Predictors of Mortality and Orotracheal Intubation in Patients with Pulmonary Barotrauma Due to COVID-19: An Italian Multicenter Observational Study during Two Years of the Pandemic
Abstract
1. Introduction
- -
- To describe the incidence and the clinical characteristics of COVID-19 patients with pneumothorax and/or pneumomediastinum.
- -
- To identify the factors associated with the risk of death within 28 days of developing pneumothorax and/or pneumomediastinum in COVID-19 patients.
- -
- To identify the factors associated with the risk of OTI after barotrauma in patients in non-invasive mechanical ventilation, as well as the probability of OTI within the first 3 days from the event.
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Predictors of Mortality in COVID-19 Patients with Pulmonary Barotrauma
3.3. Predictors and Probability of OTI within 3 Days from Barotrauma Occurring during Non-Mechanical Ventilation
4. Discussion
4.1. Clinical Characteristics
4.2. Risk of Death
4.3. Risk of Intubation
4.4. Limits and Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization WHO. Coronavirus Disease (COVID-19) Dashboard with Vaccination Data|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 20 November 2022).
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Oliveira, E.; Parikh, A.; Lopez-Ruiz, A.; Carrilo, M.; Goldberg, J.; Cearras, M.; Fernainy, K.; Andersen, S.; Mercado, L.; Guan, J.; et al. ICU Outcomes and Survival in Patients with Severe COVID-19 in the Largest Health Care System in Central Florida. PLoS ONE 2021, 16, e0249038. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef]
- Menga, L.S.; Cese, L.D.; Bongiovanni, F.; Lombardi, G.; Michi, T.; Luciani, F.; Cicetti, M.; Timpano, J.; Ferrante, M.C.; Cesarano, M.; et al. High Failure Rate of Noninvasive Oxygenation Strategies in Critically Ill Subjects with Acute Hypoxemic Respiratory Failure Due to COVID-19. Respir. Care 2021, 66, 705–714. [Google Scholar] [CrossRef]
- Tobin, M.J.; Jubran, A.; Laghi, F. Noninvasive Strategies in COVID-19: Epistemology, Randomised Trials, Guidelines, Physiology. Eur. Respir. J. 2021, 57, 2004247. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; de Soyza, A.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients with Acute Hypoxemic Respiratory Failure and COVID-19. JAMA 2022, 327, 546. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 Pneumonia: Different Respiratory Treatments for Different Phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Gould, A.; Pillay, T.D.; Khorasanee, R.; Sykes, R.; Bazo-Alvarez, J.C.; Cox, C.; Shurovi, B.; Isted, A.; Simpson, T.; et al. Subcutaneous Emphysema, Pneumomediastinum, and Pneumothorax in Critically Ill Patients with Coronavirus Disease 2019: A Retrospective Cohort Study. Crit. Care Explor. 2020, 2, e0210. [Google Scholar] [CrossRef] [PubMed]
- Hamouri, S.; Samrah, S.M.; Albawaih, O.; Saleh, Z.; Smadi, M.M.; Alhazymeh, A.; Syaj, S. Pulmonary Barotrauma in COVID-19 Patients: Invasive versus Noninvasive Positive Pressure Ventilation. Int. J. Gen. Med. 2021, 14, 2017–2032. [Google Scholar] [CrossRef] [PubMed]
- Putukian, M. Pneumothorax and Pneumomediastinum. Clin. Sports Med. 2004, 23, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Kaushik, C.; Heidelman, E.; Polychronopoulou, E.; Kuo, Y.-F.; Sharma, G.; Nishi, S.P.E. Characteristics and Factors Associated with Mortality in Patients with Coronavirus Disease 2019 and Pneumothorax. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 257–268. [Google Scholar] [CrossRef]
- Palumbo, D.; Campochiaro, C.; Belletti, A.; Marinosci, A.; Dagna, L.; Zangrillo, A.; de Cobelli, F. Pneumothorax/Pneumomediastinum in Non-Intubated COVID-19 Patients: Differences between First and Second Italian Pandemic Wave. Eur. J. Intern. Med. 2021, 88, 144–146. [Google Scholar] [CrossRef]
- Greenberg, D.J.; Nabors, C.; Chandy, D.; Dhand, A. Pneumothorax and Pneumomediastinum in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19). Heart Lung 2021, 50, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Dwarakanath, A.; Horgan, L.; Jayawardena, M.; Thirumaran, M.; Johnson, O. The Clinical Course of Pneumomediastinum in Patients with SARS-CoV-2 before Invasive Mechanical Ventilation. Clin. Med. 2022, 22, 271–275. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Farrell, J.; Rostron, A.; Smith, I.; Openshaw, P.J.M.; Baillie, J.K.; Docherty, A.; Semple, M.G. COVID-19 Pneumothorax in the UK: A Prospective Observational Study Using the ISARIC WHO Clinical Characterisation Protocol. Eur. Respir. J. 2021, 58, 2100929. [Google Scholar] [CrossRef]
