Use of Fluorescence Imaging in Liver Transplant Surgery
Abstract
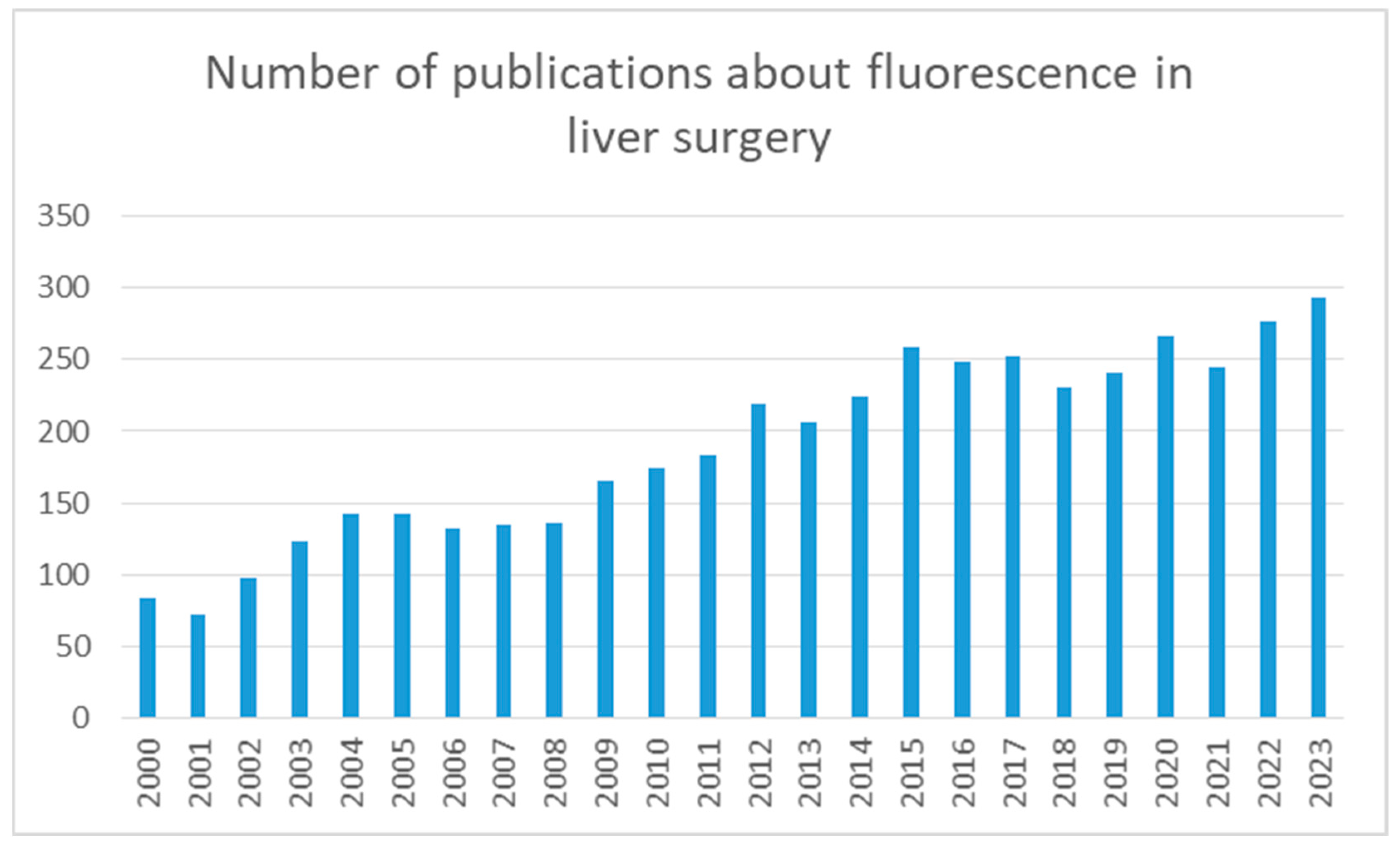
1. Introduction
2. ICG in Hepatobiliary Surgery
3. Liver Transplantation
3.1. ICG Clearance
3.2. ICG Fluorescence
4. Future Perspectives
5. Conclusions
Funding
Conflicts of Interest
References
- Dai, B.; Guissi, N.E.I.; Sulyok, L.F.; Bryski, M.G.; Wang, Y.; Wang, D.; Singhal, S.; Cai, H. Advantages of using indocyanine green in liver transplantation: A narrative review. Ann. Transl. Med. 2022, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Panaro, F.; Benedetti, E.; Pineton de Chambrun, G.; Habibeh, H.; Leon, P.; Bouyabrine, H.; Herrero, A.; Navarro, F. Indocyanine green fluorescence angiography during liver and pancreas transplantation: A tool to integrate perfusion statement’s evaluation. Hepatobiliary Surg. Nutr. 2018, 7, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.S.; Ly, M.; Liu, K.; Majumdar, A.; McCaughan, G.; Crawford, M.; Pulitano, C. Current and Potential Applications for Indocyanine Green in Liver Transplantation. Transplantation 2022, 106, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Tygstrup, N. Determination of hepatic blood flow in man by cardio green. Scand. J. Clin. Lab. Investig. 1960, 12, 353–356. [Google Scholar] [CrossRef]
- Indocyanine Green Kit. PULSION. 2010. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15096 (accessed on 9 April 2024).
