The Present and Future of the Clinical Use of Physiological Traits for the Treatment of Patients with OSA: A Narrative Review
Abstract
1. Introduction
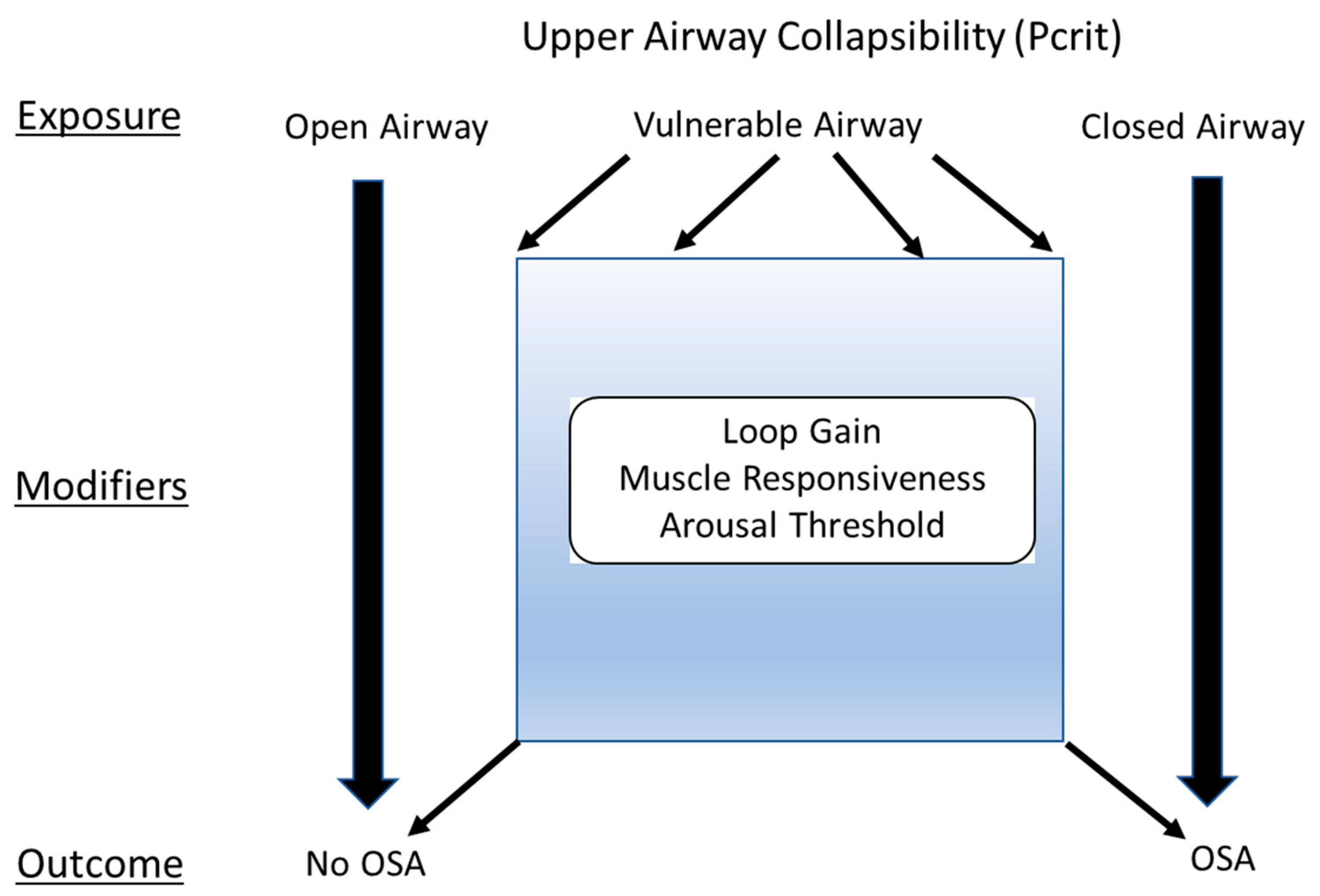
2. OSA Traits