- Miró, Ò.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberetchs, E.J.; Jacob, J.; Alquezar-Arbe, A.; et al. Frequency, Risk Factors, Clinical Characteristics, and Outcomes of Spontaneous Pneumothorax in Patients with Coronavirus Disease 2019. A Case-Control, Emergency Medicine-Based Multicenter Study. Spanish Investigators on Emergency Situations Team (SIESTA) Network. Chest 2021, 159, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Melhorn, J.; Achaiah, A.; Conway, F.M.; Thompson, E.M.F.; Skyllberg, E.W.; Durrant, J.; Hasan, N.A.; Madani, Y.; Naran, P.; Vijayakumar, B.; et al. Pneumomediastinum in COVID-19: A Phenotype of Severe COVID-19 Pneumonitis? The Results of the United Kingdom (POETIC) Survey. Eur. Respir. J. 2022, 60, 2102522. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Mehta, M.; Shaw, M.; Hussain, N.; Joseph, S.; Vancheeswaran, R. Incidence and Clinical Features of Pneumomediastinum and Pneumothorax in COVID-19 Pneumonia. J. Intensive Care Med. 2022, 37, 1015–1018. [Google Scholar] [CrossRef]
- Zantah, M.; Dominguez Castillo, E.; Townsend, R.; Dikengil, F.; Criner, G.J. Pneumothorax in COVID-19 Disease-Incidence and Clinical Characteristics. Respir. Res. 2020, 21, 236. [Google Scholar] [CrossRef]
- Akram, J.; Yousaf, Z.; Alabbas, Y.; Almoyaaf, M.I.A.; Ibrahim, A.S.S.; Kharma, N. Epidemiological and Outcome Analysis of COVID-19-Associated Pneumothorax: Multicentre Retrospective Critical Care Experience from Qatar. BMJ Open 2022, 12, e053398. [Google Scholar] [CrossRef]
- Chopra, A.; Al-Tarbsheh, A.H.; Shah, N.J.; Yaqoob, H.; Hu, K.; Feustel, P.J.; Ortiz-Pacheco, R.; Patel, K.M.; Oweis, J.; Kozlova, N.; et al. Pneumothorax in Critically Ill Patients with COVID-19 Infection: Incidence, Clinical Characteristics and Outcomes in a Case Control Multicenter Study. Respir. Med. 2021, 184, 106464. [Google Scholar] [CrossRef]
- Rajdev, K.; Spanel, A.J.; McMillan, S.; Lahan, S.; Boer, B.; Birge, J.; Thi, M. Pulmonary Barotrauma in COVID-19 Patients with ARDS on Invasive and Non-Invasive Positive Pressure Ventilation. J. Intensive Care Med. 2021, 36, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Geraci, T.C.; Williams, D.; Chen, S.; Grossi, E.; Chang, S.; Cerfolio, R.J.; Bizekis, C.; Zervos, M. Incidence, Management, and Outcomes of Patients with COVID-19 and Pneumothorax. Ann. Thorac. Surg. 2022, 114, 401–407. [Google Scholar] [CrossRef]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. Increased Incidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020, 297, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, D.H.L.; Abu Hilal, M.; Bnà, C.; Prezioso, C.; Cavallo, E.; Nencini, N.; Crisci, S.; Fusina, F.; Natalini, G. Pneumomediastinum and Subcutaneous Emphysema in COVID-19: Barotrauma or Lung Frailty? ERJ Open Res. 2020, 6, 00385–02020. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A.; Breitman, I.; Bienstock, J.; Badami, A.; Kovatch, I.; Dresner, L.; Schwartzman, A. Pulmonary Barotrauma in Mechanically Ventilated Coronavirus Disease 2019 Patients: A Case Series. Ann. Med. Surg. 2021, 61, 24–29. [Google Scholar] [CrossRef]
- Taha, M.; Elahi, M.; Wahby, K.; Samavati, L. Incidence and Risk Factors of COVID-19 Associated Pneumothorax. PLoS ONE 2022, 17, e0271964. [Google Scholar] [CrossRef]
- Housman, B.; Jacobi, A.; Carollo, A.; Nobel, T.; Eber, C.; Acquah, S.; Powell, C.; Kaufman, A.; Lee, D.S.; Nicastri, D.; et al. COVID-19 Ventilator Barotrauma Management: Less Is More. Ann. Transl. Med. 2020, 8, 1575. [Google Scholar] [CrossRef]
- Steinberger, S.; Finkelstein, M.; Pagano, A.; Manna, S.; Toussie, D.; Chung, M.; Bernheim, A.; Concepcion, J.; Gupta, S.; Eber, C.; et al. Barotrauma in COVID 19: Incidence, Pathophysiology, and Effect on Prognosis. Clin. Imaging 2022, 90, 71–77. [Google Scholar] [CrossRef]
- Belletti, A.; Landoni, G.; Zangrillo, A. Pneumothorax and Barotrauma in Invasively Ventilated Patients with COVID-19. Respir. Med. 2021, 187, 106552. [Google Scholar] [CrossRef]
- Laffey, J.G.; Bellani, G.; Pham, T.; Fan, E.; Madotto, F.; Bajwa, E.K.; Brochard, L.; Clarkson, K.; Esteban, A.; Gattinoni, L.; et al. Potentially Modifiable Factors Contributing to Outcome from Acute Respiratory Distress Syndrome: The LUNG SAFE Study. Intensive Care Med. 2016, 42, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin Definition of ARDS: An Expanded Rationale, Justification, and Supplementary Material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Fan, E.; del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Prevalence of SARS-CoV-2 Variants in Italy (ISS Flash Survey)|COVID-19 Data Portal. Available online: https://www.covid19dataportal.it/highlights/highlight36/ (accessed on 15 March 2023).
