A Simple and Safe Method for Checking the Position of Central Venous Catheters—A New and Reliable Threshold for Right Atrial Swirl Sign in Microbubbles Tests
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Primary and Secondary Outcome Parameters
2.3. Central Venous Catheter Insertion
2.4. Microbubbles Test
2.5. Chest X-ray
2.6. Statistics
3. Results
4. Discussion
4.1. Common Modalities for Confirmation of Central Venous Catheter Position and Exclusion of Pneumothorax
4.2. The Current Study Situation on Push-to-Bubbles Time in Microbubbles Tests
4.3. Clinical Implementation of Micro Bubbles Test
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seldinger, S.I. Catheter replacement of the needle in percutaneous arteriography: A new technique. Acta Radiol. 1953, 39, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Korsten, P.; Mavropoulou, E.; Wienbeck, S.; Ellenberger, D.; Patschan, D.; Zeisberg, M.; Vasko, R.; Tampe, B.; Müller, G.A. The “rapid atrial swirl sign” for assessing central venous catheters: Performance by medical residents after limited training. PLoS ONE 2018, 13, e0199345. [Google Scholar] [CrossRef]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst. Rev. 2015, 1, CD006962. [Google Scholar] [CrossRef]
- Lalu, M.M.; Fayad, A.; Ahmed, O.; Bryson, G.L.; Fergusson, D.A.; Barron, C.C.; Sullivan, P.; Thompson, C. Ultrasound-Guided Subclavian Vein Catheterization: A Systematic Review and Meta-Analysis. Crit. Care Med. 2015, 43, 1498–1507. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Rupp, S.M.; Tung, A.; Connis, R.T.; Domino, K.B.; Grant, M.D.; Mark, J.B. Practice Guidelines for Central Venous Access 2020: An Updated Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology 2020, 132, 8–43. [Google Scholar] [CrossRef]
- Ablordeppey, E.A.; Drewry, A.M.; Beyer, A.B.; Theodoro, D.L.; Fowler, S.A.; Fuller, B.M.; Carpenter, C.R. Diagnostic Accuracy of Central Venous Catheter Confirmation by Bedside Ultrasound Versus Chest Radiography in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Schummer, W.; Schummer, C.; Rose, N.; Niesen, W.-D.; Sakka, S.G. Mechanical complications and malpositions of central venous cannulations by experienced operators. A prospective study of 1794 catheterizations in critically ill patients. Intensive Care Med. 2007, 33, 1055–1059. [Google Scholar] [CrossRef]
- Stonelake, P.A.; Bodenham, A.R. The carina as a radiological landmark for central venous catheter tip position. Br. J. Anaesth. 2006, 96, 335–340. [Google Scholar] [CrossRef]
- Cadman, A.; Lawrance, J.A.L.; Fitzsimmons, L.; Spencer-Shaw, A.; Swindell, R. To clot or not to clot? That is the question in central venous catheters. Clin. Radiol. 2004, 59, 349–355. [Google Scholar] [CrossRef]
- McGee, D.C.; Gould, M.K. Preventing complications of central venous catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef]
