Fresh Frozen Homologous Rib Cartilage: A Narrative Review of a New Trend in Rhinoplasty
Abstract
1. Introduction
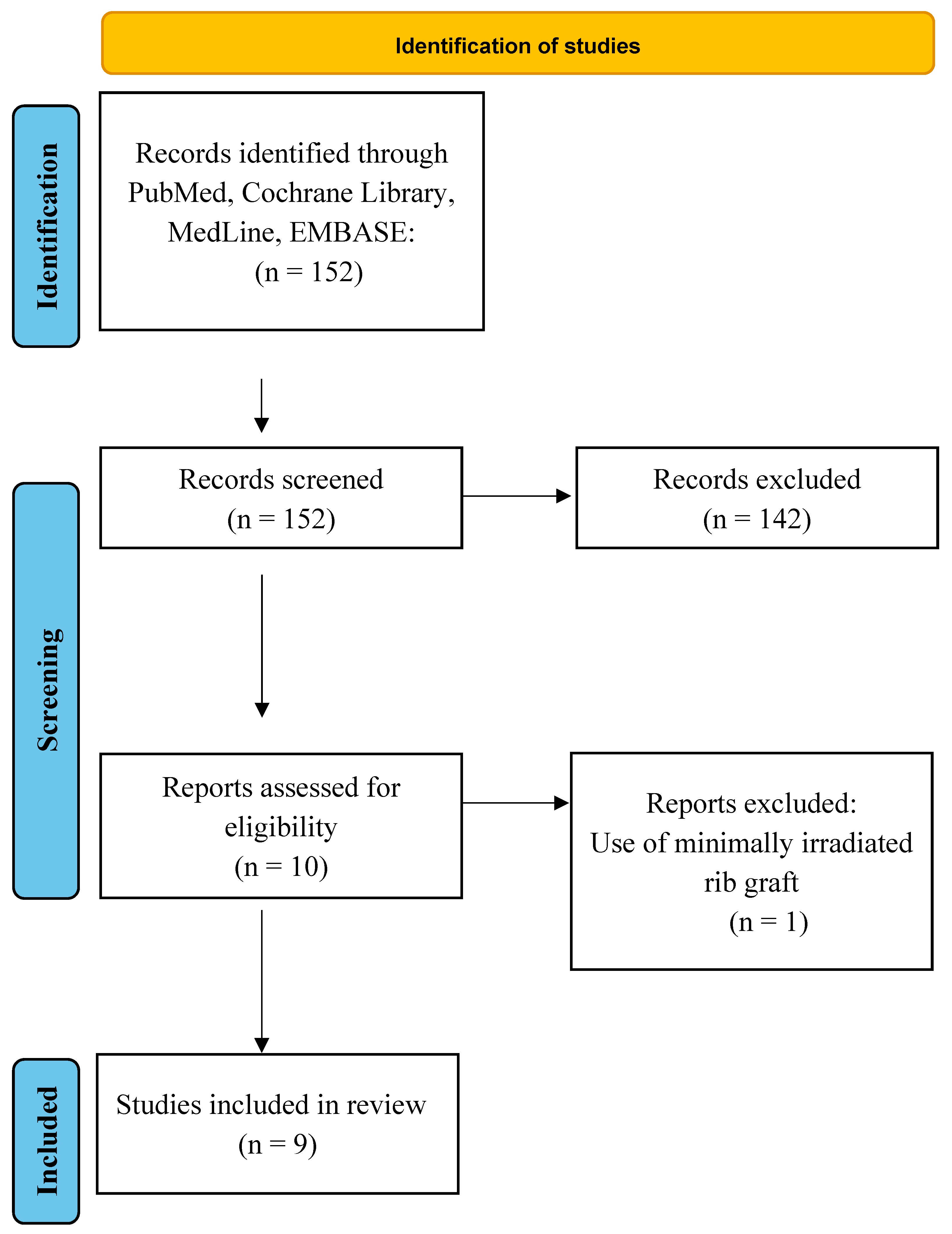
2. Materials and Methods
3. Results
Clinical Findings
4. Discussion
4.1. Autograft and Allograft to Restore the Lost Cartilaginous Structure
4.2. Harvesting Technique
4.3. Future Possibilities beyond Allogenic Rib Graft
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asps Plastic Surgery Statistics Report 2020; ASPS National Clearinghouse of Plastic Surgery Procedural Statistics: Arlington Heights, IL, USA, 2020.