- Russell, J.A. Vasopressor Therapy in Critically Ill Patients with Shock. Intensive Care Med. 2019, 45, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for Kidney Function and Disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA Score—Development, Utility and Challenges of Accurate Assessment in Clinical Trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz, R.; Villarejo, F.; Irrazabal, C.; Ciapponi, A. High versus Low Positive End-Expiratory Pressure (PEEP) Levels for Mechanically Ventilated Adult Patients with Acute Lung Injury and Acute. Respiratory Distress Syndrome. Cochrane Database Syst. Rev. 2021, 2021, CD009098. [Google Scholar] [CrossRef]
- López Vega, J.M.; Parra Gordo, M.L.; Diez Tascón, A.; Ossaba Vélez, S. Pneumomediastinum and Spontaneous Pneumothorax as an Extrapulmonary Complication of COVID-19 Disease. Emerg. Radiol. 2020, 27, 727–730. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Sedhai, Y.R.; Budhathoki, P.; Adhikari, A.; Pokharel, N.; Dhakal, R.; Kafle, S.; Yadullahi Mir, W.A.; Acharya, R.; Kashiouris, M.G.; et al. Pulmonary Barotrauma in COVID-19: A Systematic Review and Meta-Analysis. Ann. Med. Surg. 2022, 73, 103221. [Google Scholar] [CrossRef]
- Falasca, L.; Nardacci, R.; Colombo, D.; Lalle, E.; di Caro, A.; Nicastri, E.; Antinori, A.; Petrosillo, N.; Marchioni, L.; Biava, G.; et al. Postmortem Findings in Italian Patients with COVID-19: A Descriptive Full Autopsy Study of Cases with and without Comobidities. J. Infect. Dis. 2020, 222, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Plata-Menchaca, E.P.; Nuvials, F.X.; Roca, O.; Ferrer, R. Risk Factors and Outcomes of Ventilator-Associated Pneumonia in COVID-19 Patients: A Propensity Score Matched Analysis. Crit. Care 2021, 25, 235. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem Examination of Patients with COVID-19. JAMA J. Am. Med. Assoc. 2020, 323, 2518–2520. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Schnyder, P. The Macklin Effect: A Frequent Etiology for Pneumomediastinum in Severe Blunt Chest Trauma. Chest 2001, 120, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Favelle, O.; Derogis, V.; Cottier, J.P. Spontaneous Pneumomediastinum Due to the Macklin Effect: Less Is More. Intern. Emerg. Med. 2015, 10, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Carteaux, G.; Parfait, M.; Combet, M.; Haudebourg, A.-F.; Tuffet, S.; Mekontso Dessap, A. Patient-Self Inflicted Lung Injury: A Practical Review. J. Clin. Med. 2021, 10, 2738. [Google Scholar] [CrossRef] [PubMed]
- Grieco, D.L.; Menga, L.S.; Eleuteri, D.; Antonelli, M. Patient Self-Inflicted Lung Injury: Implications for Acute Hypoxemic Respiratory Failure and ARDS Patients on Non-Invasive Support. Minerva Anestesiol. 2019, 85, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Das, A.; Saffaran, S.; Yehya, N.; Scott, T.E.; Chikhani, M.; Laffey, J.G.; Hardman, J.G.; Camporota, L.; Bates, D.G. High Risk of Patient Self-Inflicted Lung Injury in COVID-19 with Frequently Encountered Spontaneous Breathing Patterns: A Computational Modelling Study. Ann. Intensive Care 2021, 11, 109. [Google Scholar] [CrossRef]
- Lal, A.; Mishra, A.K.; Akhtar, J.; Nabzdyk, C. Pneumothorax and Pneumomediastinum in COVID-19 Acute Respiratory Distress Syndrome. Monaldi Arch. Chest Dis. 2021, 91, 1608. [Google Scholar] [CrossRef]
- Vincent, J.-L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA Score to Assess the Incidence of Organ Dysfunction/Failure in Intensive Care Units. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Motiejunaite, J.; Deniau, B.; Blet, A.; Gayat, E.; Mebazaa, A. Inotropes and Vasopressors Are Associated with Increased Short-Term Mortality but Not Long-Term Survival in Critically Ill Patients. Anaesth. Crit. Care Pain. Med. 2022, 41, 101012. [Google Scholar] [CrossRef] [PubMed]
- Burki, N.K.; Lee, L.-Y. Mechanisms of Dyspnea. Chest 2010, 138, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Christophe, J.-J.; Ishikawa, T.; Imai, Y.; Takase, K.; Thiriet, M.; Yamaguchi, T. Hemodynamics in the Pulmonary Artery of a Patient with Pneumothorax. Med. Eng. Phys. 2012, 34, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Frohlich, S.; McLoughlin, P. Pulmonary Vascular Dysfunction in ARDS. Ann. Intensive Care 2014, 4, 28. [Google Scholar] [CrossRef]
