Anatomical and Functional Impacts of Congenital Bilateral Visual Deprivation on the Visual Pathway—A Comprehensive Review
Abstract
1. Introduction
Magnetic Resonance Imaging
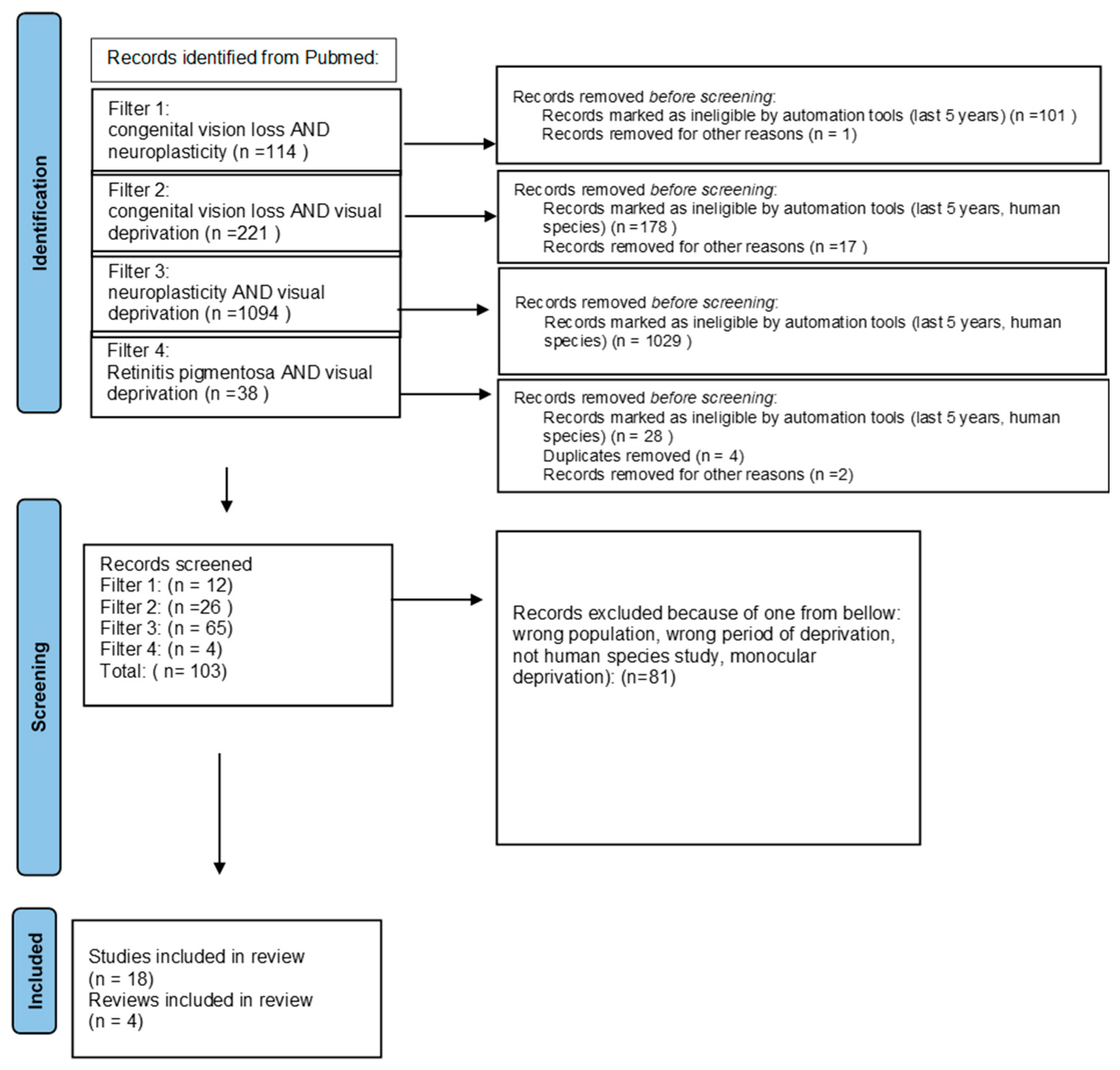
2. Materials and Methods
3. Results
3.1. Development of Brain Neuroplasticity
3.2. Early Onset of Congenital Visual Deprivation
3.3. Late Onset of Congenital Visual Deprivation
3.3.1. Retinitis Pigmentosa
3.3.2. Usher Syndrome
3.3.3. Stargardt Disease
3.4. Early Onset and Late Onset of Blindness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, F.Y.; Fatemi, A.; Johnston, M.V. Cerebral Plasticity: Windows of Opportunity in the Developing Brain. Eur. J. Paediatr. Neurol. 2017, 21, 23–48. [Google Scholar] [CrossRef]
- de Oliveira, R.M.W. Neuroplasticity. J. Chem. Neuroanat. 2020, 108, 101822. [Google Scholar] [CrossRef]
- Levin, N.; Dumoulin, S.O.; Winawer, J.; Dougherty, R.F.; Wandell, B.A. Cortical maps and white matter tracts following long period of visual deprivation and retinal image restoration. Neuron 2010, 65, 21–31. [Google Scholar] [CrossRef]
- Innocenti, G.M. Defining Neuroplasticity. Handb. Clin. Neurol. 2022, 184, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, R.F.; Koch, V.M.; Brewer, A.A.; Fischer, B.; Modersitzki, J.; Wandell, B.A. Visual Field Representations and Locations of Visual Areas V1/2/3 in Human Visual Cortex. J. Vis. 2023, 3, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ferreira, F.; Fox, M.; Harel, N.; Hattangadi-Gluth, J.; Horn, A.; Jbabdi, S.; Kahan, J.; Oswal, A.; Sheth, S.A.; et al. Clinical Applications of Magnetic Resonance Imaging Based Functional and Structural Connectivity. Neuroimage 2021, 244, 118649. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Nissen, M.J.; Klein, R.M. Visual Dominance: An Information-Processing Account of Its Origins and Significance. Psychol. Rev. 1976, 83, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Hartcher-O’Brien, J.; Levitan, C.; Spence, C. Extending Visual Dominance over Touch for Input off the Body. Brain Res. 2010, 1362, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Chialvo, D.R.; Kaiser, M.; Hilgetag, C.C. Organization, Development and Function of Complex Brain Networks. Trends Cogn. Sci. 2004, 8, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Wandell, B.A.; Smirnakis, S.M. Plasticity and Stability of Visual Field Maps in Adult Primary Visual Cortex. Nat. Rev. Neurosci. 2009, 10, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Morland, A.B. Organization of the Central Visual Pathways Following Field Defects Arising from Congenital, Inherited, and Acquired Eye Disease. Annu. Rev. Vis. Sci. 2015, 1, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.D.; Zhou, F.Q.; Huang, X.; Xing, Y.Q.; Shen, Y. Altered Intra- and Inter-Regional Functional Connectivity of the Visual Cortex in Individuals with Peripheral Vision Loss Due to Retinitis Pigmentosa. Vision. Res. 2019, 159, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, N.; Authié, C.N.; Sanda, N.; Mohand-Saïd, S.; Sahel, J.A.; Safran, A.B.; Habas, C.; Amedi, A. Increased Functional Connectivity between Language and Visually Deprived Areas in Late and Partial Blindness. Neuroimage 2016, 136, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.D.; Woodall, R.L.; Kitching, R.E.; Baseler, H.A.; Morland, A.B. Using Magnetic Resonance Imaging to Assess Visual Deficits: A Review. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2016, 36, 240–265. [Google Scholar] [CrossRef]
