contribution of various organs through platforms like the liver on chip (Figure 5).
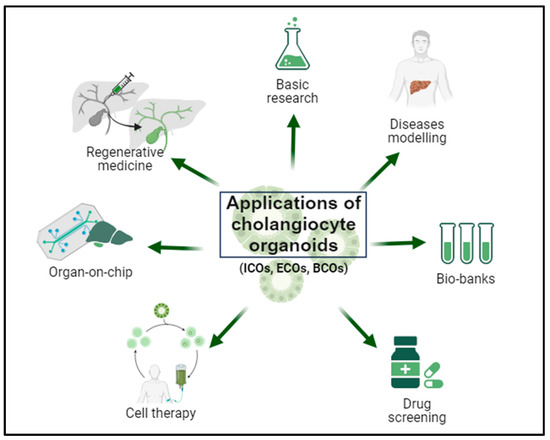
Figure 5.
Biomedical applications of cholangiocyte organoids. Cholangiocyte organoids can be derived from intrahepatic bile duct biopsies (intrahepatic cholangiocyte organoids; ICOs), extrahepatic bile duct biopsies (extrahepatic cholangiocyte organoids; ECOs), and bile samples (bile-derived cholangiocyte organoids; BCOs). Organoids offer a broad spectrum of applications, including basic research on liver physiopathology, disease modeling, biobank establishment, pharmacological screening, implementation of personalized therapies, utilization in organ-on-chip systems, and application in regenerative medicine.
6.2.1. Basic Research and Disease Modeling
Cholangiocyte organoids can be employed in basic research, enabling the study of liver cell differentiation, including the development and maturation stages of cholangiocytes [60]. Furthermore, 3D cultures derived from healthy patient samples allow the modeling of liver diseases, including cholangiopathies, by recreating the physiological microenvironment (including cell–cell and cell–ECM interactions) [7].
For instance, bile-derived organoids (BCOs) have been identified as a novel method for studying the pathogenesis and therapy of cholangiopathies, such as PSC. While tissue-derived organoids are limited by sample availability (usually collected during transplants), BCOs are easier to obtain since bile can be regularly collected [88,101]. Additionally, they exhibit a biliary phenotype and altered expression of genes associated with immune regulation [102]. BCOs from PSC patients, when stimulated with the pro-inflammatory cytokine IL-17A, were observed to secrete high levels of chemokine CCL20, confirming its key role in biliary duct damage in patients with chronic liver inflammatory diseases [103]. This discovery is valuable as it opens avenues for studying pharmacological therapies for these organoids, making them more amenable to susceptibility testing. However, while BCO technology presents limitations due to the not fully matured origin epithelia and the ability to obtain them only from patients with biliary duct stenosis, these organoids preserve more properties of the biliary tree compared to iPSCs; hence, further comparative studies are warranted [104].
Moreover, Chen et al. [105] utilized primary cholangiocytes isolated from mouse bile ducts and decellularized liver scaffolds to develop functional ductal organoids (FDOs) and construct a network structure resembling a biliary tree. The study demonstrated that cholangiocytes in FDOs could have a future in clinical therapy, as they can be used for disease modeling and generating bioengineered livers [105].
The simulation of pathological conditions is a widely explored area for organoid use, even enabling the replication of congenital and hereditary diseases through genetic manipulation techniques [100]. Additionally, organoids derived from bile ducts open up the possibility of creating human organoid biobanks (healthy or diseased), thus enabling future studies on diseases, pharmacological screenings, and personalized medicine [9].
6.2.2. Drug Screening and Organ-on-Chip System
Organoids derived from the cells of diseased individuals can be used to develop personalized gene therapies [90]. In fact, cells within the organoid can be employed to test the effects of drugs, thus aiding in identifying potential therapies and possible hepatotoxic effects they can cause, also thanks to the secretion of enzymes into the bile, which are useful for the hepatic metabolism of many hydrophobic drugs [7,106,107].
The lack of adequate in vitro models prompted the group of Shi et al. to use intrahepatic cholangiocyte organoids to recreate necroptosis, a common mode of programmed cell death in cholangiopathy [108]. They demonstrated that ICOs can serve as a useful platform for the in vitro study of biliary cytotoxicity and preclinical assessment of drugs, with significant implications for the development of therapies for cholangiopathies.
Recently, organ-on-chip (OOC) technology has been developed, representing an innovative preclinical system for the in vitro evaluation of human organ responses to anti-tumor therapies [109]. Combined with organoid cultures, it can enable in vivo drug screening [110]. The advantage of chips lies in their ability to recreate the multicellular structure, chemical gradients, vascular systems, and mechanical properties of human organs [111,112].
Organoids for tumor studies are limited as they lack components of the immune system, can be contaminated by normal organoids, and require components necessary for organoid development (growth factors, extracellular matrix, and serum). On the other hand, OOCs also presents various limitations due to their lower 3D complexity compared to organoids and longer processing times [113,114]. The combination of organoids and OOCs can allow the integration and combination of different tumor cell lines and immune cells, recreating and assessing immune microenvironment interactions in a more physiologically relevant and comprehensive manner. This has the potential to predict targeted therapy for the patient, revolutionizing preclinical tools in precision medicine [115,116].
6.2.3. Regenerative Medicine
The incidence of liver diseases is on the rise globally, and currently, LT is the only therapeutic option for end-stage liver diseases. The development of regenerative medicine techniques holds great potential, with the biliary system being a particularly interesting target due to its significant regenerative potential throughout the liver and its minimally invasive accessibility through ERCP [117,118]. Organoid culture allows the propagation of highly functional cells that retain their original functions and demonstrate the ability to engraft and regenerate the liver or bile ducts [119]. Notably, organoids adapt well to large-scale expansion, enabling the generation of autonomous mini-liver structures with a vascular network, addressing many challenges in regenerative medicine [120]. The presence of hepatic organoids fuels the prospect of implementing autologous organ transplants, where healthy patient liver tissues can be expanded and subsequently transplanted, thereby reducing the risk of adverse immune reactions [121].