- Kornbau, C.; Lee, K.C.; Hughes, G.D.; Firstenberg, M.S. Central line complications. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 170–178. [Google Scholar] [CrossRef]
- Wirsing, M.; Schummer, C.; Neumann, R.; Steenbeck, J.; Schmidt, P.; Schummer, W. Is traditional reading of the bedside chest radiograph appropriate to detect intraatrial central venous catheter position? Chest 2008, 134, 527–533. [Google Scholar] [CrossRef]
- Hsu, J.-H.; Wang, C.-K.; Chu, K.-S.; Cheng, K.-I.; Chuang, H.-Y.; Jaw, T.-S.; Wu, J.-R. Comparison of radiographic landmarks and the echocardiographic SVC/RA junction in the positioning of long-term central venous catheters. Acta Anaesthesiol. Scand. 2006, 50, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Knio, Z.O.; Mahmood, F.; Oren-Grinberg, A.; Leibowitz, A.; Bose, R.; Shaefi, S.; Mitchell, J.D.; Ahmed, M.; Bardia, A.; et al. Ultrasound as a Screening Tool for Central Venous Catheter Positioning and Exclusion of Pneumothorax. Crit. Care Med. 2017, 45, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Corradi, F.; Guarracino, F.; Santori, G.; Brusasco, C.; Tavazzi, G.; Via, G.; Mongodi, S.; Mojoli, F.; Biagini, R.U.D.; Isirdi, A.; et al. Ultrasound localization of central vein catheter tip by contrast-enhanced transthoracic ultrasonography: A comparison study with trans-esophageal echocardiography. Crit. Care 2022, 26, 113. [Google Scholar] [CrossRef]
- Cortellaro, F.; Mellace, L.; Paglia, S.; Costantino, G.; Sher, S.; Coen, D. Contrast enhanced ultrasound vs chest X-ray to determine correct central venous catheter position. Am. J. Emerg. Med. 2014, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Duran-Gehring, P.E.; Guirgis, F.W.; McKee, K.C.; Goggans, S.; Tran, H.; Kalynych, C.J.; Wears, R.L. The bubble study: Ultrasound confirmation of central venous catheter placement. Am. J. Emerg. Med. 2015, 33, 315–319. [Google Scholar] [CrossRef]
- Gidaro, A.; Casella, F.; Lugli, F.; Cogliati, C.; Calloni, M.; Samartin, F.; Brena, N.; Pace, G. Contrast enhanced ultrasound as a new tool to estimate the performance of midline catheters in the single patient. J. Vasc. Access 2023, 24, 284–288. [Google Scholar] [CrossRef]
- Iacobone, E.; Elisei, D.; Gattari, D.; Carbone, L.; Capozzoli, G. Transthoracic echocardiography as bedside technique to verify tip location of central venous catheters in patients with atrial arrhythmia. J. Vasc. Access 2020, 21, 861–867. [Google Scholar] [CrossRef]
- Meggiolaro, M.; Scatto, A.; Zorzi, A.; Roman-Pognuz, E.; Lauro, A.; Passarella, C.; Bonaccorso, G. Confirmation of correct central venous catheter position in the preoperative setting by echocardiographic “bubble-test”. Minerva Anestesiol. 2015, 81, 989–1000. [Google Scholar]
- Weekes, A.J.; Johnson, D.A.; Keller, S.M.; Efune, B.; Carey, C.; Rozario, N.L.; Norton, H.J. Central vascular catheter placement evaluation using saline flush and bedside echocardiography. Acad. Emerg. Med. 2014, 21, 65–72. [Google Scholar] [CrossRef]