- Kosior-Jarecka, E.; Pankowska, A.; Polit, P.; Stępniewski, A.; Symms, M.R.; Kozioł, P.; Żarnowski, T.; Pietura, R. Volume of Lateral Geniculate Nucleus in Patients with Glaucoma in 7Tesla MRI. J. Clin. Med. 2020, 9, 2382. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.R.; Savoy, R.L. FMRI at 20: Has It Changed the World? Neuroimage 2012, 62, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- de Borst, A.W.; de Gelder, B. Mental Imagery Follows Similar Cortical Reorganization as Perception: Intra-Modal and Cross-Modal Plasticity in Congenitally Blind. Cereb. Cortex 2019, 29, 2859–2875. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Guerreiro, M.J.S.; Kekunnaya, R.; Röder, B. Top-down Modulation of Visual Cortical Processing after Transient Congenital Blindness. Neuropsychologia 2022, 174, 108338. [Google Scholar] [CrossRef]
- Topalidis, P.; Zinchenko, A.; Gädeke, J.C.; Föcker, J. The Role of Spatial Selective Attention in the Processing of Affective Prosodies in Congenitally Blind Adults: An ERP Study. Brain Res. 2020, 1739, 146819. [Google Scholar] [CrossRef]
- Setti, W.; Cuturi, L.F.; Engel, I.; Picinali, L.; Gori, M. The Influence of Early Visual Deprivation on Audio-Spatial Working Memory. Neuropsychology 2022, 36, 55–63. [Google Scholar] [CrossRef]
- Crollen, V.; Warusfel, H.; Noël, M.P.; Collignon, O. Early Visual Deprivation Does Not Prevent the Emergence of Basic Numerical Abilities in Blind Children. Cognition 2021, 210, 104586. [Google Scholar] [CrossRef]
- Cappagli, G.; Cuturi, L.F.; Signorini, S.; Morelli, F.; Cocchi, E.; Gori, M. Early Visual Deprivation Disrupts the Mental Representation of Numbers in Visually Impaired Children. Sci. Rep. 2022, 12, 22538. [Google Scholar] [CrossRef]
- Rimmele, J.M.; Gudi-Mindermann, H.; Nolte, G.; Röder, B.; Engel, A.K. Working Memory Training Integrates Visual Cortex into Beta-Band Networks in Congenitally Blind Individuals. Neuroimage 2019, 194, 259–271. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, L.; Guo, R.; Jiao, S.; Song, X.; Feng, S.; Wang, K.; Li, M.; Luo, Y.; Han, Z. The Influence of Visual Deprivation on the Development of the Thalamocortical Network: Evidence from Congenitally Blind Children and Adults. Neuroimage 2022, 264, 119722. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Shi, L.; Liu, X.; Hou, F.; Zhou, J.; Luo, J.; Hu, Q.; Li, H. Alterations of the Brain Microstructure and Corresponding Functional Connectivity in Early-Blind Adolescents. Neural Plast. 2019, 2019, 2747460. [Google Scholar] [CrossRef]
- Lubinus, C.; Orpella, J.; Keitel, A.; Gudi-Mindermann, H.; Engel, A.K.; Roeder, B.; Rimmele, J.M. Data-Driven Classification of Spectral Profiles Reveals Brain Region-Specific Plasticity in Blindness. Cereb. Cortex 2021, 31, 2505–2522. [Google Scholar] [CrossRef]
- Chebat, D.R.; Schneider, F.C.; Ptito, M. Neural Networks Mediating Perceptual Learning in Congenital Blindness. Sci. Rep. 2020, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Heimler, B.; Amedi, A. Rapid Plasticity in the Ventral Visual Stream Elicited by a Newly Learnt Auditory Script in Congenitally Blind Adults. Neuropsychologia 2023, 190, 108685. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Leclaire, C.; Manescu, S.; Collignon, O.; Lepore, F.; Frasnelli, J. Altered Morphological Traits along Central Olfactory Centres in Congenitally Blind Subjects. Eur. J. Neurosci. 2022, 56, 4486–4500. [Google Scholar] [CrossRef] [PubMed]
- Kuhli-Hattenbach, C.; Fronius, M.; Kohnen, T. Timing of Congenital Cataract Surgery: Amblyopia versus Aphakic Glaucoma. Ophthalmologe 2020, 117, 190–198. [Google Scholar] [CrossRef]
- Senna, I.; Piller, S.; Gori, M.; Ernst, M. The Power of Vision: Calibration of Auditory Space after Sight Restoration from Congenital Cataracts. Proceedings. Biol. Sci. 2022, 289, 20220768. [Google Scholar] [CrossRef] [PubMed]