- Lohser, J.; Slinger, P. Lung Injury After One-Lung Ventilation. Anesth. Analg. 2015, 121, 302–318. [Google Scholar] [CrossRef]
- Mekontso Dessap, A.; Charron, C.; Devaquet, J.; Aboab, J.; Jardin, F.; Brochard, L.; Vieillard-Baron, A. Impact of Acute Hypercapnia and Augmented Positive End-Expiratory Pressure on Right Ventricle Function in Severe Acute Respiratory Distress Syndrome. Intensive Care Med. 2009, 35, 1850–1858. [Google Scholar] [CrossRef]
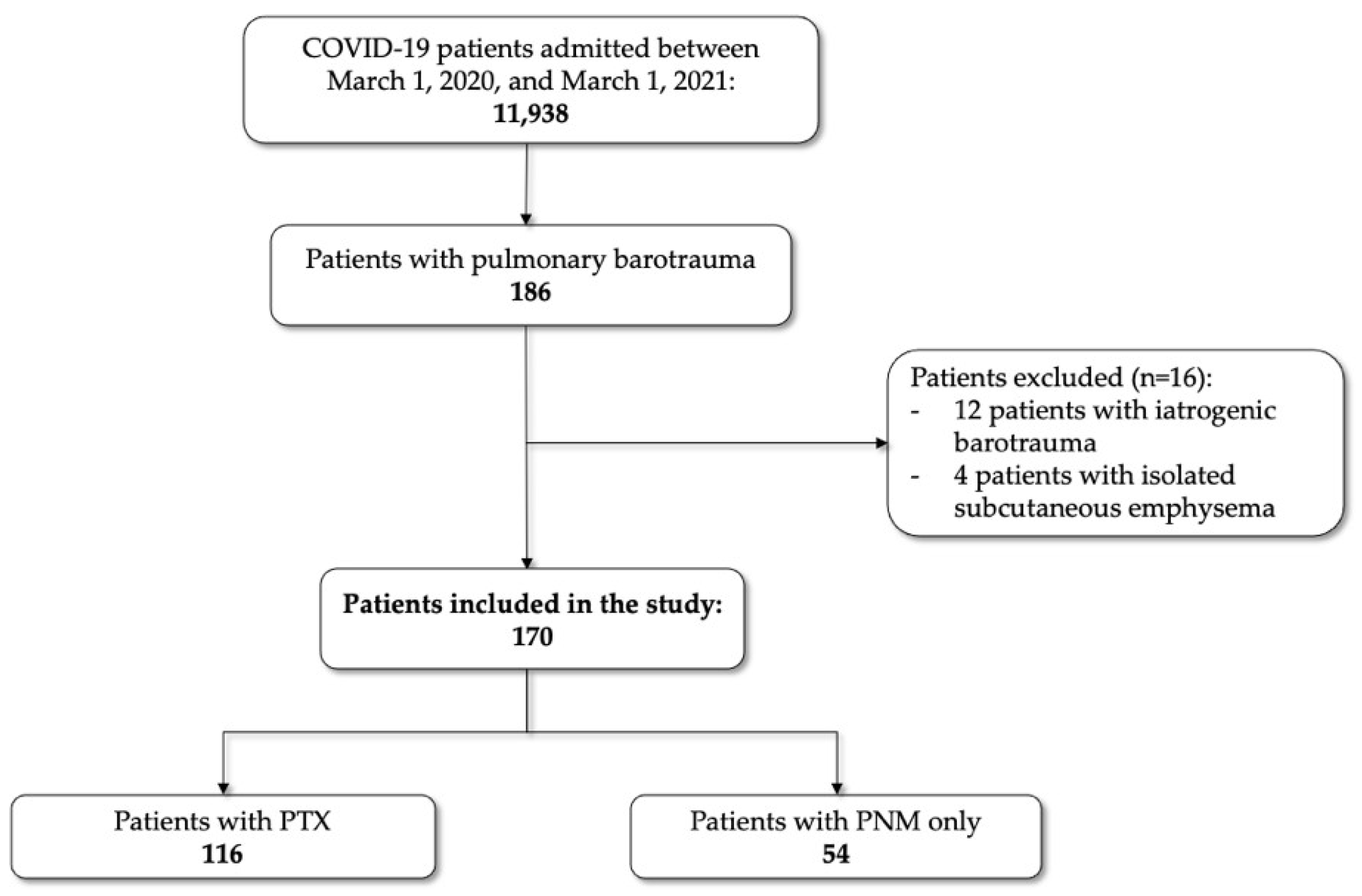
| Total | PTX | PNM Only | p-Value | |
|---|---|---|---|---|
| Number of patients (%) | 116 | 54 | ||
| Age, median (IQR) | 65.5 (56–73) | 66 (56–73) | 64.5 (57–70) | 0.421 |
| Male, n (%) | 130 | 82 (70.7) | 48 (88.9) | 0.009 |
| Female, n (%) | 40 | 34 (29.3) | 6 (11.1) | |
| BMI, kg/m2, median (IQR) | 27.0 (24–30) | 27.1 (24.1–30) | 26.1 (24.5–30.5) | 0.799 |
| SOFA score *, median (IQR) | 5 (3–8) | 6 (3–9) | 4 (3–6) | <0.001 |
| SARS-CoV-2 Variant of Concern | ||||
| Alfa | 101 (59.4) | 66 (56.9) | 35 (64.8) | 0.347 |
| Delta | 50 (29.4) | 36 (31.0) | 14 (25.9) | |
| Omicron | 19 (11.2) | 14 (12.1) | 5 (9.2) | |
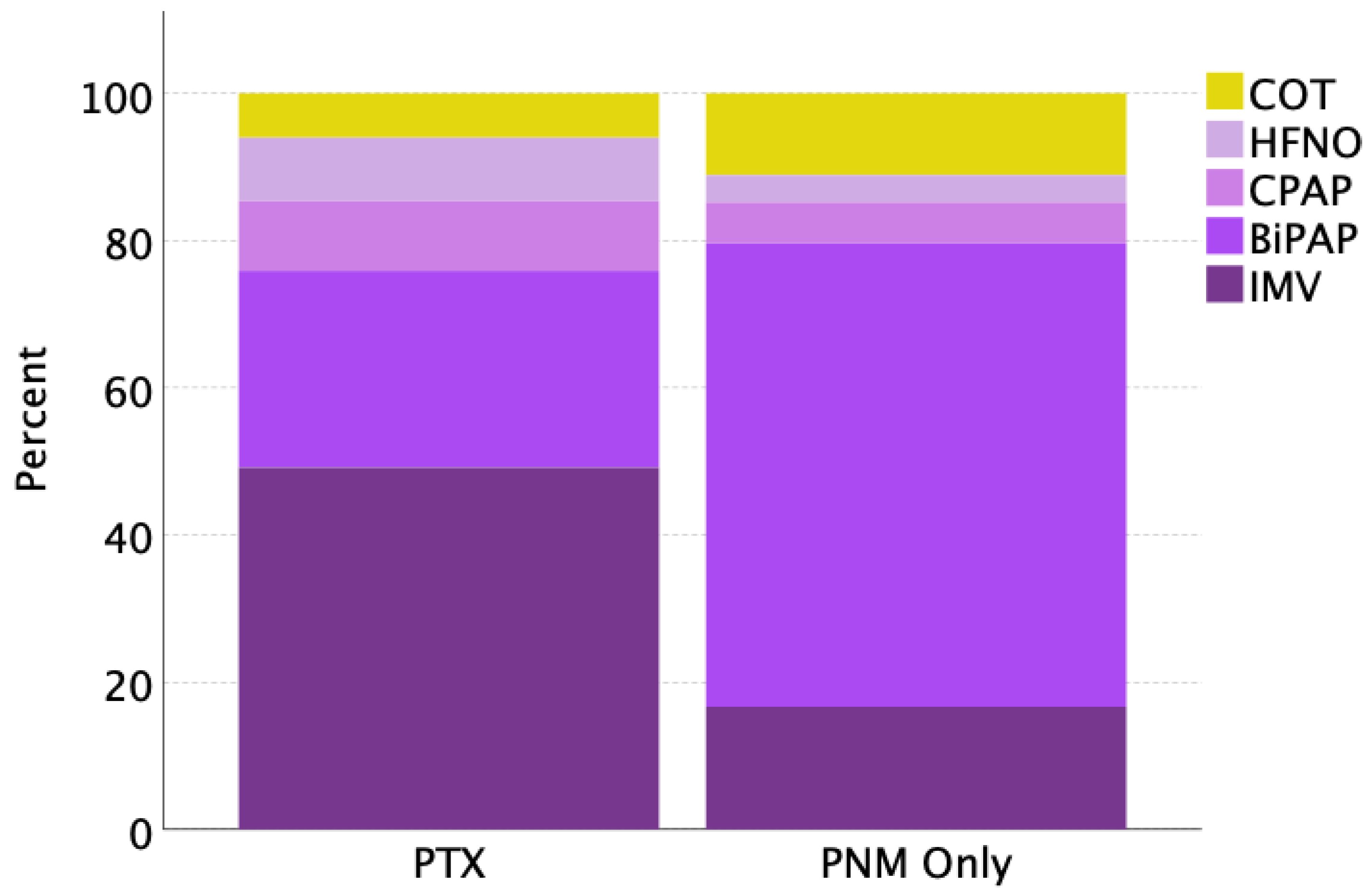
| Respiratory support | ||||
| During conventional oxygen therapy, n (%) | 13 (7.6) | 7 (6.0) | 6 (11.1) | <0.001 |
| During HFNO, n (%) | 12 (7.0) | 10 (8.6) | 2 (3.7) | |
| During CPAP, n (%) | 14 (8.2) | 11 (9.4) | 3 (5.5) | |
| During BiPAP, n (%) | 65 (38.2) | 31 (26.7) | 34 (62.9) | |
| During IMV, n (%) | 66 (38.8) | 57 (49.1) | 9 (16.7) | |
| Subcutaneous emphysema, n (%) | 71 (41.7) | 45 (38.7) | 26 (48.1) | 0.252 |
| Chest drain insertion, n (%) | 92 (54) | 91 (78.4) | 1 (1.8) | <0.001 |
| Days of hospitalization until PTX/PNM, median (IQR) | 9 (4–19) | 10.5 (5–21) | 7 (4–11) | 0.003 |
| Hospital length of stay, median (IQR) | 27 (17–43.7) | 26 (14–44.5) | 31.5 (22–41) | 0.628 |