Furthermore, exogenous cell therapy has emerged as an alternative to LT to understand and harness the regenerative capacity of hepatocytes and biliary epithelial cells, acting as stem cells to restore damaged epithelial populations. Identifying key signals can provide targeted therapeutic pathways and enable the development of therapies to enhance liver regeneration [74,122].
In 2013, Huch et al. pioneered the generation and implantation of bile duct-derived organoids in nude Balb/c mice, which differentiated into functional hepatocytes [123]. A study showed the use of extrahepatic cholangiocyte organoids for the reconstruction of the extrahepatic biliary tree in mice: ECOs transplanted into immunocompromised mice were observed to maintain gene expression and express key biliary markers, which allow for self-organization into bile duct-like tubes and repair of damaged biliary epithelia [99]. Subsequent studies demonstrated the high engraftment rate (80%) and survival (90 days) of liver organoids generated from both murine and human primary hepatocytes after transplantation into immunodeficient mice with damaged livers [124,125].
Cholangiocyte plasticity represents the strong point of this technique and potentially the future of regenerative medicine. Cholangiocytes have different transcriptional profiles, based on their location within the biliary tree, but the organoids lose these properties as they lack stimuli [120]. Following various local and environmental stimuli, it is possible to reconstitute the expression of specific markers and restore the different regional conformations [11]. Organoids derived from cholangiocytes have demonstrated the ability to regenerate up to 50% of the biliary tree in mice with injuries, and they have been successfully transplanted into ex situ perfused human livers, providing the first demonstration of the efficacy of organoids in regenerative medicine in human organs [8]. Sampaziotis et al. demonstrated that the transplantation of cholangiocytic organoids in a different region, compared to the original one, still allows the damage to be repaired. It was observed that the transplanted organoids (ICOs) at the level of the intrahepatic biliary tree formed a cell population made up of native and transplanted cholangiocytes, with the potential for regeneration of about 40–85%. As demonstrated, ICOs represent an important experimental tool for cholangiopathy pathogenesis investigation [126].
The development of cholangiocyte organoid systems can overcome the limitations of in vitro cholangiocyte cultures. There are significant challenges, as cells intended for transplantation must be highly functional to survive in hostile environments, such as bile, integrate into the vascular system, and engraft in the long term. Additionally, cell therapies are limited by the lack of integration with large-scale automated production platforms, but the use of robotic systems and bioreactors can overcome this limitation [127,128]. Finally, the use of a matrix, such as Matrigel, which can be potentially risky due to its chemically undefined nature [129], is driving the development of hydrogel matrices based on biological or synthetic polymers showing an acceptable safety profile, including genetic stability without the risk of carcinogenesis, to transition from clinical experimentation to practical applications [118].
The use of scRNA-seq (single-cell RNA sequencing) to characterize the transcriptional profile and phenotypic state of cells during regeneration can be crucial to solving another problem: understanding whether cholangiocytes undergo direct transdifferentiation or pass through an “intermediate progenitor” state after dedifferentiation [74]. Several recent studies have applied scRNA-seq to organoids to model organ development, tissue regeneration, and diseases. These studies demonstrate that the combination of these two cutting-edge technologies allows for the identification of rare or novel cell types and genetic markers in an organ, opening new perspectives in understanding and applying these technologies [122].
6.3. Challenges in Organoid Clinical Applications: From Bench to Bedside
There are several translational barriers, including regulatory, ethical, and technical challenges, which currently need to be addressed for the translation of organoid technology into clinical applications [130]. These challenges require a multidisciplinary approach among scientists, clinicians, ethicists, and regulatory agencies to develop standardized protocols, ameliorate culture techniques, and establish clear ethical guidelines. Table 3 resumes these multifaceted issues and recommends measures that need to be taken to ensure the clinical application of this technology.

Table 3.
Organoids from bench to bedside: regulatory, ethical, and technical challenges.
The potential of organoid-based approaches to improve patient care and outcomes in diverse disease settings poses regulatory and ethical issues [131]. Regulatory agencies are needed to define and implement guidelines and standards for the use of organoids in research and clinical applications through preclinical studies and clinical trials. Major ethical concerns are related to the use of human tissues or stem cells, development of an informed consent model for organoid donors, protection of donors’ identities and their personal information, and commercialization and patentability of organoids [132]. Moreover, the transplantation of organoids [131], use of gene editing [133], creation of chimeras [134], and long-term storage in biobanks [135,136] raise psychological and ethical concerns in society that should be considered and regulated to allow the bench-to-bedside translation of organoids.
Advances in culture techniques and biomaterials hold promise for overcoming these challenges and unlocking the full potential of organoid research. A major limitation is the maintenance of cellular functionality and viability over time due to culture conditions, such as media composition and growth factors; as the volume of organoids increases, so does the demand for nutrients and oxygen [130,137]. At the same time, the accumulation of toxic metabolites causes cell death and necrosis. The integration of a vascular system into organoids to allow the transport of nutrients and metabolic waste is important for recapitulating normal human physiological conditions and for long-term organoid culture [126,138,139]. This problem can be addressed using bioreactors that improve nutrient supply, and co-culturing organoids with endothelial cells or progenitors can promote vascularization during organoid formation. It has already been observed by Jin et al. [140] that vascularized hepatic organoids cultivated using chip technology have improved intercellular interactions and metabolic activity. It seems that the matrix, such as Matrigel, also influences organoid development, limiting their clinical applications, as it has a non-human origin, variable biochemical properties, and potential contamination risks [141,142,143]. Therefore, significant efforts are underway to develop synthetic matrices or hydrogels that are safer and more effective for organoid culture and their use in preclinical and therapeutic studies.
7. Conclusions
Biliary tract diseases, including primary sclerosing cholangitis, are a major cause of fibrosis, cirrhosis, and, in severe cases, LT. The lack of effective therapies for biliary diseases has led to the evaluation of new therapeutic options in the field of biliary regenerative medicine, which have the potential to radically change our management of these patients [144,145].