- de Heering, A.; Dormal, G.; Pelland, M.; Lewis, T.; Maurer, D.; Collignon, O. A Brief Period of Postnatal Visual Deprivation Alters the Balance between Auditory and Visual Attention. Curr. Biol. 2016, 26, 3101–3105. [Google Scholar] [CrossRef] [PubMed]
- Rouger, J.; Lagleyre, S.; Fraysse, B.; Deneve, S.; Deguine, O.; Barone, P. Evidence That Cochlear-Implanted Deaf Patients Are Better Multisensory Integrators. Biol. Sci. 2007, 104, 7295–7300. [Google Scholar] [CrossRef] [PubMed]
- Burton, H. Visual Cortex Activity in Early and Late Blind People. J. Neurosci. 2003, 23, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, L.; Lin, D.; Chen, W.; Zhu, Y.; Chen, C.; Chan, K.C.; Liu, Y.; Chen, W. Visual Restoration after Cataract Surgery Promotes Functional and Structural Brain Recovery. EBioMedicine 2018, 30, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.R.; Keller, G.B. A Cortical Circuit for Audio-Visual Predictions. Nat. Neurosci. 2022, 25, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zohary, E.; Harari, D.; Ullman, S. Gaze Following Requires Early Visual Experience. Proc. Natl. Acad. Sci. USA 2022, 119, e2117184119. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.; Mondloch, C.J.; Lewis, T.L. Effects of Early Visual Deprivation on Perceptual and Cognitive Development. Prog. Brain Res. 2007, 164, 87–104. [Google Scholar] [CrossRef]
- Pehere, N.K.; Chandrasekhar, G.; Kekunnaya, R. The Critical Period for Surgical Treatment of Dense Congenital Bilateral Cataracts. J. AAPOS 2009, 13, 526–527. [Google Scholar] [CrossRef]
- Feng, Y.; Collignon, O.; Maurer, D.; Yao, K.; Gao, X. Brief Postnatal Visual Deprivation Triggers Long-Lasting Interactive Structural and Functional Reorganization of the Human Cortex. Front Med. 2021, 8, 752021. [Google Scholar] [CrossRef]
- Guerreiro, M.J.S.; Erfort, M.V.; Henssler, J.; Putzar, L.; Röder, B. Increased Visual Cortical Thickness in Sight-Recovery Individuals. Hum. Brain Mapp. 2015, 36, 5265–5274. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-Syndromic Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Zhang, M.; Ouyang, W.; Wang, H.; Meng, X.; Li, S.; Yin, Z.Q. Quantitative Assessment of Visual Pathway Function in Blind Retinitis Pigmentosa Patients. Clin. Neurophysiol. 2021, 132, 392–403. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, F.H.; Pan, P.; Liu, Z.R.; Ji, Y.H. Visual Deprivation Induce Cross-Modal Enhancement of Olfactory Perception. Biochem. Biophys. Res. Commun. 2017, 486, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Striem, E.; Pundak, G.; Orlov, T.; Zohary, E. Superior Serial Memory in the Blind: A Case of Cognitive Compensatory Adjustment. Curr. Biol. 2007, 17, 1129–1133. [Google Scholar] [CrossRef]
- Kanjlia, S.; Loiotile, R.E.; Harhen, N.; Bedny, M. ‘Visual’ Cortices of Congenitally Blind Adults Are Sensitive to Response Selection Demands in a Go/No-Go Task. Neuroimage 2021, 236, 118023. [Google Scholar] [CrossRef]
- Rita Machado, A.; Carvalho Pereira, A.; Ferreira, F.; Ferreira, S.; Quendera, B.; Silva, E.; Castelo-Branco, M. Structure-Function Correlations in Retinitis Pigmentosa Patients with Partially Preserved Vision: A Voxel-Based Morphometry Study. Sci. Rep. 2017, 7, 11411. [Google Scholar] [CrossRef]
- Hofstetter, S.; Sabbah, N.; Mohand-Saïd, S.; Sahel, J.A.; Habas, C.; Safran, A.B.; Amedi, A. The Development of White Matter Structural Changes during the Process of Deterioration of the Visual Field. Sci. Rep. 2019, 9, 2085. [Google Scholar] [CrossRef]