- Engel, E.; Schraml, R.; Maisch, T.; Kobuch, K.; König, B.; Szeimies, R.-M.; Hillenkamp, J.; Bäumler, W.; Vasold, R. Light-induced decomposition of indocyanine green. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, S.K.; Lim, J.; Lee, J.M.; Cho, J.H.; Choi, Y.; Yi, N.J.; Lee, K.W.; Suh, K.S. Demarcating the Exact Midplane of the Liver Using Indocyanine Green Near-Infrared Fluorescence Imaging During Laparoscopic Donor Hepatectomy. Liver Transpl. 2021, 27, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Schols, R.M.; Dip, F.; Lo Menzo, E.; Haddock, N.T.; Landin, L.; Lee, B.T.; Malagón, P.; Masia, J.; Mathes, D.W.; Nahabedian, M.Y.; et al. Delphi survey of intercontinental experts to identify areas of consensus on the use of indocyanine green angiography for tissue perfusion assessment during plastic and reconstructive surgery. Surgery 2022, 172, S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Global Laparoscopy and Endoscopy Devices Market: Focus on Surgical Procedures (Cholecystectomy and Hysterectomy) and Product Types (Arthroscopes, Neuroendoscopes, Cystoscope, and Bronchoscopes)—Analysis and Forecast, 2018–2025. Available online: https://www.prnewswire.com/news-releases/global-laparoscopy-and-endoscopy-devices-market-focus-on-surgical-procedures-cholecystectomy-and-hysterectomy-and-product-types-arthroscopes-neuroendoscopes-cystoscope-and-bronchoscopes---analysis-and-forecast-2018-2025-300714922.html (accessed on 11 April 2024).
- Wang, X.; Teh, C.S.C.; Ishizawa, T.; Aoki, T.; Cavallucci, D.; Lee, S.Y.; Panganiban, K.M.; Perini, M.V.; Shah, S.R.; Wang, H.; et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg. 2021, 274, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, L.S.F.; Handgraaf, H.J.M.; Huurman, V.A.L.; Lam, H.D.; Mieog, J.S.D.; van der Made, W.J.; van de Velde, C.J.H.; Vahrmeijer, A.L. The Best Approach for Laparoscopic Fluorescence Cholangiography: Overview of the Literature and Optimization of Dose and Dosing Time. Surg Innov. 2017, 24, 386–396. [Google Scholar] [CrossRef]
- Symeonidis, S.; Mantzoros, I.; Anestiadou, E.; Ioannidis, O.; Christidis, P.; Bitsianis, S.; Zapsalis, K.; Karastergiou, T.; Athanasiou, D.; Apostolidis, S.; et al. Biliary Anatomy Visualization and Surgeon Satisfaction Using Standard Cholangiography versus Indocyanine Green Fluorescent Cholangiography during Elective Laparoscopic Cholecystectomy: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 864. [Google Scholar] [CrossRef]
- Ishizawa, T.; Bandai, Y.; Kokudo, N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: An initial experience. Arch Surg. 2009, 144, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Osayi, S.N.; Wendling, M.R.; Drosdeck, J.M.; Chaudhry, U.I.; Perry, K.A.; Noria, S.F.; Narula, V.K.; Mikami, D.J.; Needleman, B.J.; Muscarell, P., II; et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg. Endosc. 2015, 29, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Ishizawa, T.; Tani, K.; Harada, N.; Ichida, A.; Shimizu, A.; Aoki, T.; Sakamoto, Y.; Sugawara, Y.; Hasegawa, K.; et al. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg. Endosc. 2014, 28, 2504–2508. [Google Scholar] [CrossRef]
- Rossi, G.; Tarasconi, A.; Baiocchi, G.; De’ Angelis, G.L.; Gaiani, F.; Di Mario, F.; Catena, F.; Dalla Valle, R. Fluorescence guided surgery in liver tumors: Applications and advantages. Acta Biomed. 2018, 89, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, M.; Ishizawa, T.; Mise, Y.; Inoue, Y.; Ito, H.; Takahashi, Y.; Saiura, A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017, 31, 5111–5118. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, G.; Barabino, M.; Santambrogio, R.; Lecchi, F.; Di Gioia, G.; Opocher, E.; Bianchi, P.P. Correlation Between Indocyanine Green Fluorescence Patterns and Grade of Differentiation of Hepatocellular Carcinoma: A Western Prospective Cohort Study. Surg. Innov. 2023, 30, 770–778. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef]