- Neaman, K.C.; Boettcher, A.K.; Do, V.H.; Mulder, C.; Baca, M.; Renucci, J.D.; VanderWoude, D.L. Cosmetic Rhinoplasty: Revision Rates Revisited. Aesthetic Surg. J. 2013, 33, 31–37. [Google Scholar] [CrossRef]
- Loghmani, S.; Loghmani, A.; Maraki, F. Secondary Rhinoplasty: Aesthetic and Functional Concerns. Plast. Surg. 2019, 27, 217–222. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Dayan, E.; Durand, P.D.; Brito, I.; Gronet, E. Warping Characteristics of Rib Allograft Cartilage. Plast. Reconstr. Surg. 2020, 146, 37e–42e. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Shanmugakrishnan, R.R.; Mohan, R. Rhinoplasty Refinements: Revision Rhinoplasty Using Fresh Frozen Costal Cartilage Allograft. Plast. Reconstr. Surg. 2020, 145, 1050e–1053e. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Shanmuga Krishnan, R.R.; Rohrich, R.J. Role of Fresh Frozen Cartilage in Revision Rhinoplasty. Plast. Reconstr. Surg. 2019, 144, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Ullrich, P.; Joshi, C.; Hassan, A.; Weissman, J.; Galiano, R. The Utilization of Fresh Frozen Cartilage in Asian Rhinoplasty: A New Approach. Plast. Reconstr. Surg.—Glob. Open 2021, 9, 2–3. [Google Scholar] [CrossRef]
- Milkovich, J.; Ahmad, J. A Canadian Experience with Off-the-Shelf, Aseptically Processed, Costal Cartilage Segment Allografts in Complex Rhinoplasty. Aesthetic Surg. J. Open Forum 2022, 4, ojac085. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Abraham, J.; Alleyne, B.; Bellamy, J.; Mohan, R. Fresh Frozen Rib Cartilage Grafts in Revision Rhinoplasty: A 9-Year Experience. Plast. Reconstr. Surg. 2022, 150, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Cason, R.; Rohrich, R.J. Role of Rib Graft for Tip Shaping in Primary Rhinoplasty: A Retrospective Case Series of 30 Patients. Plast. Reconstr. Surg. 2023. publish ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Weissman, J.P.; Williams, T.; Ullrich, P.J.; Joshi, C.; Huffman, K.; Galiano, R.D. Prospective Clinical Trial Evaluating the Outcomes Associated with the Use of Fresh Frozen Allograft Cartilage in Rhinoplasty. Plast. Reconstr. Surg.—Glob. Open 2023, 11, e5315. [Google Scholar] [CrossRef]
- Hanna, S.A.; Mattos, D.; Datta, S.; Reish, R.G. Outcomes of the Use of Fresh Frozen Costal Cartilage in Rhinoplasty. Plast. Reconstr. Surg. 2023. ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Nassimizadeh, A.; Nassimizadeh, M.; Wu, J.; Yoo, D.B. Correction of the Over-Resected Nose. Facial Plast. Surg. Clin. N. Am. 2019, 27, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Robotti, E.; Daniel, R.K.; Leone, F. Cone-Beam Computed Tomography: A User-Friendly, Practical Roadmap to the Planning and Execution of Every Rhinoplasty—A 5-Year Review. Plast. Reconstr. Surg. 2021, 147, 749e–762e. [Google Scholar] [CrossRef] [PubMed]