- Ferreira, S.; Duarte, I.C.; Paula, A.; Pereira, A.C.; Ribeiro, J.C.; Quental, H.; Reis, A.; Silva, E.D.; Castelo-Branco, M. Decreased Activity of Piriform Cortex and Orbitofrontal Hyperactivation in Usher Syndrome, a Human Disorder of Ciliary Dysfunction. Brain Imaging Behav. 2022, 16, 1176–1185. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Oliveiros, B.; Pereira, P.; António, N.; Hummel, T.; Paiva, A.; Silva, E.D. Accelerated Age-Related Olfactory Decline among Type 1 Usher Patients. Sci. Rep. 2016, 6, 28309. [Google Scholar] [CrossRef] [PubMed]
- Zrada, S.E.; Braat, K.; Doty, R.L.; Laties, A.M. Olfactory Loss in Usher Syndrome: Another Sensory Deficit? Am. J. Med. Genet. 1996, 64, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.N.; Ribeiro, J.C.; Pereira, A.C.; Ferreira, S.; Duarte, I.C.; Castelo-Branco, M. Fer Evidence for Impaired Olfactory Function and Structural Brain Integrity in a Disorder of Ciliary Function, Usher Syndrome. Neuroimage Clin. 2019, 22, 101757. [Google Scholar] [CrossRef] [PubMed]
- Zaw, K.; Carvalho, L.S.; Aung-Htut, M.T.; Fletcher, S.; Wilton, S.D.; Chen, F.K.; McLenachan, S. Pathogenesis and Treatment of Usher Syndrome Type IIA. Asia-Pac. J. Ophthalmol. 2022, 11, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, G.B.; Bodensteiner, J.B.; Thompson, J.N., Jr.; Kimberling, W.J.; Craft, J.M. Volumetric Neuroimaging in Usher Syndrome: Evidence of Global Involvement. Am. J. Med. Genet. 1998, 79, 1–4. [Google Scholar] [CrossRef]
- van der Heijden, K.; Formisano, E.; Valente, G.; Zhan, M.; Kupers, R.; de Gelder, B. Reorganization of Sound Location Processing in the Auditory Cortex of Blind Humans. Cereb. Cortex 2020, 30, 1103–1116. [Google Scholar] [CrossRef]
- Melillo, P.; Prinster, A.; Di Iorio, V.; Olivo, G.; D’Alterio, F.M.; Cocozza, S.; Orrico, A.; Quarantelli, M.; Testa, F.; Brunetti, A.; et al. Visual Cortex Activation in Patients with Stargardt Disease. Invest. Ophthalmol. Vis. Sci. 2018, 59, 1503–1511. [Google Scholar] [CrossRef]
- Sanda, N.; Cerliani, L.; Authié, C.N.; Sabbah, N.; Sahel, J.A.; Habas, C.; Safran, A.B.; Thiebaut de Schotten, M. Visual Brain Plasticity Induced by Central and Peripheral Visual Field Loss. Brain Struct. Funct. 2018, 223, 3473–3485. [Google Scholar] [CrossRef]
- Ptito, M.; Paré, S.; Dricot, L.; Cavaliere, C.; Tomaiuolo, F.; Kupers, R. A Quantitative Analysis of the Retinofugal Projections in Congenital and Late-Onset Blindness. Neuroimage Clin. 2021, 32, 102809. [Google Scholar] [CrossRef]
- Cavaliere, C.; Aiello, M.; Soddu, A.; Laureys, S.; Reislev, N.L.; Ptito, M.; Kupers, R. Organization of the Commissural Fiber System in Congenital and Late-Onset Blindness. Neuroimage Clin. 2020, 25, 102133. [Google Scholar] [CrossRef]
- Battal, C.; Gurtubay-Antolin, A.; Rezk, M.; Mattioni, S.; Bertonati, G.; Occelli, V.; Bottini, R.; Targher, S.; Maffei, C.; Jovicich, J.; et al. Structural and Functional Network-Level Reorganization in the Coding of Auditory Motion Directions and Sound Source Locations in the Absence of Vision. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 4652–4668. [Google Scholar] [CrossRef]
- Rączy, K.; Urbańczyk, A.; Korczyk, M.; Szewczyk, J.M.; Sumera, E.; Szwed, M. Orthographic Priming in Braille Reading as Evidence for Task-Specific Reorganization in the Ventral Visual Cortex of the Congenitally Blind. J. Cogn. Neurosci. 2019, 31, 1065–1078. [Google Scholar] [CrossRef]
- Jonak, K.; Krukow, P.; Jonak, K.E.; Radzikowska, E.; Baj, J.; Niedziałek, A.; Pankowska, A.; Symms, M.; Stępniewski, A.; Podkowiński, A.; et al. Decreased Volume of Lateral and Medial Geniculate Nuclei in Patients with Lhon Disease—7 Tesla Mri Study. J. Clin. Med. 2020, 9, 2914. [Google Scholar] [CrossRef]
