Association between Left Atrial Appendage Morphology and Clot Histology in Patients with Embolic Ischemic Stroke: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging Protocol
2.3. CTA Analysis
2.4. Treatment
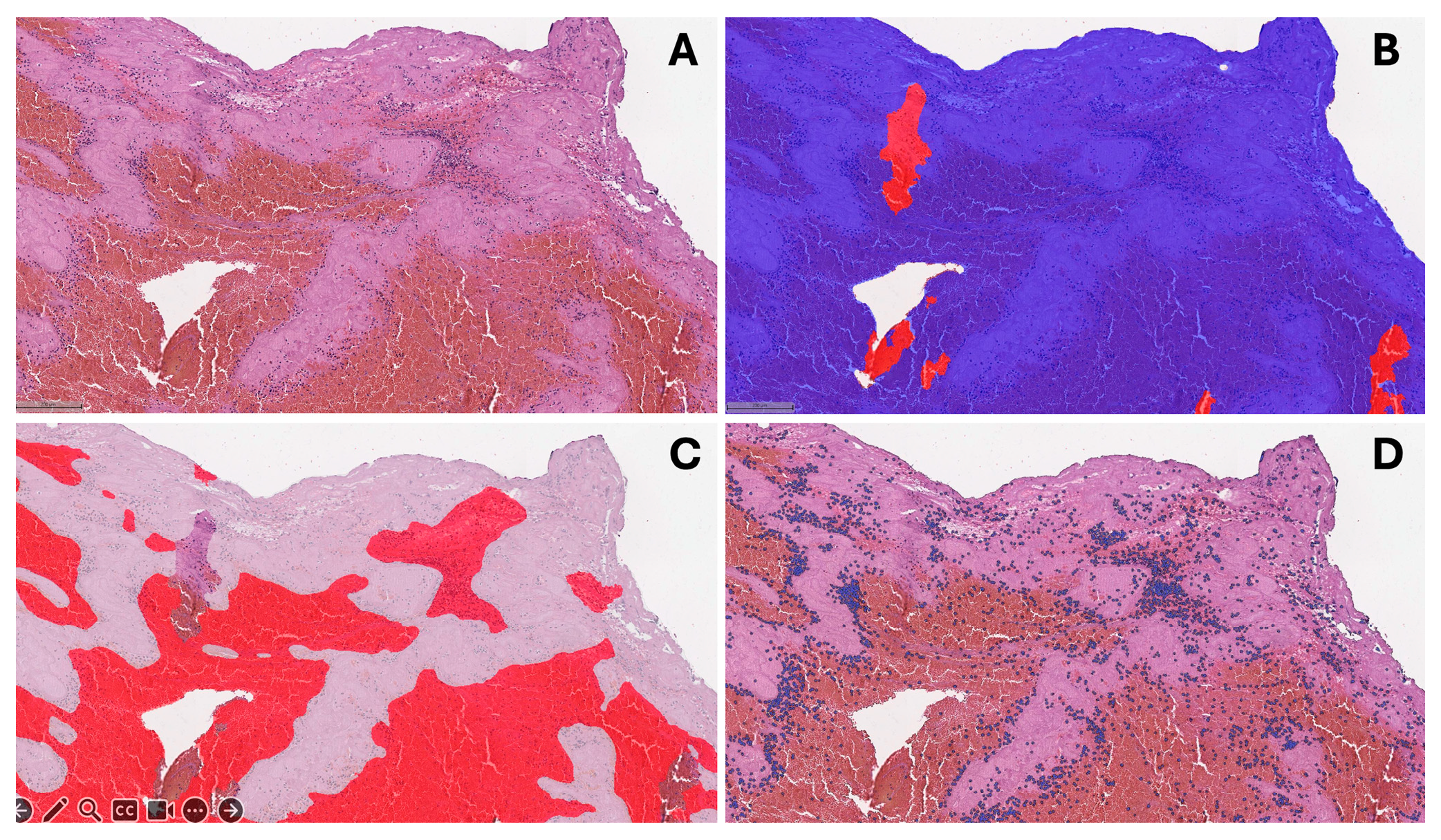
2.5. Histological Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, M.; Yoshimoto, T.; Tanaka, K.; Koge, J.; Shiozawa, M.; Nishii, T.; Ohta, Y.; Fukuda, T.; Satow, T.; Kataoka, H.; et al. Mechanical Thrombectomy Up to 24 Hours in Large Vessel Occlusions and Infarct Velocity Assessment. J. Am. Heart Assoc. 2021, 10, e022880. [Google Scholar] [CrossRef]
- Hart, R.G.; Catanese, L.; Perera, K.S.; Ntaios, G.; Connolly, S.J. Embolic Stroke of Undetermined Source. Stroke 2017, 48, 867–872. [Google Scholar] [CrossRef]
- Staessens, S.; François, O.; Brinjikji, W.; Doyle, K.M.; Vanacker, P.; Andersson, T.; De Meyer, S.F. Studying Stroke Thrombus Composition After Thrombectomy: What Can We Learn? Stroke 2021, 52, 3718–3727. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Rossi, R.; Mereuta, O.M.; Jabrah, D.; Okolo, A.; Douglas, A.; Molina Gil, S.; Pandit, A.; McCarthy, R.; Gilvarry, M.; et al. Per-Pass Analysis of Acute Ischemic Stroke Clots: Impact of Stroke Etiology on Extracted Clot Area and Histological Composition. J. Neurointerv. Surg. 2020, 13, 1111–1116. [Google Scholar] [CrossRef]
- Hund, H.M.; Boodt, N.; Hansen, D.; Haffmans, W.A.; Lycklama à Nijeholt, G.J.; Hofmeijer, J.; Dippel, D.W.J.; van der Lugt, A.; van Es, A.C.G.M.; van Beusekom, H.M.M.; et al. Association between Thrombus Composition and Stroke Etiology in the MR CLEAN Registry Biobank. Neuroradiology 2023, 65, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Chang, A.D.; Akiki, R.; Collins, S.; Novack, T.; Hemendinger, M.; Schomer, A.; Grory, B.M.; Cutting, S.; Burton, T.; et al. The Left Atrial Appendage Morphology Is Associated with Embolic Stroke Subtypes Using a Simple Classification System: A Proof of Concept Study. J. Cardiovasc. Comput. Tomogr. 2020, 14, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Balmus, M.; Nechipurenko, D.; Ataullakhanov, F.; Williams, S.; Lip, G.; Nordsletten, D.; Aslanidi, O.; de Vecchi, A. Left Atrial Appendage Morphology Impacts Thrombus Formation Risks in Multi-Physics Atrial Models. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 13–15 September 2021; Volume 48, pp. 1–4. [Google Scholar] [CrossRef]