- Starr, N.C.; Zachary Porterfield, J.; Harryman, C.; Gupta, N. The Use of Autologous and Cadaveric Grafts in Rhinoplasty: A Survey Study. Aesth. Plast. Surg. 2022, 46, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.P.; Landecker, A.; Gunter, J.P. Harvesting Rib Cartilage Grafts for Secondary Rhinoplasty. Plast. Reconstr. Surg. 2008, 121, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.H.; Park, M.-H.; Oh, S.; Jin, H.-R. Complications Associated With Autologous Rib Cartilage Use in Rhinoplasty: A Meta-Analysis. JAMA Facial Plast. Surg. 2015, 17, 49–55. [Google Scholar] [CrossRef]
- Fedok, F.G. Costal Cartilage Grafts in Rhinoplasty. Clin. Plast. Surg. 2016, 43, 201–212. [Google Scholar] [CrossRef]
- Vila, P.M.; Jeanpierre, L.M.; Rizzi, C.J.; Yaeger, L.H.; Chi, J.J. Comparison of Autologous vs Homologous Costal Cartilage Grafts in Dorsal Augmentation Rhinoplasty: A Systematic Review and Meta-Analysis. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 347. [Google Scholar] [CrossRef]
- Kadakia, N.; Nguyen, C.; Motakef, S.; Hill, M.; Gupta, S. Is Irradiated Homologous Costal Cartilage Reliable? A Meta-Analysis of Complication Rates in Rhinoplasty. Plast. Surg. 2022, 30, 212–221. [Google Scholar] [CrossRef]
- Wee, J.H.; Mun, S.J.; Na, W.S.; Kim, H.; Park, J.H.; Kim, D.-K.; Jin, H.-R. Autologous vs Irradiated Homologous Costal Cartilage as Graft Material in Rhinoplasty. JAMA Facial Plast. Surg. 2017, 19, 183–188. [Google Scholar] [CrossRef]
- Chen, K.; Schultz, B.D.; Mattos, D.; Reish, R.G. Optimizing the Use of Autografts, Allografts, and Alloplastic Materials in Rhinoplasty. Plast. Reconstr. Surg. 2022, 150, 675e–683e. [Google Scholar] [CrossRef] [PubMed]
- Drake, V.E.; Mowery, A.J.; Nellis, J.C. An Update on Rib Grafting in Rhinoplasty: Which Rib Is Right? Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 209–214. [Google Scholar] [CrossRef]
- Read-Fuller, A.M.; Yates, D.M.; Radwan, A.; Schrodt, A.M.; Finn, R.A. The Use of Allogeneic Cartilage for Grafting in Functional and Reconstructive Rhinoplasty. J. Oral Maxillofac. Surg. 2018, 76, 1560.e1–1560.e7. [Google Scholar] [CrossRef] [PubMed]
- Lavernia, L.; Brown, W.E.; Wong, B.J.F.; Hu, J.C.; Athanasiou, K.A. Toward Tissue-Engineering of Nasal Cartilages. Acta Biomater. 2019, 88, 42–56. [Google Scholar] [CrossRef] [PubMed]
| Author | Population | Male | Female | Mean Age (Years) | Mean Follow-Up (Months) | Previous Rhinoplasty |
|---|---|---|---|---|---|---|
| Rohrich (2020) [5] | 50 patients | Not specified | Not specified | Not specified | Not specified | Not specified |
| Mohan (2019) [6] | 50 patients | 12 | 38 | 40 (range 21–70) | 3.35 | Not specified |
| Wan (2021) [7] | 5 patients | 0 | 5 | Not specified | 14.2 | Not specified |
| Milkovich (2022) [8] | 21 patients | 4 | 17 | 39 (range 27–58) | 15 | 10 out of 21 |
| Rohrich (2022) [9] | 226 patients | 41 | 185 | 40.6 (range 19–74) | 12.2 | 104 out of 226 |
| Novak (2023) [10] | 30 patients | 6 | 24 | Not specified | Minimum 6 months | 0 out of 30 |