- Vezzani, A.; Brusasco, C.; Palermo, S.; Launo, C.; Mergoni, M.; Corradi, F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: An alternative to chest radiography. Crit. Care Med. 2010, 38, 533–538. [Google Scholar] [CrossRef]
- Wen, M.; Stock, K.; Heemann, U.; Aussieker, M.; Küchle, C. Agitated saline bubble-enhanced transthoracic echocardiography: A novel method to visualize the position of central venous catheter. Crit. Care Med. 2014, 42, e231–e233. [Google Scholar] [CrossRef]
- Weekes, A.J.; Keller, S.M.; Efune, B.; Ghali, S.; Runyon, M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg. Med. J. EMJ 2016, 33, 176–180. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Bodenham, A.R. Safe placement of central venous catheters: Where should the tip of the catheter lie? Br. J. Anaesth. 2000, 85, 188–191. [Google Scholar] [CrossRef]
- Ruesch, S.; Walder, B.; Tramèr, M.R. Complications of central venous catheters: Internal jugular versus subclavian access—A systematic review. Crit. Care Med. 2002, 30, 454–460. [Google Scholar] [CrossRef]
- Ablordeppey, E.A.; Drewry, A.M.; Theodoro, D.L.; Tian, L.; Fuller, B.M.; Griffey, R.T. Current Practices in Central Venous Catheter Position Confirmation by Point of Care Ultrasound: A Survey of Early Adopters. Shock 2019, 51, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Tocino, I.M.; Miller, M.H.; Fairfax, W.R. Distribution of pneumothorax in the supine and semirecumbent critically ill adult. AJR Am. J. Roentgenol. 1985, 144, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ball, C.G.; Kirkpatrick, A.W.; Laupland, K.B.; Fox, D.L.; Litvinchuk, S.; Dyer, D.M.M.; Anderson, I.B.; Hameed, S.M.; Kortbeek, J.B.; Mulloy, R. Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am. J. Surg. 2005, 189, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Pittiruti, M.; La Greca, A.; Scoppettuolo, G. The electrocardiographic method for positioning the tip of central venous catheters. J. Vasc. Access 2011, 12, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Schummer, W.; Herrmann, S.; Schummer, C.; Funke, F.; Steenbeck, J.; Fuchs, J.; Uhlig, T.; Reinhart, K. Intra-atrial ECG is not a reliable method for positioning left internal jugular vein catheters. Br. J. Anaesth. 2003, 91, 481–486. [Google Scholar] [CrossRef]
- Urban, T.; Wappler, F.; Sakka, S.G. Kasuistik: Fehlerhafte Lage eines zentralen Venenkatheters trotz korrekter intravasaler EKG-Ableitung—Positives P-Wellenpotenzial bei intraarterieller Katheterfehllage. Anästhesiol. Intensiv. Notfallmed. Schmerzther. 2011, 46, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Schummer, W.; Schummer, C.; Schelenz, C.; Brandes, H.; Stock, U.; Müller, T.; Leder, U.; Hüttemann, E. Central venous catheters--the inability of ‘intra-atrial ECG’ to prove adequate positioning. Br. J. Anaesth. 