- Hart, R.G.; Diener, H.-C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J. Embolic Strokes of Undetermined Source: The Case for a New Clinical Construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Popkirov, S.; Schlegel, U.; Weber, W.; Kleffner, I.; Altenbernd, J. Cardiac Imaging Within Emergency CT Angiography for Acute Stroke Can Detect Atrial Clots. Front. Neurol. 2019, 10, 457398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Biase, L.D.; Horton, R.P.; Nguyen, T.; Morhanty, P.; Natale, A. Left Atrial Appendage Studied by Computed Tomography to Help Planning for Appendage Closure Device Placement. J. Cardiovasc. Electrophysiol. 2010, 21, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takatsuki, S.; Inagawa, K.; Katsumata, Y.; Nishiyama, T.; Nishiyama, N.; Fukumoto, K.; Aizawa, Y.; Tanimoto, Y.; Tanimoto, K.; et al. Anatomical Characteristics of the Left Atrial Appendage in Cardiogenic Stroke with Low CHADS2 Scores. Heart Rhythm 2013, 10, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Dargazanli, C.; Fahed, R.; Blanc, R.; Gory, B.; Labreuche, J.; Duhamel, A.; Marnat, G.; Saleme, S.; Costalat, V.; Bracard, S.; et al. Modified Thrombolysis in Cerebral Infarction 2C/Thrombolysis in Cerebral Infarction 3 Reperfusion Should Be the Aim of Mechanical Thrombectomy. Stroke 2018, 49, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the Left Atrial Appendage Morphology Correlate With the Risk of Stroke in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Lupercio, F.; Carlos Ruiz, J.; Briceno, D.F.; Romero, J.; Villablanca, P.A.; Berardi, C.; Faillace, R.; Krumerman, A.; Fisher, J.D.; Ferrick, K.; et al. Left Atrial Appendage Morphology Assessment for Risk Stratification of Embolic Stroke in Patients with Atrial Fibrillation: A Meta-Analysis. Heart Rhythm 2016, 13, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Negrotto, S.M.; Lugo, R.M.; Metawee, M.; Kanagasundram, A.N.; Chidsey, G.; Baker, M.T.; Michaud, G.F.; Piana, R.N.; Benjamin Shoemaker, M.; Ellis, C.R. Left Atrial Appendage Morphology Predicts the Formation of Left Atrial Appendage Thrombus. J. Cardiovasc. Electrophysiol. 2021, 32, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Pearce, L.A.; Veltkamp, R.; Sharma, M.; Kasner, S.E.; Korompoki, E.; Milionis, H.; Mundl, H.; Berkowitz, S.D.; Connolly, S.J.; et al. Potential Embolic Sources and Outcomes in Embolic Stroke of Undetermined Source in the NAVIGATE-ESUS Trial. Stroke 2020, 51, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Fukushima, N.; Kato, K.; Ejima, K.; Sato, H.; Fukushima, K.; Saito, C.; Hayashi, K.; Arai, K.; Manaka, T.; et al. Correlation between Left Atrial Appendage Morphology and Flow Velocity in Patients with Paroxysmal Atrial Fibrillation. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 59–66. [Google Scholar] [CrossRef]