| Wan (2023) [11] | 25 patients | 5 | 20 | 38.8 (range 20–83) | 14.8 | 4 out of 25 |
| Hanna (2023) [12] | 282 patients | 27 | 225 | 35.8 (range 15–68) | 20.3 | 242 |
| Author | Complications | Warping | Resorption | Infection | Extrusion/Displacement | Other |
|---|---|---|---|---|---|---|
| Rohrich (2020) [5] | 1 out of 50 | Not specified | 0 | 1 out of 50 | Not specified | Not specified |
| Mohan (2019) [6] | 1 out of 50 | 0 | 0 | 1 out of 50 | 0 | 0 |
| Wan (2021) [7] | 1 out of 5 | 0 | 0 | 0 | 0 | 1 pathological scarring |
| Milkovich (2022) [8] | 1 out of 21 | 0 | 1 | 0 | 0 | 0 |
| Rohrich (2022) [9] | 21 out of 226 | 6 | Not specified | 6 | 0 | 9 tip erythema |
| Wan (2023) [11] | 6 out of 25 | 2 | 2 | 0 | 0 | 2 pathological scarring |
| Hanna (2023) [12] | 12 out of 282 | 0 | 0 | 6 out of 282 | 0 | 0 |
| Author | Septal Extension | Columellar Strut | Alar Contour | Dorsal Onlay | Spreader | Infratip | Diced Cartilage |
|---|---|---|---|---|---|---|---|
| Rohrich (2020) [5] | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified |
| Mohan (2019) [6] | 6% | 28% | 88% | 30% | 16% | 16% | 0% |
| Wan (2021) [7] | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified |
| Milkovich (2022) [8] | 61.9% | 42.8% | 76.1% | 9.5% | 47.6% | 9.5% | 4.8% |
| Rohrich (2022) [9] | 40% | 23% | 49% | 12% | Not specified | Not specified | Not specified |
| Novak (2023) [10] | 100% | 0% | 0% | 16.7% | 0% | 0% | 0% |
| Wan (2023) [11] | 13% | 10% | 5% | 10% | 45% | 17% | Not specified |
| Hanna (2023) [12] | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, G.; Audino, G.; Dell’Aversana Orabona, G.; Committeri, U.; Troise, S.; Arena, A.; Vaira, L.A.; De Luca, P.; Scarpa, A.; Elefante, A.; et al. Fresh Frozen Homologous Rib Cartilage: A Narrative Review of a New Trend in Rhinoplasty. J. Clin. Med. 2024, 13, 1715. https://doi.org/10.3390/jcm13061715
Salzano G, Audino G, Dell’Aversana Orabona G, Committeri U, Troise S, Arena A, Vaira LA, De Luca P, Scarpa A, Elefante A, et al. Fresh Frozen Homologous Rib Cartilage: A Narrative Review of a New Trend in Rhinoplasty. Journal of Clinical Medicine. 2024; 13(6):1715. https://doi.org/10.3390/jcm13061715
Chicago/Turabian StyleSalzano, Giovanni, Giovanni Audino, Giovanni Dell’Aversana Orabona, Umberto Committeri, Stefania Troise, Antonio Arena, Luigi Angelo Vaira, Pietro De Luca, Alfonso Scarpa, Andrea Elefante, and et al. 2024. "Fresh Frozen Homologous Rib Cartilage: A Narrative Review of a New Trend in Rhinoplasty" Journal of Clinical Medicine 13, no. 6: 1715. https://doi.org/10.3390/jcm13061715
APA StyleSalzano, G., Audino, G., Dell’Aversana Orabona, G., Committeri, U., Troise, S., Arena, A., Vaira, L. A., De Luca, P., Scarpa, A., Elefante, A., Romano, A., Califano, L., & Piombino, P. (2024). Fresh Frozen Homologous Rib Cartilage: A Narrative Review of a New Trend in Rhinoplasty. Journal of Clinical Medicine, 13(6), 1715. https://doi.org/10.3390/jcm13061715