- Ishizawa, T.; Masuda, K.; Urano, Y.; Kawaguchi, Y.; Satou, S.; Kaneko, J.; Hasegawa, K.; Shibahara, J.; Fukayama, M.; Tsuji, S.; et al. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 440–448. [Google Scholar] [CrossRef]
- Morita, Y.; Sakaguchi, T.; Unno, N.; Shibasaki, Y.; Suzuki, A.; Fukumoto, K.; Inaba, K.; Baba, S.; Takehara, Y.; Suzuki, S.; et al. Detection of hepatocellular carcinomas with near-infrared fluorescence imaging using indocyanine green: Its usefulness and limitation. Int. J. Clin. Oncol. 2013, 18, 232–2341. [Google Scholar] [CrossRef]
- Ishizawa, T.; Zuker, N.B.; Kokudo, N.; Gayet, B. Positive and negative staining of hepatic segments by use of fluorescent imaging techniques during laparoscopic hepatectomy. Arch Surg. 2012, 147, 393–394. [Google Scholar] [CrossRef]
- Suh, K.S.; Hong, S.K.; Lee, S.; Hong, S.Y.; Suh, S.; Han, E.S.; Yang, S.M.; Choi, Y.; Yi, N.J.; Lee, K.W. Pure laparoscopic living donor liver transplantation: Dreams come true. Am. J. Transplant. 2022, 22, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse reactions due to indocyanine green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef] [PubMed]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transplant. 2003, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Man, K.; Fan, S.T. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br. J. Surg. 1997, 84, 1255–1259. [Google Scholar] [PubMed]
- Coppola, A.; Bianco, G.; Lai, Q.; Marrone, G.; Caimano, M.; Agnes, S.; Spoletini, G. Indocyanine green clearance test in liver transplantation: Defining cut-off levels for graft viability assessment during organ retrieval and for the prediction of post-transplant graft function recovery—The Liver Indocyanine Green (LivInG) Trial Study Protocol. BMJ Open 2022, 12, e063081. [Google Scholar] [CrossRef]
- Tang, Y.; Han, M.; Chen, M.; Wang, X.; Ji, F.; Zhao, Q.; Zhang, Z.; Ju, W.; Wang, D.; He, X.; et al. Donor indocyanine green clearance test predicts graft quality and early graft prognosis after liver transplantation. Dig. Dis. Sci. 2017, 62, 3212–3220. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, K.; Goto, F.; Morishita, Y. Measurement of blood concentration of indocyanine green by pulse dye densitometry—Comparison with the conventional spectrophotometric method. J. Clin. Monit. Comput. 1998, 14, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Plevris, J.N.; Jalan, A.R.; Finlayson, N.D.; Hayes, P.C. A pilot study of indocyanine green clearance as an early predictor of graft function. Transplantation 1994, 58, 196–200. [Google Scholar] [CrossRef]
- Cherchi, V.; Vetrugno, L.; Terrosu, G.; Zanini, V.; Ventin, M.; Pravisani, R.; Tumminelli, F.; Brollo, P.P.; Boscolo, E.; Peressutti, R.; et al. Association between the donor to recipient ICG-PDR variation rate and the functional recovery of the graft after orthotopic liver transplantation: A case series. PLoS ONE 2021, 16, e0256786. [Google Scholar] [CrossRef]
- Food and Drug Administration. Highlights of Prescribing Information—SPY AGENTTM GREEN (Indocyanine Green for Injection), for Intravenous or Interstitial Use. November 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211580s000lbl.pdf (accessed on 20 December 2019).