- Touj, S.; Gallino, D.; Chakravarty, M.M.; Bronchti, G.; Piché, M. Structural Brain Plasticity Induced by Early Blindness. Eur. J. Neurosci. 2021, 53, 778–795. [Google Scholar] [CrossRef]
- Bridge, H.; Watkins, K.E. Structural and Functional Brain Reorganisation Due to Blindness: The Special Case of Bilateral Congenital Anophthalmia. Neurosci. Biobehav. Rev. 2019, 107, 765–774. [Google Scholar] [CrossRef]
- López-Bendito, G.; Aníbal-Martínez, M.; Martini, F.J. Cross-Modal Plasticity in Brains Deprived of Visual Input before Vision. Annu. Rev. Neurosci. 2022, 45, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Heimler, B.; Amedi, A. Are Critical Periods Reversible in the Adult Brain? Insights on Cortical Specializations Based on Sensory Deprivation Studies. Neurosci. Biobehav. Rev. 2020, 116, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Röder, B.; Kekunnaya, R.; Guerreiro, M.J.S. Neural Mechanisms of Visual Sensitive Periods in Humans. Neurosci. Biobehav. Rev. 2021, 120, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.; Kanjlia, S.; Omaki, A.; Bedny, M. “Visual” Cortex of Congenitally Blind Adults Responds to Syntactic Movement. J. Neurosci. 2015, 35, 12859–12868. [Google Scholar] [CrossRef] [PubMed]
- Sadato, N.; Pascual-Leone, A.; Grafman, J. Activation of the Primary Visual Cortex by Braille Reading in Blind Subjects. Nature 1996, 380, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Wanet-Defalque, M.C.; Veraart, C.; De Volder, A.; Metz, R.; Michel, C.; Dooms, G.; Goffinet, A. High Metabolic Activity in the Visual Cortex of Early Blind Human Subjects. Brain Res. 1988, 446, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Abboud, S.; Cohen, L. Distinctive Interaction between Cognitive Networks and the Visual Cortex in Early Blind Individuals. Cereb. Cortex 2019, 29, 4725–4742. [Google Scholar] [CrossRef] [PubMed]
- Crollen, V.; Spruyt, T.; Mahau, P.; Bottini, R.; Collignon, O. How Visual Experience and Task Context Modulate the Use of Internal and External Spatial Coordinate for Perception and Action. J. Exp. Psychology. Hum. Percept. Perform. 2019, 45, 354–362. [Google Scholar] [CrossRef]
- Crollen, V.; Noël, M.P.; Honoré, N.; Degroote, V.; Collignon, O. Investigating the Respective Contribution of Sensory Modalities and Spatial Disposition in Numerical Training. J. Exp. Child. Psychol. 2020, 190, 104729. [Google Scholar] [CrossRef]
- Crollen, V.; Lazzouni, L.; Rezk, M.; Bellemare, A.; Lepore, F.; Noël, M.P.; Seron, X.; Collignon, O. Recruitment of the Occipital Cortex by Arithmetic Processing Follows Computational Bias in the Congenitally Blind. Neuroimage 2019, 186, 549–556. [Google Scholar] [CrossRef]
- Dennis, M.; Spiegler, B.J.; Juranek, J.J.; Bigler, E.D.; Snead, O.C.; Fletcher, J.M. Age, Plasticity, and Homeostasis in Childhood Brain Disorders. Neurosci. Biobehav. Rev. 2013, 37, 2760–2773. [Google Scholar] [CrossRef] [PubMed]
| Order of Appearance in PubMed | Reference Number | Type of the Study/Used Methodology | Studied Group | Aim of the Study | Main Results and Conclusions |
|---|---|---|---|---|---|
| 1 | Hofstetter et al., 2019 [48] | Neuroimaging/MRI Diffusion tensor imaging (DTI) | Blind retinitis pigmentosa (RP) patients (RP-BL) (n = 10) RP patients with tunnel vision (n = 10) | To study the progression of white matter plasticity in a state of deteriorating visual field perception. | The results demonstrated gradual changes in diffusivity that are indicative of degenerative processes in the primary visual pathway comprising the optic tract and the optic radiation. This reorganization may point to microstructural plasticity underlying adaptive behavior and cross-modal integration after partial visual deprivation. |