Significant advancements in the culture of hepatic and cholangiocyte organoids, along with in-depth regenerative medicine techniques, hold considerable promise for improving the lives of patients with advanced liver diseases. Cholangiocyte organoids represent a formidable technology for better understanding the molecular pathways underlying cholangiopathies and allows for the exploration of personalized therapeutic approaches for each patient and the repair of damaged biliary epithelia.
The application of organoids is still under investigation in humans, and there are still many challenges regarding the adaptation and translation of regenerative concepts and tools into truly regenerative therapies. This includes the development of reproducible methods for the composition and functionality of organoids and the enhancement of cellular maturation to ensure greater resemblance to pathophysiological processes. However, the results obtained to date are promising and represent a potential paradigm shift in the management of acute and chronic liver and biliary diseases [146].
Author Contributions
Conceptualization, S.B. and S.D.T.; methodology, P.G.V., S.B., D.P., C.M., F.F., E.M., L.S. and G.B. (Giuseppe Bianco); writing—original draft preparation, S.B., L.S., P.G.V., D.P., C.M., F.F., G.B. (Giuseppe Bianco) and E.M.; writing—review and editing, S.D.T., G.B. (Giuseppina Basta) and D.G.; project administration, S.D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AIRCS-Italian Association for Sclerosing Cholangitis Research (DSB.AD008.822, GAE P0002886).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
For Literature Search Strategy, We performed a systematic literature search using the PubMed, SCOPUS, Web of Science, Google Scholar, and MedRxiv/BioRxiv (preprints) databases. We included scientific publications and preprint articles and book chapters. We screened all reference lists of the most pertinent studies in order to identify any missing publications. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Act A | Activin A |
| AdSCs | organ-restricted adult stem cells |
| AMAs | anti-mitochondrial antibodies |
| ANAs | specific anti-nuclear antibodies |
| AS | anastomotic stenosis |
| BA | biliary atresia |
| BCOs | organoids and cholangiocyte organoids derived from bile |
| BMP | bone morphogenic protein |
| BTPCs | biliary tree progenitor cells |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| DAMPs | damage-associated molecular patterns |
| DCDs | donors after cardiac death |
| DR | ductular reaction |
| ECM | extracellular matrix |
| ECOs | extrahepatic cholangiocytes |
| EGF | epidermal growth factor |
| ELTR | liver transplant registry |
| ERCP | endoscopic retrograde cholangiopancreatography |
| ESCs | embryonic stem cells |
| FDOs | functional ductal organoids |
| FGF19 | fibroblast growth factor 19 |
| FSK | forskolin |
| GCOs | gallbladder cholangiocyte organoids |
| HGF | hepatocyte growth factor |
| HMP | hypothermic machine perfusion |
| HNF1β | hepatocyte nuclear factor 1β |
| HPC | hepatic progenitor cell |
| IBD | inflammatory bowel disease |
| IC | ischemic cholangiopathy |
| ICOs | intrahepatic cholangiocyte organoids |
| Ig | immunoglobulin |
| IL-6 | interleukin-6 |
| iPSCs | pluripotent stem cells |
| IRI | ischemia-reperfusion injury |
| ISC | IgC4-related sclerosing cholangitis |
| ITBLs | ischemic-type biliary lesions |
| LT | liver transplantation |
| MP | machine perfusion |
| NAS | non-anastomotic stenosis |
| NMP | normothermic machine perfusion |
| norUDCA | 24-norursodeoxycholic acid |
| NOX | NADPH oxidase |
| NRP | normothermic regional perfusion |
| OCA | obeticholic acid |
| OOC | organ on a chip |
| OSM | oncostatin M |
| PBC | primary biliary cholangitis |
| PBGs | peribiliary glands |
| PDGFB | platelet-derived growth factor |
| PSC | primary sclerosing cholangitis |
| ROS | oxygen-containing reactive species |
| S1RPs | signal regulatory proteins |
| scRNA-seq | single-cell RNA sequencing |
| SOX9 | SRY-box transcription factor 9 |
| TGFβ | transforming growth factor beta |
| TNFα | tumor necrosis factor alpha |
| UDCA | ursodeoxycholic acid |
References
- Tam, P.K.H.; Yiu, R.S.; Lendahl, U.; Andersson, E.R. Cholangiopathies—Towards a molecular understanding. EBioMedicine 2018, 35, 381–393. [Google Scholar] [CrossRef]
- Chapman, M.H.; Thorburn, D.; Hirschfield, G.M.; Webster, G.G.J.; Rushbrook, S.M.; Alexander, G.; Collier, J.; Dyson, J.K.; Jones, D.E.; Patanwala, I.; et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019, 68, 1356–1378. [Google Scholar] [CrossRef]
- Tanaka, A.; Mori, M.; Kubota, K.; Naitoh, I.; Nakazawa, T.; Takikawa, H.; Unno, M.; Kamisawa, T.; Kawa, S.; Okazaki, K. Epidemiological features of immunoglobulin G4-related sclerosing cholangitis in Japan. J. Hepatobiliary Pancreat. Sci. 2020, 27, 598–603. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Fiorotto, R.; Cadamuro, M.; Spirli, C.; Mariotti, V.; Kaffe, E.; Scirpo, R.; Fabris, L. Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Deeb, M.; Karlsen, T.H.; Hirschfield, G.M. The 6 C’s of primary sclerosing cholangitis. J. Hepatol. 2020, 73, 1255–1256. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Kenigsberg, E.; Jaitin, D.A.; David, E.; Paul, F.; Tanay, A.; Amit, I. MARS-seq2.0: An experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing. Nat. Protoc. 2019, 14, 1841–1862. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; Muraro, D.; Tysoe, O.C.; Sawiak, S.; Beach, T.E.; Godfrey, E.M.; Upponi, S.S.; Brevini, T.; Wesley, B.T.; Garcia-Bernardo, J.; et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 2021, 371, 839–846. [Google Scholar] [CrossRef]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Masyuk, A.I.; Masyuk, T.V.; O’Hara, S.P.; LaRusso, N.F. Physiology of cholangiocytes. Compr. Physiol. 2013, 3, 541–565. [Google Scholar]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Bogert, P.T.; LaRusso, N.F. Cholangiocyte biology. Curr. Opin. Gastroenterol. 2007, 23, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, Y.; Harada, K.; Nakanuma, Y. Canals of Hering loss relates to the progression of the histological stages of primary biliary cirrhosis. J. Clin. Pathol. 2015, 68, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.C.; Ng, I.O. Hepatic progenitor cells: Their role and functional significance in the new classification of primary liver cancers. Liver Cancer 2013, 2, 84–92. [Google Scholar]
- Arias, I.M.; Alter, H.J.; Boyer, J.L.; Cohen, D.E.; Shafritz, D.A.; Thorgeirsson, S.S.; Wolkoff, A.W. The Liver: Biology and Pathobiology, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; p. 1144. [Google Scholar]
- Sell, S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 2001, 33, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Strazzabosco, M.; Fabris, L. Functional anatomy of normal bile ducts. Anat. Rec. 2008, 291, 653–660. [Google Scholar] [CrossRef]
- Petroni, D.; Fabbri, C.; Babboni, S.; Menichetti, L.; Basta, G.; Del Turco, S. Extracellular Vesicles and Intercellular Communication: Challenges for In Vivo Molecular Imaging and Tracking. Pharmaceutics 2023, 15, 1639. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.S.; Lim, W.T.; Choi, H.S. Biology of Cholangiocytes: From Bench to Bedside. Gut Liver 2016, 10, 687–698. [Google Scholar] [CrossRef]
- Yang, F.; Gaudio, E.; Onori, P.; Wise, C.; Alpini, G.; Glaser, S.S. Mechanisms of Biliary Damage. J. Cell Death 2010, 3, 13–21. [Google Scholar] [CrossRef]
- Xia, X.; Demorrow, S.; Francis, H.; Glaser, S.; Alpini, G.; Marzioni, M.; Fava, G.; Lesage, G. Cholangiocyte injury and ductopenic syndromes. Semin. Liver Dis. 2007, 27, 401–412. [Google Scholar] [CrossRef]
- Lillemoe, K.D. Current management of bile duct injury. Br. J. Surg. 2008, 95, 403–405. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Tabibian, J.H.; Splinter, P.L.; LaRusso, N.F. The dynamic biliary epithelia: Molecules, pathways, and disease. J. Hepatol. 2013, 58, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Glaser, S.; Kennedy, L.; Liangpunsakul, S.; Meng, F.; Francis, H.; Alpini, G. Preclinical insights into cholangiopathies: Disease modeling and emerging therapeutic targets. Expert Opin. Ther. Targets 2019, 23, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Kawakami, H.; Kim, J.H. IgG4-related sclerosing cholangitis: All we need to know. J. Gastroenterol. 2016, 51, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.N.; Strazzabosco, M.; Larusso, N.F. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology 2004, 127, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Karlsen, T.H.; LaRusso, N.F. Cholangiocytes and the environment in primary sclerosing cholangitis: Where is the link? Gut 2017, 66, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Prokopic, M.; Beuers, U. Management of primary sclerosing cholangitis and its complications: An algorithmic approach. Hepatol. Int. 2021, 15, 6–20. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis—A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef] [PubMed]
- Manganis, C.D.; Chapman, R.W.; Culver, E.L. Review of primary sclerosing cholangitis with increased IgG4 levels. World J. Gastroenterol. 2020, 26, 3126–3144. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Melandro, F.; Torri, F.; Martinelli, C.; Cappello, V.; Babboni, S.; Silvestrini, B.; De Simone, P.; Basta, G.; Del Turco, S. Extended criteria grafts and emerging therapeutics strategy in liver transplantation. The unstable balance between damage and repair. Transplant. Rev. 2021, 35, 100639. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, P.; Donati, F.; Pacciardi, F.; Ghinolfi, D.; Falaschi, F. Biliary complications after liver transplantation: Assessment with MR cholangiopancreatography and MR imaging at 3T device. Eur. J. Radiol. 2018, 106, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Cantu, P.; Parzanese, I.; Balassone, V.; Di Sario, A.; Soggiu, F.; Lombardi, G.; Barbaro, F.; Pisani, A.; Baldan, A.; Cariani, G.; et al. Management of biliary anastomotic strictures after liver transplantation (BASALT study): A nationwide Italian survey. Liver Transpl. 2017, 23, 257–261. [Google Scholar] [CrossRef]
- Buis, C.I.; Hoekstra, H.; Verdonk, R.C.; Porte, R.J. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J. Hepatobiliary Pancreat. Surg. 2006, 13, 517–524. [Google Scholar] [CrossRef]
- Biancofiore, G.; Bindi, M.; Ghinolfi, D.; Lai, Q.; Bisa, M.; Esposito, M.; Meacci, L.; Mozzo, R.; Spelta, A.; Filipponi, F. Octogenarian donors in liver transplantation grant an equivalent perioperative course to ideal young donors. Dig. Liver Dis. 2017, 49, 676–682. [Google Scholar] [CrossRef]
- Pezzati, D.; Ghinolfi, D.; Balzano, E.; De Simone, P.; Coletti, L.; Roffi, N.; Rreka, E.; Meacci, L.; Campani, D.; Mazzoni, A.; et al. Salvage of an Octogenarian Liver Graft Using Normothermic Perfusion: A Case Report. Transplant. Proc. 2017, 49, 726–728. [Google Scholar] [CrossRef]