| Requirement of vasopressor/inotropic therapy 1, n (%) | 69 (40.6) | 56 (48.3) | 13 (24.0) | 0.003 |
| Respiratory parameters * | ||||
| PCO2, mmHg | 44 (37–56) | 46 (38–58) | 41 (36–50) | 0.004 |
| P/F ratio, mmHg | 119 (90–156) | 111 (85–155) | 130 (103–157) | 0.405 |
| PEEP, cmH2O | 10 (8–11) | 9 (8–10) | 10 (8–12) | 0.630 |
| FiO2, % | 65 (55–90) | 70 (54–100) | 60 (55–70) | 0.010 |
| SpO2, % | 91 (89–94) | 91 (88–94) | 91 (90–94) | 0.332 |
| Comorbidities, n (%) | ||||
| Arterial hypertension | 98 (57.6) | 66 (56.9) | 32 (59.2) | 0.733 |
| Cardiovascular diseases | 40 (23.5) | 30 (25.8) | 10 (18.5) | 0.296 |
| Diabetes | 29 (17.0) | 26 (22.4) | 3 (5.5) | 0.006 |
| Obesity a | 54 (31.7) | 41 (35.) | 13 (24.0) | 0.143 |
| Chronic pulmonary diseases | 39 (22.9) | 27 (23.3) | 12 (22.2) | 0.880 |
| Kidney diseases b | 10 (5.8) | 9 (7.7) | 1 (1.8) | 0.129 |
| Neoplasia c | 16 (9.4) | 11 (9.5) | 5 (9.2) | 0.963 |
| Outcome within 28 days from PTX/PNM | ||||
| Mortality, patients (%) | 102 (60) | 78 (67.2) | 24 (44.4) | 0.005 |
| All Patients | Exitus a, No. (%) | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | aOR (95% CI) | p | |||
| Variables | 170 | 99 (58.2) | ||||
| Age ≥ 65 years | 102 | 59 (57.8) | 1.45 (0.78–2.69) | 0.272 | ||
| Male, n (%) (versus female) | 130 | 73 (56.1) | 0.49 (0.22–1.05) | 0.069 | ||
| SOFA score * ≥ 4 | 113 | 82 (72.6) | 4.89 (2.47–9.69) | <0.001 | 3.22 (1.27–8.17) | 0.013 |
| Pneumothorax with or without PNM (versus PNM only) | 116 | 78 (67.2) | 2.56 (1.32–4.97) | 0.005 | ||
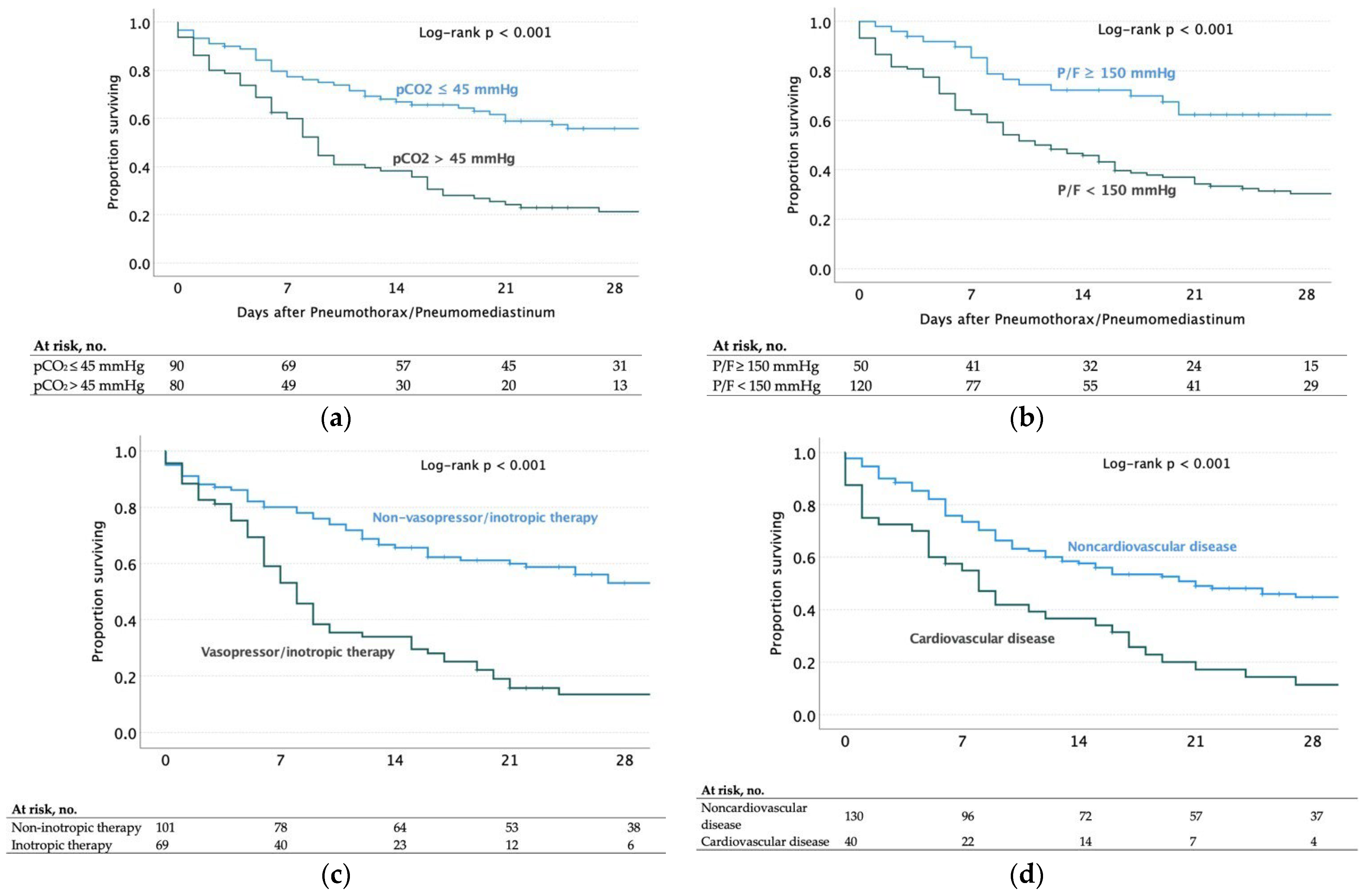
| Requirement of vasopressor/inotropic Therapy 1 | 69 | 58 (84.0) | 6.83 (3.21–14.53) | <0.001 | 11.8 (3.97–35.2) | <0.001 |
| SARS-CoV-2 Variant of Concern | ||||||
| Alfa | 101 | 61 (60.4) | 1 | |||
| Delta | 50 | 32 (62) | 1.07 (0.53–2.14) | 0.849 | ||
| Omicron | 19 | 11 (52.6) | 0.73 (0.27–1.95) | 0.614 | ||
| Respiratory parameters * | ||||||
| PCO2 > 45 mmHg | 80 | 63 (78.7) | 4.84 (2.45–9.55) | <0.001 | 2.72 (1.16–6.41) | 0.021 |
| P/F ratio < 150 mmHg | 120 | 85 (70.8) | 4.71 (2.32–9.54) | <0.001 | 10.9 (3.76–31.8) | <0.001 |