| 2 | Zhou et al., 2019 [25] | Neuroimaging/MRI Behavioral (training with a visual-to-tactile sensory substitution system) | Congenitally blind (CB) (n = 12) | To correlate the performance of congenitally blind individuals (CB) with the size of brain structures. | There are no volumetric differences in the dorsal stream structures between CB and controls; this brain network is likely to be recruited in both groups. |
| 3 | Rączy et al., 2019 [60] | Neuroimaging/MRI (and individual in-ear recordings) | EB (early-blind) participants (n = 16) | To functionally and structurally investigate how early blindness affects brain networks involved in spatial hearing. | 10 |
| 4 | de Borst and de Gelder, 2019 [61] | Neuroimaging/MRI (fMRI scans before and after 2-h behavioral training) Behavioral/training using an auditory script based on a visual-to-auditory sensory-substitution-device (SSD) | Congenitally blind (n = 11) | To characterize the temporal dynamics of computation-specific neural specialization in the deprived visual cortex when processing an atypical sensory input | Observations after 2 h of SSD training indicate the recruitment of the deprived ventral visual stream by auditory stimuli; computation-selective cross-modal recruitment requires longer training to establish. |
| 5 | Rimmele et al., 2019 [23] | Electroencephalography and Behavioral (to detect rare pseudowords) | Congenitally blind individuals (n = 8) | To investigate that spatial selective attention is necessary for the processing of affective prosodies after visual deprivation from birth |
Blind individuals were more efficient in detecting deviant syllables at the attended loudspeaker and had higher auditory N1 amplitude than controls. The results provide evidence for “emotion-general“ auditory spatial selective attention effects in congenitally blind individuals and suggest a potential reorganization of the voice processing brain system following visual deprivation from birth. |
| 6 | Chebat et al., 2020 [27] | Neuroimaging/MRI and Magnetoencephalography/ | Congenital blind (CB) (n = 26) | To characterize alterations in spectral profiles in CB in order to map visual deprivation-related spectral changes to areas in the brain. | The results suggest that the power increases in the theta-to-beta frequency bands in auditory and frontal brain regions may reflect adaptive sensory or higher cognitive processing in blind individuals, while altered spectral profiles in visual brain regions (a lower alpha and a gamma peak) may indicate a change in the excitation–inhibition balance. |
| 7 | Cavaliere et al., 2020 [62] | Neuroimaging/MRI | Early-blind adolescents (EBAs) (n = 23) | To investigate the effects of residual light perception on brain microstructure and function in EBAs. | The study demonstrated significant microstructural and functional alterations in EBAs with and without residual light perception. These findings provide additional evidence that early visual deprivation may lead to functional neuroplasticity earlier than structural neuroplasticity in EBAs. |
| 8 | Topalidis et al., 2020 [19] | Neuroimaging/MRI (fMRI scans during behavioral tasks) Behavioral/(3D picture and voice training) | Congenitally blind (n = 8) | To show that auditory versus tactile perception evokes similar intra-modal discriminative patterns in congenitally blind compared to sighted participants |