- Buis, C.I.; Verdonk, R.C.; Van der Jagt, E.J.; van der Hilst, C.S.; Slooff, M.J.; Haagsma, E.B.; Porte, R.J. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007, 13, 708–718. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; Gastaca, M.; Minambres, E.; Colmenero, J.; Fondevila, C.; in representation of the SETH Working Group on DCD. Donation after circulatory death liver transplantation: Consensus statements from the Spanish Liver Transplantation Society. Transpl. Int. 2020, 33, 902–916. [Google Scholar] [CrossRef]
- de Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef]
- Schlegel, A.; Mergental, H.; Fondevila, C.; Porte, R.J.; Friend, P.J.; Dutkowski, P. Machine perfusion of the liver and bioengineering. J. Hepatol. 2023, 78, 1181–1198. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef]
- Machado, I.F.; Palmeira, C.M.; Rolo, A.P. Preservation of Mitochondrial Health in Liver Ischemia/Reperfusion Injury. Biomedicines 2023, 11, 948. [Google Scholar] [CrossRef]
- Rampes, S.; Ma, D. Hepatic ischemia-reperfusion injury in liver transplant setting: Mechanisms and protective strategies. J. Biomed. Res. 2019, 33, 221–234. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Tincani, G.; Rreka, E.; Roffi, N.; Coletti, L.; Balzano, E.; Catalano, G.; Meli, S.; Carrai, P.; Petruccelli, S.; et al. Dual aortic and portal perfusion at procurement prevents ischaemic-type biliary lesions in liver transplantation when using octogenarian donors: A retrospective cohort study. Transpl. Int. 2019, 32, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, M.; Croome, K.P.; Hernandez-Alejandro, R.; Pirenne, J.; Cortes-Cerisuelo, M.; Minambres, E.; Abt, P.L. Donor Warm Ischemia Time in DCD Liver Transplantation-Working Group Report from the ILTS DCD, Liver Preservation, and Machine Perfusion Consensus Conference. Transplantation 2021, 105, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Nikeghbalian, S.; Shamsaeefar, A.; Kazemi, K.; Fattahi, M.R.; Malek-Hosseini, S.A. Hepatic steatosis and liver fat contents in liver transplant recipients are associated with serum adipokines and insulin resistance. Sci. Rep. 2020, 10, 12701. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Dutkowski, P. Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. Int. J. Mol. Sci. 2018, 19, 3567. [Google Scholar] [CrossRef]
- Liu, Q.; Nassar, A.; Farias, K.; Buccini, L.; Baldwin, W.; Mangino, M.; Bennett, A.; O’Rourke, C.; Okamoto, T.; Uso, T.D.; et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014, 20, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Jassem, W.; Mergental, H.; Heaton, N.; Mirza, D.; Perera, M.T.; Quaglia, A.; Holroyd, D.; Vogel, T.; Coussios, C.C.; et al. Liver Transplantation after Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transpl. 2016, 16, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- de Jong, I.E.M.; Bodewes, S.B.; van Leeuwen, O.B.; Oosterhuis, D.; Lantinga, V.A.; Thorne, A.M.; Lascaris, B.; van den Heuvel, M.C.; Wells, R.G.; Olinga, P.; et al. Restoration of Bile Duct Injury of Donor Livers during Ex Situ Normothermic Machine Perfusion. Transplantation 2023, 107, e161–e172. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, S.; Cappello, V.; Tapeinos, C.; Moscardini, A.; Sabatino, L.; Battaglini, M.; Melandro, F.; Torri, F.; Martinelli, C.; Babboni, S.; et al. Cerium oxide nanoparticles administration during machine perfusion of discarded human livers: A pilot study. Liver Transpl. 2022, 28, 1173–1185. [Google Scholar]
- Bonaccorsi-Riani, E.; Gillooly, A.R.; Iesari, S.; Bruggenwirth, I.M.A.; Ferguson, C.M.; Komuta, M.; Xhema, D.; Daumerie, A.; Maistriaux, L.; Leuvenink, H.; et al. Delivering siRNA Compounds During HOPE to Modulate Organ Function: A Proof-of-concept Study in a Rat Liver Transplant Model. Transplantation 2022, 106, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, A.R.; Perry, J.; Martins, P.N. First Report of siRNA Uptake (for RNA Interference) during Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation 2019, 103, e56–e57. [Google Scholar] [CrossRef]
- Woud, W.W.; Arykbaeva, A.S.; Alwayn, I.P.J.; Baan, C.C.; Minnee, R.C.; Hoogduijn, M.J.; Boer, K. Extracellular Vesicles Released during Normothermic Machine Perfusion Are Associated with Human Donor Kidney Characteristics. Transplantation 2022, 106, 2360–2369. [Google Scholar] [CrossRef]
- Willemse, J.; van der Laan, L.J.W.; de Jonge, J.; Verstegen, M.M.A. Design by Nature: Emerging Applications of Native Liver Extracellular Matrix for Cholangiocyte Organoid-Based Regenerative Medicine. Bioengineering 2022, 9, 110. [Google Scholar] [CrossRef]
- Ghinolfi, D.; De Simone, P.; Lai, Q.; Pezzati, D.; Coletti, L.; Balzano, E.; Arenga, G.; Carrai, P.; Grande, G.; Pollina, L.; et al. Risk analysis of ischemic-type biliary lesions after liver transplant using octogenarian donors. Liver Transpl. 2016, 22, 588–598. [Google Scholar] [CrossRef]
- Lleo, A.; Leung, P.S.C.; Hirschfield, G.M.; Gershwin, E.M. The Pathogenesis of Primary Biliary Cholangitis: A Comprehensive Review. Semin Liver Dis. 2020, 40, 34–48. [Google Scholar] [CrossRef]
- Antoniou, A.; Raynaud, P.; Cordi, S.; Zong, Y.; Tronche, F.; Stanger, B.Z.; Jacquemin, P.; Pierreux, C.E.; Clotman, F.; Lemaigre, F.P. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 2009, 136, 2325–2333. [Google Scholar] [CrossRef]
- Manco, R.; Clerbaux, L.A.; Verhulst, S.; Bou Nader, M.; Sempoux, C.; Ambroise, J.; Bearzatto, B.; Gala, J.L.; Horsmans, Y.; van Grunsven, L.; et al. Reactive cholangiocytes differentiate into proliferative hepatocytes with efficient DNA repair in mice with chronic liver injury. J. Hepatol. 2019, 70, 1180–1191. [Google Scholar] [CrossRef]