2.1. Anatomic Contributions to Upper-Airway (UA) Collapsibility (the Exposure)
2.2. Physiological Contributors to OSA (the Effect Modifiers)
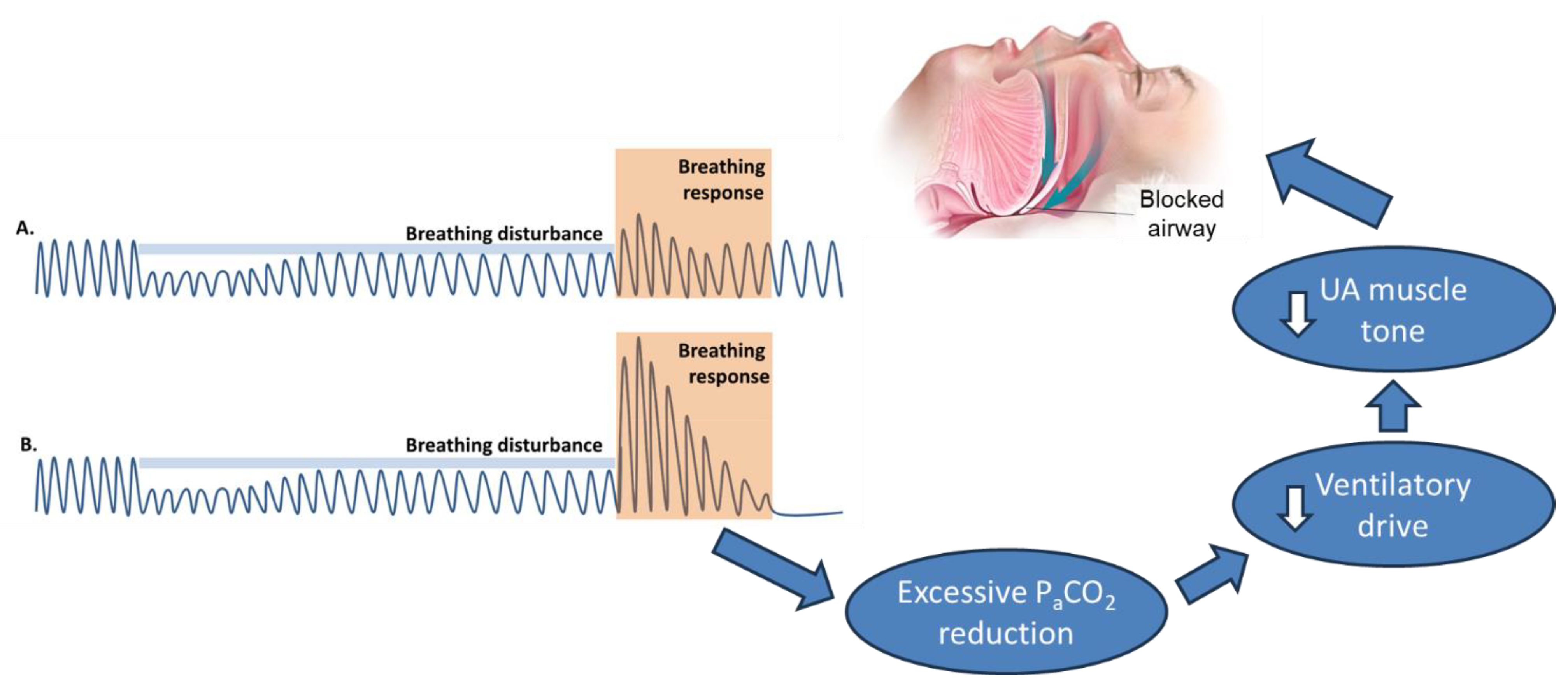
2.2.1. Ventilator Control Instability (High Loop Gain)
2.2.2. Pharyngeal Muscle Responsiveness