- Chen, L.; Xu, C.; Chen, W.; Zhang, C. Left Atrial Appendage Orifice Area and Morphology Is Closely Associated with Flow Velocity in Patients with Nonvalvular Atrial Fibrillation. BMC Cardiovasc. Disord. 2021, 21, 442. [Google Scholar] [CrossRef]
- Petersen, M.; Roehrich, A.; Balzer, J.; Shin, D.-I.; Meyer, C.; Kelm, M.; Kehmeier, E.S. Left Atrial Appendage Morphology Is Closely Associated with Specific Echocardiographic Flow Pattern in Patients with Atrial Fibrillation. Europace 2014, 17, 539–545. [Google Scholar] [CrossRef]
- Kishima, H.; Mine, T.; Ashida, K.; Sugahara, M.; Kodani, T.; Masuyama, T. Does Left Atrial Appendage Morphology Influence Left Atrial Appendage Flow Velocity? Circ. J. 2015, 79, 1706–1711. [Google Scholar] [CrossRef]
- Shimada, M.; Akaishi, M.; Kobayashi, T. Left Atrial Appendage Morphology and Cardiac Function in Patients with Sinus Rhythm. J. Echocardiogr. 2020, 18, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Dhillon, G.; Pourafkari, M.; DaBreo, D.; Jaff, Z.; Appireddy, R.; Jin, A.; Boissé Lomax, L.; Durafourt, B.A.; Boyd, J.G.; et al. Non–ECG-Gated Cardiac CT Angiography in Acute Stroke Is Feasible and Detects Sources of Embolism. Int. J. Stroke 2023, 19, 189–198. [Google Scholar] [CrossRef] [PubMed]



| Chicken-Wing-Type LAA (n = 24) | Non-Chicken-Wing-Type LAA (n = 55) | p Value | |
|---|---|---|---|
| Age, years (median [IQR]) | 75 (66–82) | 76 (69–84) | 0.543 |
| Female, n (%) | 14 (58.3) | 29 (52.7) | 0.645 |
| NIHSS score on admission, points (median [IQR]) | 14 (9–18) | 12 (8–16) | 0.269 |
| ASPECTS on admission, points (median [IQR]) | 8 (6–9) | 8 (7–10) | 0.205 |
| Atrial fibrillation, n (%) | 14 (58.3) | 39 (70.9) | 0.274 |
| Hypertension, n (%) | 17 (70.8) | 48 (87.3) | 0.109 |
| Diabetes mellitus, n (%) | 4 (16.7) | 16 (29.1) | 0.243 |
| CTA features | |||
| LA volume, mL (median [IQR]) | 98.5 (87.3–112.5) | 129.5 (95.3–161) | 0.067 |
| LAA volume, mL (median [IQR]) | 11.1 (8.6–15.9) | 12 (7.3–17.0) | 0.850 |
| LAA thrombus, n (%) | 0 (0) | 6 (10.9) | 0.170 |
| LAA stasis, n (%) | 6 (25.0) | 20 (36.4) | 0.323 |
| Occlusion site, n (%) | |||
| Distal ICA | 2 (8.3) | 7 (12.7) | – |
| M1 | 11 (45.8) | 26 (47.3) | – |
| M2 | 6 (25.0) | 17 (30.9) | – |
| Basilar artery | 3 (12.5) | 3 (5.5) | – |
| Distal VA (V4 segment) | 2 (8.3) | 2 (3.6) | – |
| Intracranial clot length, mm (median [IQR]) | 12 (10–15) | 10 (6–15) | 0.205 |
| IVT, n (%) | 5 (20.8) | 10 (18.2) | 0.764 |
| Chicken-Wing-Type LAA (n = 24) | Non-Chicken-Wing-Type LAA (n = 55) | p Value * | |