| Comorbidities, n (%) | ||||||
| Arterial hypertension | 98 | 60 (61.2) | 1.12 (0.60–2.09) | 0.704 | ||
| Cardiovascular diseases | 34 | 40 (85) | 5.67 (2.03–13.14) | <0.001 | 7.92 (2.48–25.2) | <0.001 |
| Diabetes | 29 | 22 (75.8) | 2.39 (0.96–5.97) | 0.056 | 1.18 (0.36–3.82) | 0.077 |
| Obesity b | 54 | 36 (66.7) | 1.51 (0.77–2.97) | 0.226 | ||
| Chronic pulmonary diseases | 39 | 23 (58.9) | 0.94 (0.45–1.95) | 0.882 | ||
| Chronic renal failure c | 10 | 8 (80) | 2.80 (0.57–13.6) | 0.319 | ||
| Neoplasm d | 16 | 12 (75) | 2.13 (0.65–6.91) | 0.198 |
| Non-MV Patients | Orotracheal Intubation a, No. (%) | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | aOR (95% CI) | p | |||
| Dichotomous variables | 104 | 65 (62.5) | ||||
| Age ≥ 65 years | 57 | 39 (68.4) | 1.75 (0.78–3.90) | 0.170 | ||
| Male, n (%) (versus female) | 83 | 51 (61.4) | 0.79 (0.29–2.18) | 0.659 | ||
| SOFA score * ≥ 4 | 52 | 40 (76.9) | 3.60 (1.54–8.37) | 0.004 | 3.10 (1.24–7.73) | 0.015 |
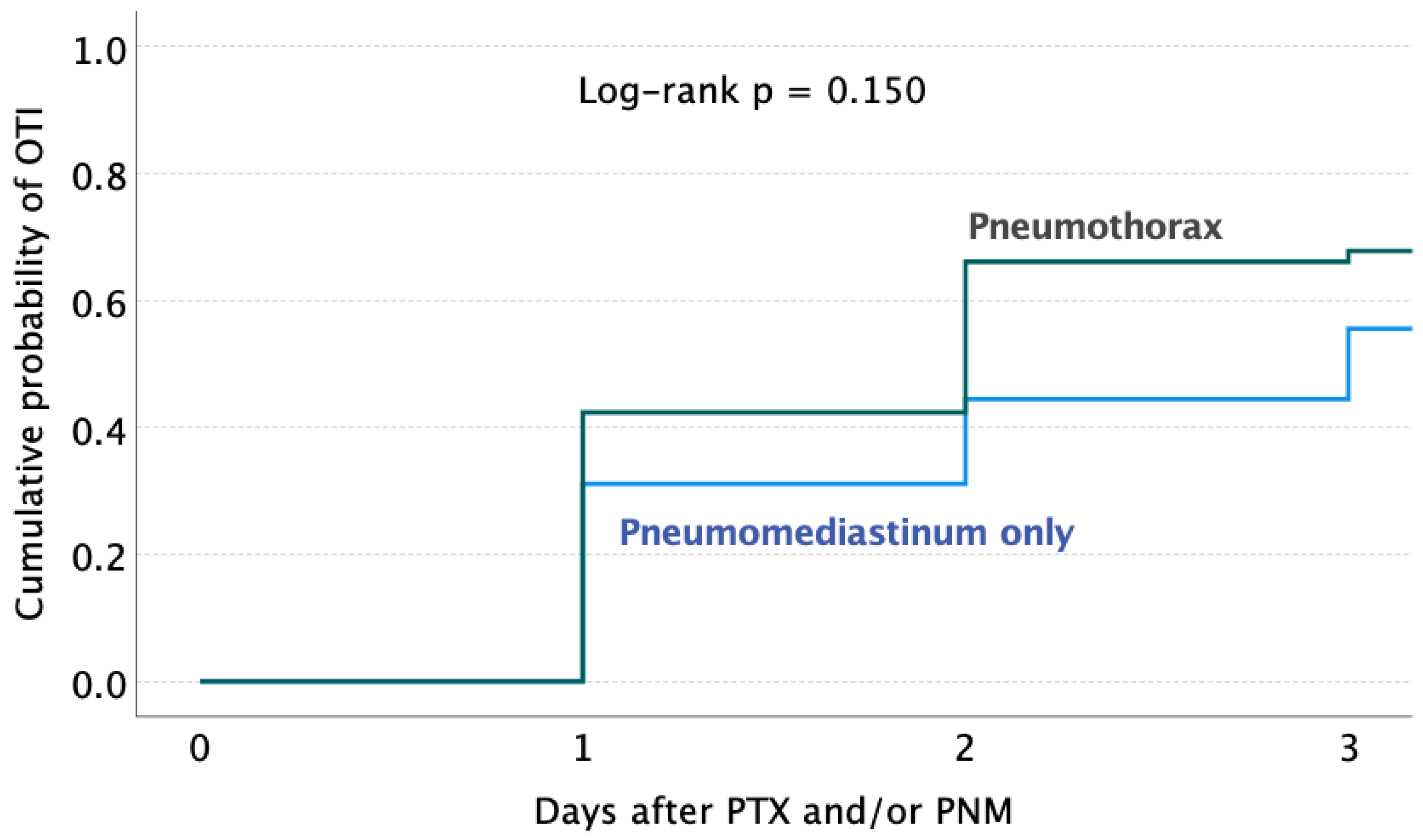
| Pneumothorax with or without PNM (versus PNM only) | 59 | 21 (35.6) | 1.77 (0.75–4.17) | 0.199 | ||
| Vasopressor/inotropic therapy 1 | 19 | 16 (84.2) | 3.91 (1.06–14.4) | 0.031 | 1.97 (0.47–8.14) | 0.347 |
| SARS-CoV-2 Variant of Concern | ||||||
| Alfa | 61 | 35 (57.4) | 1 | |||
| Delta | 32 | 25 (78.1) | 1.83 (0.76–4.40) | 0.195 | ||
| Omicron | 11 | 5 (45.5) | 0.62 (0.17–2.25) | 0.522 | ||
| Respiratory parameters * | ||||||
| PCO2 > 45 mmHg | 32 | 28 (87.5) | 6.62 (2.10–20.8) | <0.001 | 6.01 (1.83–19.7) | 0.003 |
| P/F ratio < 150 mmHg | 77 | 54 (70.1) | 3.41 (1.37–8.48) | 0.007 | 2.87 (1.03–7.93) | 0.042 |
| Comorbidities, n (%) | ||||||
| Arterial hypertension | 60 | 39 (65.0) | 1.28 (0.57–2.86) | 0.539 | ||
| Cardiovascular diseases | 27 | 21 (77.8) | 2.62 (0.95–7.23) | 0.057 | 1.32 (0.41–4.20) | <0.001 |
| Diabetes | 16 | 13 (81.2) | 2.39 (0.96–5.97) | 0.056 | 1.75 (0.41–7.45) | 0.639 |
| Obesity b | 28 | 19 (67.8) | 1.37 (0.55–3.44) | 0.497 | ||
| Chronic pulmonary diseases | 26 | 15 (57.7) | 0.76 (0.30–1.88) | 0.559 | ||