2004, 93, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Schummer, W.; Schummer, C.; Paxian, M.; Stock, U.; Richter, K.; Bauer, M. Extravasale Lage von zentralen Venenkathetern bei korrekter EKG-Ableitung. Anasthesiol. Intensivmed. Notfallmedizin Schmerzther. AINS 2005, 40, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Ryu, H.-G.; Yoon, S.-Z.; Kim, J.-H.; Bahk, J.-H. Transesophageal echocardiographic evaluation of ECG-guided central venous catheter placement. Can. J. Anaesth. J. Can. D’anesthesie 2006, 53, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Schummer, W.; Schummer, C.; Schelenz, C.; Schmidt, P.; Fröber, R.; Hüttemann, E. Optimierte Positionierung zentraler Venenkatheter durch eine modifizierte Anwendung der intravasalen Elektrokardiographie. Validierung mithilfe der transösophagealen Echokardiographie. Der Anaesthesist 2005, 54, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Chaney, M.A.; Minhaj, M.M.; Patel, K.; Muzic, D. Transoesophageal echocardiography and central line insertion. Ann. Card. Anaesth. 2007, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Nakanishi, T.; Sakamoto, S.; Toriumi, T. Confirmation of optimal guidewire length for central venous catheter placement using transesophageal echocardiography. J. Clin. Anesth. 2016, 35, 58–61. [Google Scholar] [CrossRef]
- Smit, J.M.; Raadsen, R.; Blans, M.J.; Petjak, M.; van de Ven, P.M.; Tuinman, P.R. Bedside ultrasound to detect central venous catheter misplacement and associated iatrogenic complications: A systematic review and meta-analysis. Crit. Care 2018, 22, 65. [Google Scholar] [CrossRef]
- Zieleskiewicz, L.; Muller, L.; Lakhal, K.; Meresse, Z.; Arbelot, C.; Bertrand, P.-M.; Bouhemad, B.; Cholley, B.; Demory, D.; Duperret, S.; et al. Point-of-care ultrasound in intensive care units: Assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med. 2015, 41, 1638–1647. [Google Scholar] [CrossRef]
- Soldati, G.; Testa, A.; Sher, S.; Pignataro, G.; La Sala, M.; Silveri, N.G. Occult traumatic pneumothorax: Diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008, 133, 204–211. [Google Scholar] [CrossRef]
- Jalli, R.; Sefidbakht, S.; Jafari, S.H. Value of ultrasound in diagnosis of pneumothorax: A prospective study. Emerg. Radiol. 2013, 20, 131–134. [Google Scholar] [CrossRef]
- Alrajhi, K.; Woo, M.Y.; Vaillancourt, C. Test characteristics of ultrasonography for the detection of pneumothorax: A systematic review and meta-analysis. Chest 2012, 141, 703–708. [Google Scholar] [CrossRef]
- Raman, D.; Sharma, M.; Moghekar, A.; Wang, X.; Hatipoğlu, U. Utilization of Thoracic Ultrasound for Confirmation of Central Venous Catheter Placement and Exclusion of Pneumothorax: A Novel Technique in Real-Time Application. J. Intensive Care Med. 2019, 34, 594–598. [Google Scholar] [CrossRef]