|---|---|---|---|
| Histological features | |||
| Proportion of fibrin in clot, mean ± SD | 0.49 ± 0.18 | 0.67 ± 0.16 | <0.001 |
| Nucleated cells in clot, cells/mm2 (median [IQR]) | 1093 (770–1500) | 1236 (665–1700) | 0.639 |
| Recanalization | |||
| mTICI score, n (%) | |||
| 0–2A | 0 (0) | 5 (9.1) | – |
| 2B | 6 (25.0) | 15 (27.3) | – |
| 2C–3 | 18 (75.0) | 35 (63.6) | – |
| No. of passes, median (IQR) | 1 (1–2) | 1 (1–2) | 0.527 |
| FPE, n (%) | 12 (50.0) | 25 (45.5) | 0.710 |
| Variable | Estimate (95% CI) | p Value |
|---|---|---|
| Age | 0.001 (−0.003, 0.004) | 0.726 |
| Female sex | 0.016 (−0.068, 0.100) | 0.704 |
| Atrial fibrillation | −0.033 (−0.121, 0.056) | 0.467 |
| Hypertension | 0.080 (−0.028, 0.188) | 0.142 |
| Diabetes mellitus | −0.001 (−0.097, 0.096) | 0.989 |
| IVT | −0.060 (−0.165, 0.046) | 0.265 |
| Chicken-wing LAA | −0.177 (−0.259, −0.096) | <0.001 * |
| LA volume | <0.001 (−0.001, 0.001) | 0.392 |
| LAA volume | −0.001 (−0.009, 0.007) | 0.826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengvenis, G.; Drachneris, J.; Žurauskas, E.; Ekkert, A.; Berūkštis, A.; Kurminas, M.; Girčius, R.; Mikelis, K.; Afanasjev, A.; Ryliškienė, K.; et al. Association between Left Atrial Appendage Morphology and Clot Histology in Patients with Embolic Ischemic Stroke: An Exploratory Study. J. Clin. Med. 2024, 13, 1734. https://doi.org/10.3390/jcm13061734
Lengvenis G, Drachneris J, Žurauskas E, Ekkert A, Berūkštis A, Kurminas M, Girčius R, Mikelis K, Afanasjev A, Ryliškienė K, et al. Association between Left Atrial Appendage Morphology and Clot Histology in Patients with Embolic Ischemic Stroke: An Exploratory Study. Journal of Clinical Medicine. 2024; 13(6):1734. https://doi.org/10.3390/jcm13061734
Chicago/Turabian StyleLengvenis, Givi, Julius Drachneris, Edvardas Žurauskas, Aleksandra Ekkert, Andrius Berūkštis, Marius Kurminas, Rokas Girčius, Kipras Mikelis, Andrej Afanasjev, Kristina Ryliškienė, and et al. 2024. "Association between Left Atrial Appendage Morphology and Clot Histology in Patients with Embolic Ischemic Stroke: An Exploratory Study" Journal of Clinical Medicine 13, no. 6: 1734. https://doi.org/10.3390/jcm13061734
APA StyleLengvenis, G., Drachneris, J., Žurauskas, E., Ekkert, A., Berūkštis, A., Kurminas, M., Girčius, R., Mikelis, K., Afanasjev, A., Ryliškienė, K., Laurinavičius, A., & Tamošiūnas, A. E. (2024). Association between Left Atrial Appendage Morphology and Clot Histology in Patients with Embolic Ischemic Stroke: An Exploratory Study. Journal of Clinical Medicine, 13(6), 1734. https://doi.org/10.3390/jcm13061734