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1390–1400. [Google Scholar] [CrossRef]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef]
- Gouw, A.S.; Clouston, A.D.; Theise, N.D. Ductular reactions in human liver: Diversity at the interface. Hepatology 2011, 54, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, H.R.; Hantelys, F.; Gracz, A.D. Panic at the Bile Duct: How Intrahepatic Cholangiocytes Respond to Stress and Injury. Am. J. Pathol. 2023, 193, 1440–1454. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Lu, W.Y. Liver stem cells: Plasticity of the liver epithelium. World J. Gastroenterol. 2019, 25, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Jiang, J.X.; Xia, S.; Yang, D.; Ding, A.; Laselva, O.; Hernandez, M.; Cui, C.; Higuchi, Y.; Suemizu, H.; et al. Generation of functional ciliated cholangiocytes from human pluripotent stem cells. Nat. Commun. 2021, 12, 6504. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Cardinale, V.; Onori, P.; Franchitto, A.; Berloco, P.B.; Rossi, M.; Wang, Y.; Semeraro, R.; Anceschi, M.; Brunelli, R.; et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: An anatomical in situ study yielding evidence of maturational lineages. J. Anat. 2012, 220, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Zhong, A.; Short, C.; Xu, J.; Fernandez, G.E.; Malkoff, N.; Noriega, N.; Yeo, T.; Wang, L.; Mavila, N.; Asahina, K.; et al. Prominin-1 promotes restitution of the murine extrahepatic biliary luminal epithelium following cholestatic liver injury. Hepatol. Commun. 2023, 7, e0018. [Google Scholar] [CrossRef] [PubMed]
- Overi, D.; Carpino, G.; Cardinale, V.; Franchitto, A.; Safarikia, S.; Onori, P.; Alvaro, D.; Gaudio, E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int. J. Mol. Sci. 2018, 19, 2917. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Aleksieva, N.; Forbes, S.J. Epithelial Plasticity during Liver Injury and Regeneration. Cell. Stem Cell. 2020, 27, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, Y.; Han, C.; Zhang, Y.; Liu, Q.; Wei, W.; Ma, Y. The pathogenesis, models and therapeutic advances of primary biliary cholangitis. Biomed. Pharmacother 2021, 140, 111754. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kim, W.R. Natural History of Primary Biliary Cholangitis in the Ursodeoxycholic Acid Era: Role of Scoring Systems. Clin. Liver Dis. 2018, 22, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Trivella, J.; John, B.V.; Levy, C. Primary biliary cholangitis: Epidemiology, prognosis, and treatment. Hepatol. Commun. 2023, 7, e0179. [Google Scholar] [CrossRef] [PubMed]
- Gidwaney, N.G.; Pawa, S.; Das, K.M. Pathogenesis and clinical spectrum of primary sclerosing cholangitis. World J. Gastroenterol. 2017, 23, 2459–2469. [Google Scholar] [CrossRef]
- Vesterhus, M.; Karlsen, T.H. Emerging therapies in primary sclerosing cholangitis: Pathophysiological basis and clinical opportunities. J. Gastroenterol. 2020, 55, 588–614. [Google Scholar] [CrossRef]
- Chen, H.; Han, Z.; Fan, Y.; Chen, L.; Peng, F.; Cheng, X.; Wang, Y.; Su, J.; Li, D. CD4+ T-cell subsets in autoimmune hepatitis: A review. Hepatol. Commun. 2023, 7, e0269. [Google Scholar] [CrossRef]
- Keitel, V.; Droge, C.; Haussinger, D. Targeting FXR in Cholestasis. Handb. Exp. Pharmacol. 2019, 256, 299–324. [Google Scholar]
- Cazzagon, N.; Floreani, A. Primary biliary cholangitis: Treatment. Curr. Opin. Gastroenterol. 2021, 37, 99–104. [Google Scholar] [CrossRef]
- Halilbasic, E.; Fiorotto, R.; Fickert, P.; Marschall, H.U.; Moustafa, T.; Spirli, C.; Fuchsbichler, A.; Gumhold, J.; Silbert, D.; Zatloukal, K.; et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology 2009, 49, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in Liver Diseases: A Review of Their Mechanisms of Action and Clinical Applications. Handb. Exp. Pharmacol. 2019, 256, 237–264. [Google Scholar] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; Consortium, H.P.B.O.; Gehart, H.; de Koning, E.; Lemaigre, F.; Forbes, S.J.; Peng, W.C.; Huch, M.; et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell. Stem Cell. 2021, 28, 816–832. [Google Scholar] [CrossRef]
- Tysoe, O.C.; Justin, A.W.; Brevini, T.; Chen, S.E.; Mahbubani, K.T.; Frank, A.K.; Zedira, H.; Melum, E.; Saeb-Parsy, K.; Markaki, A.E.; et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 2019, 14, 1884–1925. [Google Scholar] [CrossRef] [PubMed]
- Roos, F.J.M.; Wu, H.; Willemse, J.; Lieshout, R.; Albarinos, L.A.M.; Kan, Y.Y.; Poley, J.W.; Bruno, M.J.; de Jonge, J.; Bartfai, R.; et al. Cholangiocyte organoids from human bile retain a local phenotype and can repopulate bile ducts in vitro. Clin. Transl. Med. 2021, 11, e566. [Google Scholar] [CrossRef]
- Gunther, C.; Brevini, T.; Sampaziotis, F.; Neurath, M.F. What gastroenterologists and hepatologists should know about organoids in 2019. Dig. Liver Dis. 2019, 51, 753–760. [Google Scholar] [CrossRef]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef]
- Takayama, K.; Mitani, S.; Nagamoto, Y.; Sakurai, F.; Tachibana, M.; Taniguchi, Y.; Sekiguchi, K.; Mizuguchi, H. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2016, 474, 91–96. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef]
- Ogawa, M.; Ogawa, S.; Bear, C.E.; Ahmadi, S.; Chin, S.; Li, B.; Grompe, M.; Keller, G.; Kamath, B.M.; Ghanekar, A. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 853–861. [Google Scholar] [CrossRef]