| Chronic renal failure c | 6 | 4 (66.7) | 1.21 (0.21–6.95) | 0.828 | ||
| Neoplasm d | 13 | 10 (76.9) | 2.18 (0.56–8.47) | 0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetaj, N.; De Pascale, G.; Antonelli, M.; Vargas, J.; Savino, M.; Pugliese, F.; Alessandri, F.; Giordano, G.; Tozzi, P.; Rocco, M.; et al. Predictors of Mortality and Orotracheal Intubation in Patients with Pulmonary Barotrauma Due to COVID-19: An Italian Multicenter Observational Study during Two Years of the Pandemic. J. Clin. Med. 2024, 13, 1707. https://doi.org/10.3390/jcm13061707
Tetaj N, De Pascale G, Antonelli M, Vargas J, Savino M, Pugliese F, Alessandri F, Giordano G, Tozzi P, Rocco M, et al. Predictors of Mortality and Orotracheal Intubation in Patients with Pulmonary Barotrauma Due to COVID-19: An Italian Multicenter Observational Study during Two Years of the Pandemic. Journal of Clinical Medicine. 2024; 13(6):1707. https://doi.org/10.3390/jcm13061707
Chicago/Turabian StyleTetaj, Nardi, Gennaro De Pascale, Massimo Antonelli, Joel Vargas, Martina Savino, Francesco Pugliese, Francesco Alessandri, Giovanni Giordano, Pierfrancesco Tozzi, Monica Rocco, and et al. 2024. "Predictors of Mortality and Orotracheal Intubation in Patients with Pulmonary Barotrauma Due to COVID-19: An Italian Multicenter Observational Study during Two Years of the Pandemic" Journal of Clinical Medicine 13, no. 6: 1707. https://doi.org/10.3390/jcm13061707
APA StyleTetaj, N., De Pascale, G., Antonelli, M., Vargas, J., Savino, M., Pugliese, F., Alessandri, F., Giordano, G., Tozzi, P., Rocco, M., Biava, A. M., Maggi, L., Pisapia, R., Fusco, F. M., Stazi, G. V., Garotto, G., Marini, M. C., Piselli, P., Beccacece, A., ... Nicastri, E., on behalf of PTX/PNM Study Group. (2024). Predictors of Mortality and Orotracheal Intubation in Patients with Pulmonary Barotrauma Due to COVID-19: An Italian Multicenter Observational Study during Two Years of the Pandemic. Journal of Clinical Medicine, 13(6), 1707. https://doi.org/10.3390/jcm13061707