- Galante, O.; Slutsky, T.; Fuchs, L.; Smoliakov, A.; Mizrakli, Y.; Novack, V.; Brotfein, E.; Klein, M.; Frenkel, A.; Koifman, L.; et al. Single-Operator Ultrasound-Guided Central Venous Catheter Insertion Verifies Proper Tip Placement. Crit. Care Med. 2017, 45, e994–e1000. [Google Scholar] [CrossRef]
- Avila, J.O.; Smith, B.C.; Seaberg, D.C. Use of echocardiography to identify appropriate placement of a central venous catheter wire in the vena cava prior to cannulation. Acad. Emerg. Med. 2014, 21, E1–E2. [Google Scholar] [CrossRef] [PubMed]
- Bedel, J.; Vallée, F.; Mari, A.; Riu, B.; Planquette, B.; Geeraerts, T.; Génestal, M.; Minville, V.; Fourcade, O. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: A periprocedural method to evaluate catheter placement. Intensive Care Med. 2013, 39, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Zick, G.; Eimer, C.; Renner, J.; Becher, T.; Kott, M.; Schädler, D.; Weiler, N.; Elke, G. Sonographische Visualisierung des Führungsdrahtes und Positionierung des zentralen Venenkatheters: Eine prospektive Beobachtungsstudie. Der Anaesthesist 2020, 69, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.P.; Bithal, P.K.; Toshniwal, G.R.; Prabhakar, H.; Dash, H.H. Saline flush test for bedside detection of misplaced subclavian vein catheter into ipsilateral internal jugular vein. Br. J. Anaesth. 2009, 102, 499–502. [Google Scholar] [CrossRef]
- Wilson, S.P.; Assaf, S.; Lahham, S.; Subeh, M.; Chiem, A.; Anderson, C.; Shwe, S.; Nguyen, R.; Fox, J.C. Simplified point-of-care ultrasound protocol to confirm central venous catheter placement: A prospective study. World J. Emerg. Med. 2017, 8, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Tozer, J.; Vitto, M.J.; Joyce, M.; Taylor, L.; Evans, D.P. Central Venous Catheter Confirmation by Ultrasonography: A Novel Instructional Protocol. South. Med. J. 2020, 113, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.M.; Babu, S.C.; Goyal, A.; Mateo, R.B.; Madden, R.E. Arterial misplacement of large-caliber cannulas during jugular vein catheterization: Case for surgical management. J. Am. Coll. Surg. 2004, 198, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Gibson, F.; Bodenham, A. Misplaced central venous catheters: Applied anatomy and practical management. Br. J. Anaesth. 2013, 110, 333–346. [Google Scholar] [CrossRef] [PubMed]
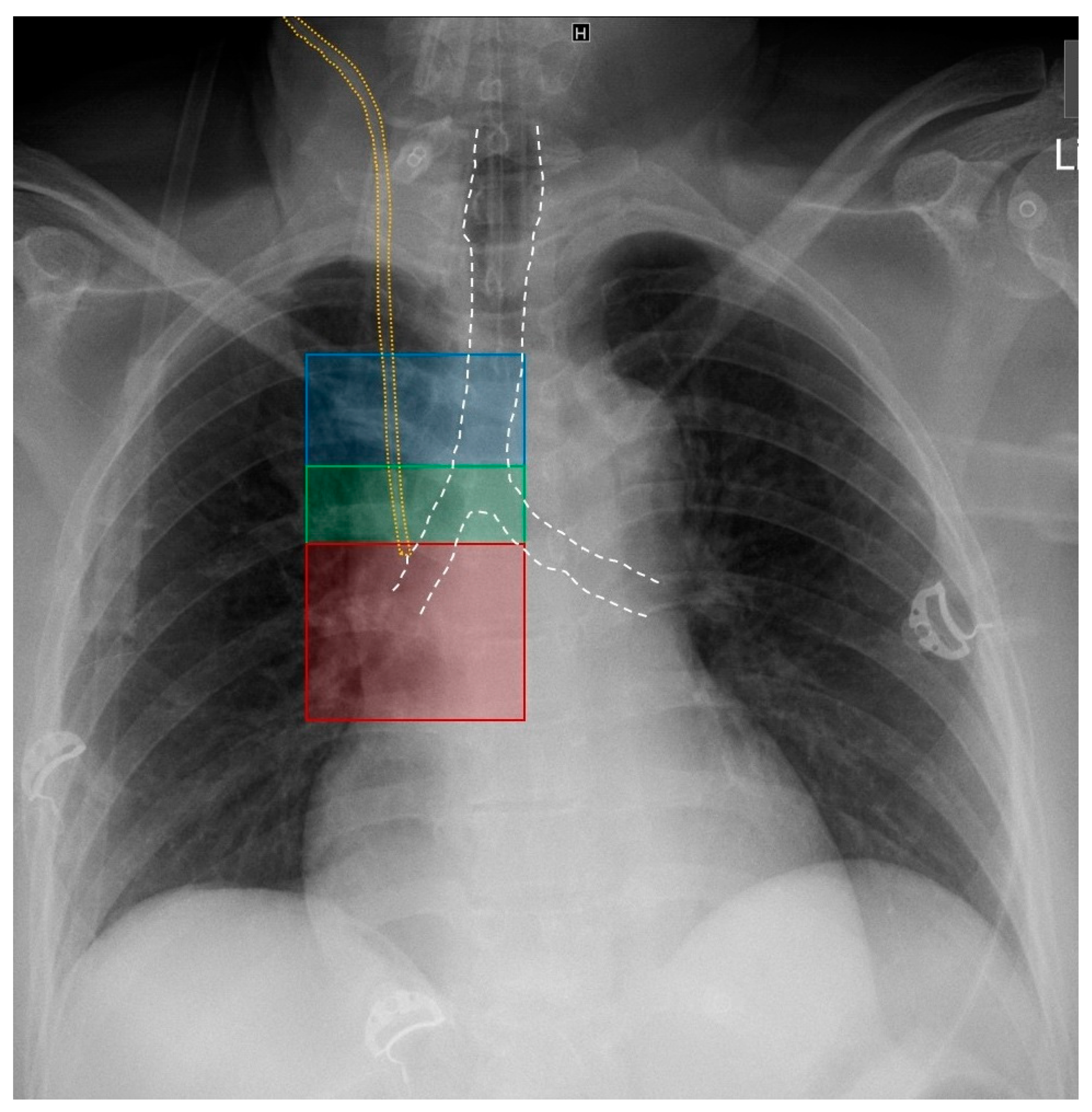
| RASS | Echocardiographic Correlate | Anatomical Correlate | Radiological Correlate | CVC Position |
|---|---|---|---|---|
| Positive RASS | Rapid (PTB time < 1 s) appearance of multiple bubbles in the RA | CVC in SVC or RA | Zone 1: above carina level (SVC) Zone 2: at carina level (terminal SVC) Zone 3: below carina level (SVC/RA transition) | Correct position |