- Mizuno, S.; Isaji, S. Indocyanine green (ICG) fluorescence imaging-guided cholangiography for donor hepatectomy in living donor liver transplantation. Am. J. Transplant. 2010, 10, 2725–2726. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, M.; Tojimbara, T.; Sato, S.; Nakamura, M.; Kawase, T.; Kai, K.; Urashima, Y.; Nakajima, I.; Fuchinoue, S.; Teraoka, S.; et al. An intraoperative fluorescent imaging system in organ transplantation. Transplant. Proc. 2004, 36, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Akamatsu, N.; Ishizawa, T.; Kaneko, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Evaluation of hepatic perfusion in the liver graft using fluorescence imaging with indocyanine green. Int. J. Surg. Case Rep. 2015, 14, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Lee, K.W.; Kim, H.S.; Yoon, K.C.; Ahn, S.W.; Choi, J.Y.; Kim, H.; Yi, N.-J.; Suh, K.-S. Optimal bile duct division using real-time indocyanine green near-infrared fluorescence cholangiography during laparoscopic donor hepatectomy. Liver Transpl. 2017, 23, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Igami, T.; Nojiri, M.; Shinohara, K.; Ebata, T.; Yokoyama, Y.; Sugawara, G.; Mizuno, T.; Yamaguchi, J.; Nagino, M. Clinical value and pitfalls of fluorescent cholangiography during single-incision laparoscopic cholecystectomy. Surg. Today 2016, 46, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Ankersmit, M.; van Dam, D.A.; van Rijswijk, A.S.; van den Heuvel, B.; Tuynman, J.B.; Meijerink, W. Fluorescent imaging with indocyanine green during laparoscopic cholecystectomy in patients at increased risk of bile duct injury. Surg. Innov. 2017, 24, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.A.; van Dam, M.A.; Cahill, R.A.; Mieog, S.; Polom, K.; Vahrmeijer, A.L.; van der Vorst, J. Fluorescence-guided surgery: Comprehensive review. BJS Open 2023, 7, zrad049. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. J. Biomed. Imaging. 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Barcali, E.; Iadanza, E.; Manetti, L.; Francia, P.; Nardi, C.; Bocchi, L. Augmented reality in surgery: A scoping review. Appl. Sci. 2022, 12, 6890. [Google Scholar] [CrossRef]
| Author | Year | Patients | Dose | Time after Injection | Procedure |
|---|---|---|---|---|---|
| Ishizawa | 2009 | 49 | 0.5 mg/kg | 3 days | Liver tumor identification |
| Ishizawa | 2009 | 1 | 2.5 mg | 2 h | Intraoperative biliary anatomy visualization |
| Morita | 2013 | 58 | 0.5 mg/kg | 3 to 28 days | Preoperative hepatic function |
| Kudo | 2014 | 17 | 0.5 mg/kg | 2 weeks | Visualization of subcapsular hepatic malignancy |
| Osayi | 2015 | 82 | 6 mg | 60 min | Intraoperative biliary anatomy visualization |
| Kawaguchi | 2015 | 1 | 0.93 mg | Intraoperative | Intraoperative hepatic perfusion evaluation in liver graft |
| Panaro | 2017 | 6 | 0.5 mg/kg | 47 s | Intraoperative fluorescence angiography |
| Terasawa | 2017 | 41 | 0.5 mg/kg | 3 days | Liver tumor identification |
| Tang | 2017 | 90 | 0.5 mg/kg | 6 h | Donor graft quality prediction |
| Kim | 2021 | 46 | 0.025 mg/kg | 85.6 min | Midplane demarcation for laparoscopic hepatectomy |
| Symeonidis | 2024 | 80 | 0.3 mg/kg | 6 h | Intraoperative biliary anatomy visualization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducas, A.; Martinino, A.; Evans, L.A.; Manueli Laos, E.G.; Giovinazzo, F.; on behalf of the SMAGEICS Group. Use of Fluorescence Imaging in Liver Transplant Surgery. J. Clin. Med. 2024, 13, 2610. https://doi.org/10.3390/jcm13092610
Ducas A, Martinino A, Evans LA, Manueli Laos EG, Giovinazzo F, on behalf of the SMAGEICS Group. Use of Fluorescence Imaging in Liver Transplant Surgery. Journal of Clinical Medicine. 2024; 13(9):2610. https://doi.org/10.3390/jcm13092610
Chicago/Turabian StyleDucas, Alvaro, Alessandro Martinino, Lorna Astrid Evans, Emiliano G. Manueli Laos, Francesco Giovinazzo, and on behalf of the SMAGEICS Group. 2024. "Use of Fluorescence Imaging in Liver Transplant Surgery" Journal of Clinical Medicine 13, no. 9: 2610. https://doi.org/10.3390/jcm13092610
APA StyleDucas, A., Martinino, A., Evans, L. A., Manueli Laos, E. G., Giovinazzo, F., & on behalf of the SMAGEICS Group. (2024). Use of Fluorescence Imaging in Liver Transplant Surgery. Journal of Clinical Medicine, 13(9), 2610. https://doi.org/10.3390/jcm13092610