- Jalan-Sakrikar, N.; De Assuncao, T.M.; Navarro-Corcuera, A.; Hamdan, F.H.; Loarca, L.; Kirkeby, L.A.; Resch, Z.T.; O’Hara, S.P.; Juran, B.D.; Lazaridis, K.N.; et al. Induced Pluripotent Stem Cells From Subjects with Primary Sclerosing Cholangitis Develop a Senescence Phenotype Following Biliary Differentiation. Hepatol. Commun. 2022, 6, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; de Brito, M.C.; Madrigal, P.; Bertero, A.; Saeb-Parsy, K.; Soares, F.A.C.; Schrumpf, E.; Melum, E.; Karlsen, T.H.; Bradley, J.A.; et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 2015, 33, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Unzu, C.; Planet, E.; Brandenberg, N.; Fusil, F.; Cassano, M.; Perez-Vargas, J.; Friedli, M.; Cosset, F.L.; Lutolf, M.P.; Wildhaber, B.E.; et al. Pharmacological Induction of a Progenitor State for the Efficient Expansion of Primary Human Hepatocytes. Hepatology 2019, 69, 2214–2231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Faria, J.; Penning, L.C.; Masereeuw, R.; Spee, B. Tissue-Engineered Bile Ducts for Disease Modeling and Therapy. Tissue Eng. Part C Methods 2021, 27, 59–76. [Google Scholar] [CrossRef]
- Roos, F.J.M.; Verstegen, M.M.A.; Munoz Albarinos, L.; Roest, H.P.; Poley, J.W.; Tetteroo, G.W.M.; IJzermans, J.N.M.; van der Laan, L.J.W. Human Bile Contains Cholangiocyte Organoid-Initiating Cells Which Expand as Functional Cholangiocytes in Non-canonical Wnt Stimulating Conditions. Front. Cell. Dev. Biol. 2020, 8, 630492. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Gieseck, R.L., 3rd; de Brito, M.C.; Berntsen, N.L.; Gomez-Vazquez, M.J.; et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Nuciforo, S.; Heim, M.H. Organoids to model liver disease. JHEP Rep. 2021, 3, 100198. [Google Scholar] [CrossRef] [PubMed]
- Shiota, J.; Samuelson, L.C.; Razumilava, N. Hepatobiliary Organoids and Their Applications for Studies of Liver Health and Disease: Are We There Yet? Hepatology 2021, 74, 2251–2263. [Google Scholar] [CrossRef]
- Jiang, X.; Karlsen, T.H. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 279–295. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Trussoni, C.E.; Krishnan, A.; Bronk, S.F.; Lorenzo Pisarello, M.J.; O’Hara, S.P.; Splinter, P.L.; Gao, Y.; Vig, P.; Revzin, A.; et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol. 2018, 69, 676–686. [Google Scholar] [CrossRef]
- Soroka, C.J.; Assis, D.N.; Alrabadi, L.S.; Roberts, S.; Cusack, L.; Jaffe, A.B.; Boyer, J.L. Bile-Derived Organoids From Patients with Primary Sclerosing Cholangitis Recapitulate Their Inflammatory Immune Profile. Hepatology 2019, 70, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, S.; Yang, H.; Liang, X.; Yao, H.; Guo, B.; Chen, D.; Jiang, J.; Shi, D.; Xin, J.; et al. Generation and metabolomic characterization of functional ductal organoids with biliary tree networks in decellularized liver scaffolds. Bioact. Mater. 2023, 26, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.C.; Tao, Y.; Proenca, S.; van Steenbeek, F.G.; Samsom, R.A.; Nijmeijer, S.M.; Sinnige, T.; van der Laan, L.J.W.; Legler, J.; Schneeberger, K.; et al. Drug Metabolism of Hepatocyte-like Organoids and Their Applicability in In Vitro Toxicity Testing. Molecules 2023, 28, 621. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef]
- Shi, S.; Verstegen, M.M.A.; Roest, H.P.; Ardisasmita, A.I.; Cao, W.; Roos, F.J.M.; de Ruiter, P.E.; Niemeijer, M.; Pan, Q.; IJzermans, J.N.M.; et al. Recapitulating Cholangiopathy-Associated Necroptotic Cell Death In Vitro Using Human Cholangiocyte Organoids. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 541–564. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Sellgren, K.L.; Butala, E.J.; Gilmour, B.P.; Randell, S.H.; Grego, S. A biomimetic multicellular model of the airways using primary human cells. Lab. Chip. 2014, 14, 3349–3358. [Google Scholar] [CrossRef]
- Esch, M.B.; Mahler, G.J.; Stokol, T.; Shuler, M.L. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab. Chip. 2014, 14, 3081–3092. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hubner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab. Chip. 2015, 15, 2688–2699. [Google Scholar] [CrossRef]
- Zheng, F.; Xiao, Y.; Liu, H.; Fan, Y.; Dao, M. Patient-Specific Organoid and Organ-on-a-Chip: 3D Cell-Culture Meets 3D Printing and Numerical Simulation. Adv. Biol. 2021, 5, e2000024. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, L.; Chen, Y.; Li, H.; Huang, M.; Dai, Z.; Wang, J.; Xiang, D.; Fu, G.; Lei, Z.; et al. Organoids and organs-on-chips: Insights into predicting the efficacy of systemic treatment in colorectal cancer. Cell. Death Discov. 2023, 9, 72. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e1916. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; de Groot, M.H.; Zhang, C.; Mosa, M.H.; Michels, B.E.; Roder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019, 38, e100928. [Google Scholar] [CrossRef]
- Brevini, T.; Tysoe, O.C.; Sampaziotis, F. Tissue engineering of the biliary tract and modelling of cholestatic disorders. J. Hepatol. 2020, 73, 918–932. [Google Scholar] [CrossRef]
- Jalan-Sakrikar, N.; Brevini, T.; Huebert, R.C.; Sampaziotis, F. Organoids and regenerative hepatology. Hepatology 2023, 77, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal. Transduct. Target Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- De Assuncao, T.M.; Jalan-Sakrikar, N.; Huebert, R.C. Regenerative Medicine and the Biliary Tree. Semin Liver Dis. 2017, 37, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ten Dam, M.J.M.; Frederix, G.W.J.; Ten Ham, R.M.T.; van der Laan, L.J.W.; Schneeberger, K. Toward Transplantation of Liver Organoids: From Biology and Ethics to Cost-effective Therapy. Transplantation 2023, 107, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, P.Y.; Shi, Y.; Li, P. Single-Cell Sequencing and Organoids: A Powerful Combination for Modelling Organ Development and Diseases. Rev. Physiol. Biochem. Pharmacol. 2021, 179, 189–210. [Google Scholar]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e1519. [Google Scholar] [CrossRef]