| Negative RASS | Slow (PTB time > 1 s) appearance and/or appearance of few bubbles in the RA | CVC in venous system (veins other than SVC or RA) | Out of zones 1–3 | Malposition |
| No appearance of bubbles in the RA | CVC in arterial system or venous system distant from heart | Out of zones 1–3 | Malposition |
| Patients (n) | 102 |
| CVCs (n) | 102 |
| Age (median, interquartile range, years) | 66 (57–76) |
| Female/male (%) | 38, 62 |
| CVC insertion site: LIJV (%) | 53.9 (55/102) |
| CVC insertion site: RIJV (%) | 44.1 (45/102) |
| CVC insertion site: LSV (%) | 2.0 (2/102) |
| Ultrasound-guidance rate (%) | 100 |
| Average number of attempts | 1 |
| Setting | ICU (100%) |
| Mechanically ventilated (%) | 100 |
| TTE views | Subxiphoid (100%) |
| TTE: technical success (%) | 100 (102/102) |
| Correctly placed catheters/RASS positive (TTE, %) | 98.0 (100/102) |
| Misplaced catheters/RASS negative (TTE, %) | 2.0 (2/102) |
| Correctly placed catheters (CXR, %) | 98.0 (100/102) |
| Correctly placed catheters in zone 1 (CXR, %) | 30.4 (31/102) |
| Correctly placed catheters in zone 2 (CXR, %) | 26.5 (27/102) |
| Correctly placed catheters in zone 3 (CXR, %) | 43.1 (44/102) |
| Misplaced catheters (CXR, %) | 2.0 (2/102) |
| Pneumothorax rate (CXR, %) | 0 (0/102) |
| Time to TTE result (min) | About 0.5 |
| Time to CXR results (min) | Within 30 |
| PTB time when RASS was positive (s) | <1 |
| PTB time when RASS was negative (s) | >2 |
| Sensitivity (%) | 100 |
| Specificity (%) | 100 |
| PPV (%) | 100 |
| NPV (%) | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ley, L.; Ghofrani, H.A.; Klingenberger, P.; Niemann, T.; Allendörfer, J.; Bandorski, D. A Simple and Safe Method for Checking the Position of Central Venous Catheters—A New and Reliable Threshold for Right Atrial Swirl Sign in Microbubbles Tests. J. Clin. Med. 2024, 13, 1657. https://doi.org/10.3390/jcm13061657
Ley L, Ghofrani HA, Klingenberger P, Niemann T, Allendörfer J, Bandorski D. A Simple and Safe Method for Checking the Position of Central Venous Catheters—A New and Reliable Threshold for Right Atrial Swirl Sign in Microbubbles Tests. Journal of Clinical Medicine. 2024; 13(6):1657. https://doi.org/10.3390/jcm13061657
Chicago/Turabian StyleLey, Lukas, Hossein Ardeschir Ghofrani, Pascal Klingenberger, Tilo Niemann, Jens Allendörfer, and Dirk Bandorski. 2024. "A Simple and Safe Method for Checking the Position of Central Venous Catheters—A New and Reliable Threshold for Right Atrial Swirl Sign in Microbubbles Tests" Journal of Clinical Medicine 13, no. 6: 1657. https://doi.org/10.3390/jcm13061657
APA StyleLey, L., Ghofrani, H. A., Klingenberger, P., Niemann, T., Allendörfer, J., & Bandorski, D. (2024). A Simple and Safe Method for Checking the Position of Central Venous Catheters—A New and Reliable Threshold for Right Atrial Swirl Sign in Microbubbles Tests. Journal of Clinical Medicine, 13(6), 1657. https://doi.org/10.3390/jcm13061657