The analyses showed that classification accuracies were significantly lower for the blind group in the primary motor cortex area 4p, for primary auditory cortex areas Te1.0 and Te1.1, primary 11somatosensory cortex areas 1, 2, and 3b, and primary motor cortex area 4a. This indicates that perception modality was not better differentiated in primary sensory cortices in the blind than in the sighted participants. |
| 9 | Lubinus et al., 2021 [26] | Magnetoencephalography/ Behavioral: memory training with (1) voices; (2) tactile motion stimuli; (3) an active training-control task. | Congenitally blind (n = 27) | To study the cross-modal reorganization of the neuronal mechanisms underlying the recruitment of the visual cortex for non-visual tasks in congenitally blind participants |
In blind participants, beta-band networks largely emerged during the training, and connectivity increased between brain areas involved in auditory working memory and, as predicted, the visual cortex. These findings highlight long-range connectivity as a key mechanism of functional reorganization following congenital blindness and provide new insights into the spectral characteristics of functional network connectivity. |
| 10 | Ptito et al., 2021 [58] | Neuroimaging/MRI | Congenitally blind (CB) (n = 22) late-blind (LB) (n = 14) | To measure the integrity of the retino-fugal system using structural MRI images |
The optic nerve, optic tract, optic chiasm, and lateral geniculate nucleus (LGN) were reduced by 50 to 60% in CB and LB. There were no differences between CB and LB. In LB, optic nerve volume correlated negatively with blindness duration. |
| 11 | Lin et al., 2022 [24] | Neuroimaging/MRI +Behavioral/verb generation and nonword reading | Congenitally blind: children (n = 8); adults (n = 22) | To investigate how the deprivation of congenital visual sensory information modulates the development of the thalamocortical network | Blind children had markedly lower (gray matter volume (GMV) values in the “visual” thalamic regions than sighted children. |
| 12 | Cappagli et al., 2022 [22] | Behavioral/localize positive and negative numbers in space |
Children with low vision (n = 3) Children with complete blindness (n = 8) | To investigate how congenital visual deprivation affects the ability to represent positive and negative numbers in horizontal and sagittal planes in visually impaired children | Long-term visual deprivation alters the ability to identify the spatial position of numbers independently of the spatial plane and the number polarity, suggesting that visual experience might have a differential role in numerical processing depending on number polarity. |
| 13 | Guerreiro et al., 2022 [18] | Neuroimaging/MRI | Patients treated for bilateral congenital cataracts (n = 9) | To explore whether early visual deprivation may affect the extent to which typically visual, motion-selective area hMT responds to moving visual stimuli | The results suggest a significantly attenuated functional selectivity of area hMT for visual motion processing in cataract-reversal individuals, consistent with the notion that visual cortical specialization depends on early visual experience. |
| 14 | Setti et al., 2022 [20] | Behavioral/acoustic simulation: recalling sequences of spatialized auditory items in the same or reverse order | Congenitally blind (n = 9) | To investigate how spatial working memory skills as well as the processing and retrieval of distal auditory spatial information are influenced by visual experience |
Blind participants had a shorter memory span in the backward than the forward order of presentation. A lack of early visual experience affects the ability to encode the surrounding space. |