- Rashidi, H.; Luu, N.T.; Alwahsh, S.M.; Ginai, M.; Alhaque, S.; Dong, H.; Tomaz, R.A.; Vernay, B.; Vigneswara, V.; Hallett, J.M.; et al. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch. Toxicol. 2018, 92, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Roest, H.P.; van den Bosch, T.P.P.; Bijvelds, M.J.C.; Boehnert, M.U.; de Jonge, J.; Dekker, S.O.; de Vries, A.A.F.; de Jonge, H.R.; Verstegen, M.M.A.; et al. Modeling bile duct ischemia and reoxygenation injury in human cholangiocyte organoids for screening of novel cholangio-protective agents. EBioMedicine 2023, 88, 104431. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Przepiorski, A.; Sander, V.; Tran, T.; Hollywood, J.A.; Sorrenson, B.; Shih, J.H.; Wolvetang, E.J.; McMahon, A.P.; Holm, T.M.; Davidson, A.J. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell. Rep. 2018, 11, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Rezakhani, S.; Yildiz, E.; Nuciforo, S.; Heim, M.H.; Lutolf, M.P.; Schoonjans, K. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 2020, 11, 3416. [Google Scholar] [CrossRef]
- Obeid, D.A.; Mir, T.A.; Alzhrani, A.; Altuhami, A.; Shamma, T.; Ahmed, S.; Kazmi, S.; Fujitsuka, I.; Ikhlaq, M.; Shabab, M.; et al. Using Liver Organoids as Models to Study the Pathobiology of Rare Liver Diseases. Biomedicines 2024, 12, 446. [Google Scholar] [CrossRef]
- Mollaki, V. Ethical Challenges in Organoid Use. BioTech 2021, 10, 12. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; the VANGUARD Consortium; Bunnik, E.M. Organoids: A systematic review of ethical issues. Stem Cell. Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef]
- Driehuis, E.; Clevers, H. CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G257–G265. [Google Scholar] [CrossRef]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell. Stem Cell. 2013, 12, 342–353. [Google Scholar] [CrossRef]
- Boers, S.N.; van Delden, J.J.; Clevers, H.; Bredenoord, A.L. Organoid biobanking: Identifying the ethics: Organoids revive old and raise new ethical challenges for basic research and therapeutic use. EMBO Rep. 2016, 17, 938–941. [Google Scholar] [CrossRef]
- Lensink, M.A.; Boers, S.N.; Jongsma, K.R.; Carter, S.E.; van der Ent, C.K.; Bredenoord, A.L. Organoids for personalized treatment of Cystic Fibrosis: Professional perspectives on the ethics and governance of organoid biobanking. J. Cyst. Fibros. 2021, 20, 443–451. [Google Scholar] [CrossRef]
- He, J.; Cui, H.; Shi, X.; Jin, Q.; Han, X.; Han, T.; Peng, J.; Guo, S.; Zhang, L.; Zhao, Y.; et al. Functional hepatobiliary organoids recapitulate liver development and reveal essential drivers of hepatobiliary cell fate determination. Life Med. 2022, 1, 345–358. [Google Scholar] [CrossRef]
- Eltaher, H.M.; Abukunna, F.E.; Ruiz-Cantu, L.; Stone, Z.; Yang, J.; Dixon, J.E. Human-scale tissues with patterned vascular networks by additive manufacturing of sacrificial sugar-protein composites. Acta Biomater. 2020, 113, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Kinstlinger, I.S.; Saxton, S.H.; Calderon, G.A.; Ruiz, K.V.; Yalacki, D.R.; Deme, P.R.; Rosenkrantz, J.E.; Louis-Rosenberg, J.D.; Johansson, F.; Janson, K.D.; et al. Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates. Nat. Biomed. Eng. 2020, 4, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, J.; Lee, J.S.; Min, S.; Kim, S.; Ahn, D.-H.; Kim, Y.-G.; Cho, S.-W. Vascularized Liver Organoids Generated Using Induced Hepatic Tissue and Dynamic Liver-specific Microenvironment as a Drug Testing Platform. Adv. Funct. Mater. 2018, 28, 1801954. [Google Scholar] [CrossRef]
- Hipwood, L.; Clegg, J.; Weekes, A.; Davern, J.W.; Dargaville, T.R.; Meinert, C.; Bock, N. Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture. Gels 2022, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Monferrer, E.; Dobre, O.; Trujillo, S.; Gonzalez Oliva, M.A.; Trubert-Paneli, A.; Acevedo-Leon, D.; Noguera, R.; Salmeron-Sanchez, M. Vitronectin-based hydrogels recapitulate neuroblastoma growth conditions. Front. Cell. Dev. Biol. 2022, 10, 988699. [Google Scholar] [CrossRef] [PubMed]
- Willemse, J.; van Tienderen, G.; van Hengel, E.; Schurink, I.; van der Ven, D.; Kan, Y.; de Ruiter, P.; Rosmark, O.; Westergren-Thorsson, G.G.; Schneeberger, K.; et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 2022, 284, 121473. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Dadgar, N.; Vaidya, A.; Simon, R.; Fulmer, C.; Siddiki, H.; Narayanan Menon, K.V.; Liu, P.; Matthew Walsh, R. Mesenchymal stem cells: A novel treatment option for primary sclerosing cholangitis. Cell. Biol. Int. 2023, 47, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Ogoke, O.; Maloy, M.; Parashurama, N. The science and engineering of stem cell-derived organoids-examples from hepatic, biliary, and pancreatic tissues. Biol. Rev. Camb. Philos. Soc. 2021, 96, 179–204. [Google Scholar] [CrossRef]
- Caiazza, C.; Parisi, S.; Caiazzo, M. Liver Organoids: Updates on Disease Modeling and Biomedical Applications. Biology 2021, 10, 835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).