| 15 | Zohary E et al., 2022 [37] | Behavioral/gaze-cueing paradigm in at least one of two experiments before and after cataract surgery | Congenital cataract + late surgery (n = 19) Congenital cataract + early surgery (n = 11) | To check if gaze understanding can be learned later if vision is extremely poor throughout early childhood | Late-operated groups failed to show eye gaze-following effects and fixated less than controls on the eyes—two spontaneous behaviors typically seen in controls. The restored vision Is effective for the head, but not the eye, confirming that gaze-following mechanisms based on eye position information develop normally despite a brief deprivation period in early development (typically 4 to 6 mo). |
| 16 | Battal et al., 2022 [59] | Neuroimaging/MRI |
Congenitally blind (CB) (n = 12) and late-blind (LB) (n = 15) | To investigate the effects of blindness on the structural and functional integrity of the corpus callosum and the anterior commissure (AC) |
The results show a larger anterior commissure (AC) for CB, decreased fractional anisotropy (FA) in the posterior part of AC (pAC), and selective reduction of the splenium of the corpus callosum (CC) in CB and LB. The results further support the findings that the splenium, a structure primarily composed of fibers connecting the visual areas of the brain, is indeed sensitive to visual deprivation in both CB and LB. |
| 17 | Chouinard-Leclaire et al., 2022 [29] | Neuroimaging/MRI | Congenitally blind (n = 16) | To investigate whether congenitally blind (CB) individuals show brain alterations in the olfactory system by comparing cortical morphology and olfactory bulb (OB) volume |
The results showed that CB individuals exhibited smaller OB and alterations of cortical density in some higher olfactory processing centers, but unchanged cortical thickness. These findings suggest that a lifelong absence of visual input leads to morphological alterations in olfactory processing areas. |
| 18 | Arbel et al., 2023 [28] | Neuroimaging/MRI | Congenitally blind (n = 15) | To investigate which areas deprived of stimuli of the brain expand, preserve their function and to what extent they acquire new ones | The results reveal a double dissociation, with tactile orthographic priming in the vOT (ventral occipitotemporal cortex) and auditory priming in general language areas/vOT in the blind group serving multiple functions, one of which, orthographic processing, overlaps with its function in the sighted group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnek-Chudzik, A.; Toro, M.D.; Rejdak, R.; Nowomiejska, K. Anatomical and Functional Impacts of Congenital Bilateral Visual Deprivation on the Visual Pathway—A Comprehensive Review. J. Clin. Med. 2024, 13, 1775. https://doi.org/10.3390/jcm13061775
Czarnek-Chudzik A, Toro MD, Rejdak R, Nowomiejska K. Anatomical and Functional Impacts of Congenital Bilateral Visual Deprivation on the Visual Pathway—A Comprehensive Review. Journal of Clinical Medicine. 2024; 13(6):1775. https://doi.org/10.3390/jcm13061775
Chicago/Turabian StyleCzarnek-Chudzik, Aleksandra, Mario Damiano Toro, Robert Rejdak, and Katarzyna Nowomiejska. 2024. "Anatomical and Functional Impacts of Congenital Bilateral Visual Deprivation on the Visual Pathway—A Comprehensive Review" Journal of Clinical Medicine 13, no. 6: 1775. https://doi.org/10.3390/jcm13061775
APA StyleCzarnek-Chudzik, A., Toro, M. D., Rejdak, R., & Nowomiejska, K. (2024). Anatomical and Functional Impacts of Congenital Bilateral Visual Deprivation on the Visual Pathway—A Comprehensive Review. Journal of Clinical Medicine, 13(6), 1775. https://doi.org/10.3390/jcm13061775