2.2.3. Low Arousal Threshold
2.2.4. Sex and Race Differences in Pathophysiology of OSA
2.2.5. Role of Comorbid Conditions in the Pathophysiology of OSA
3. Measurement of OSA Traits
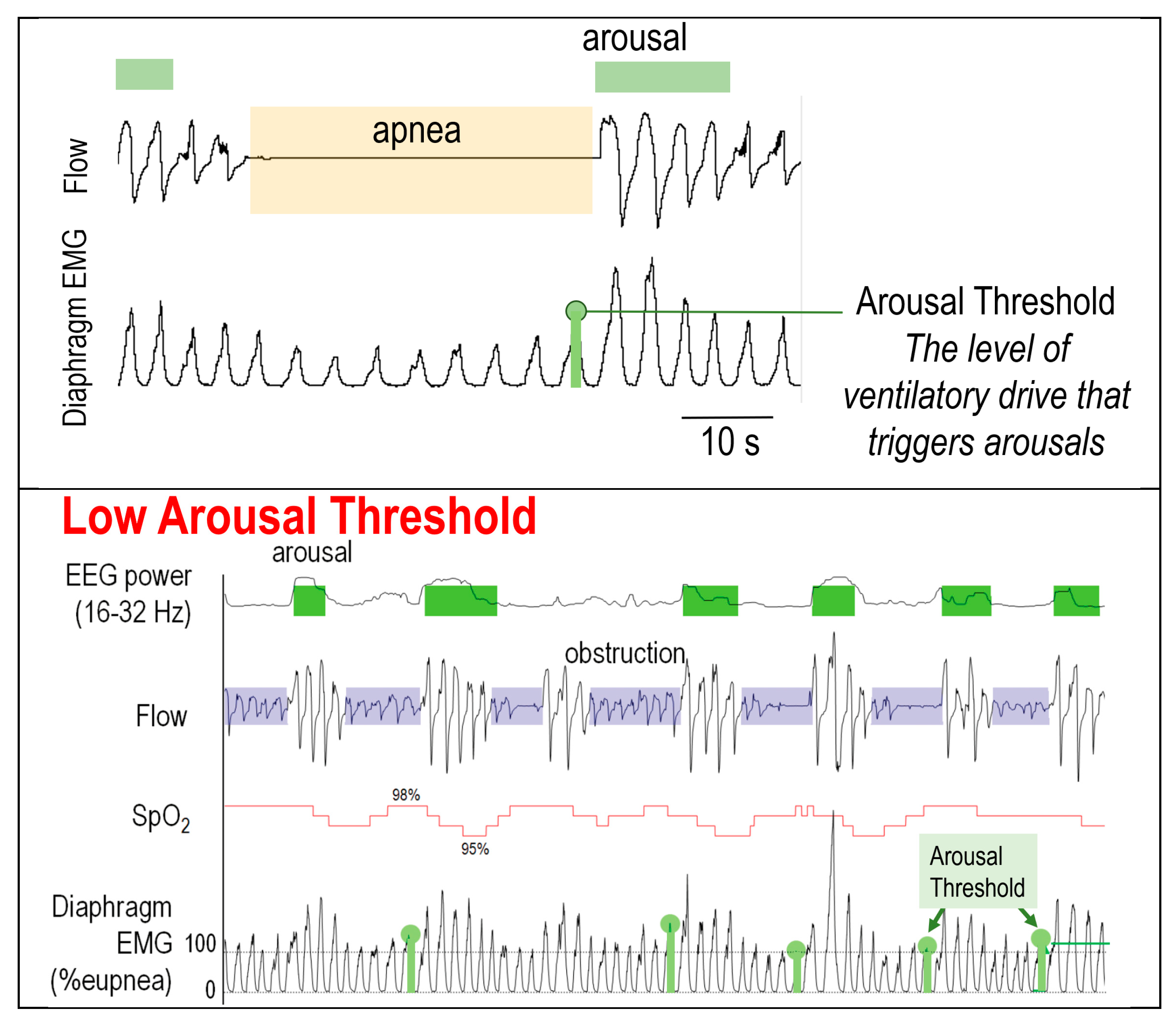
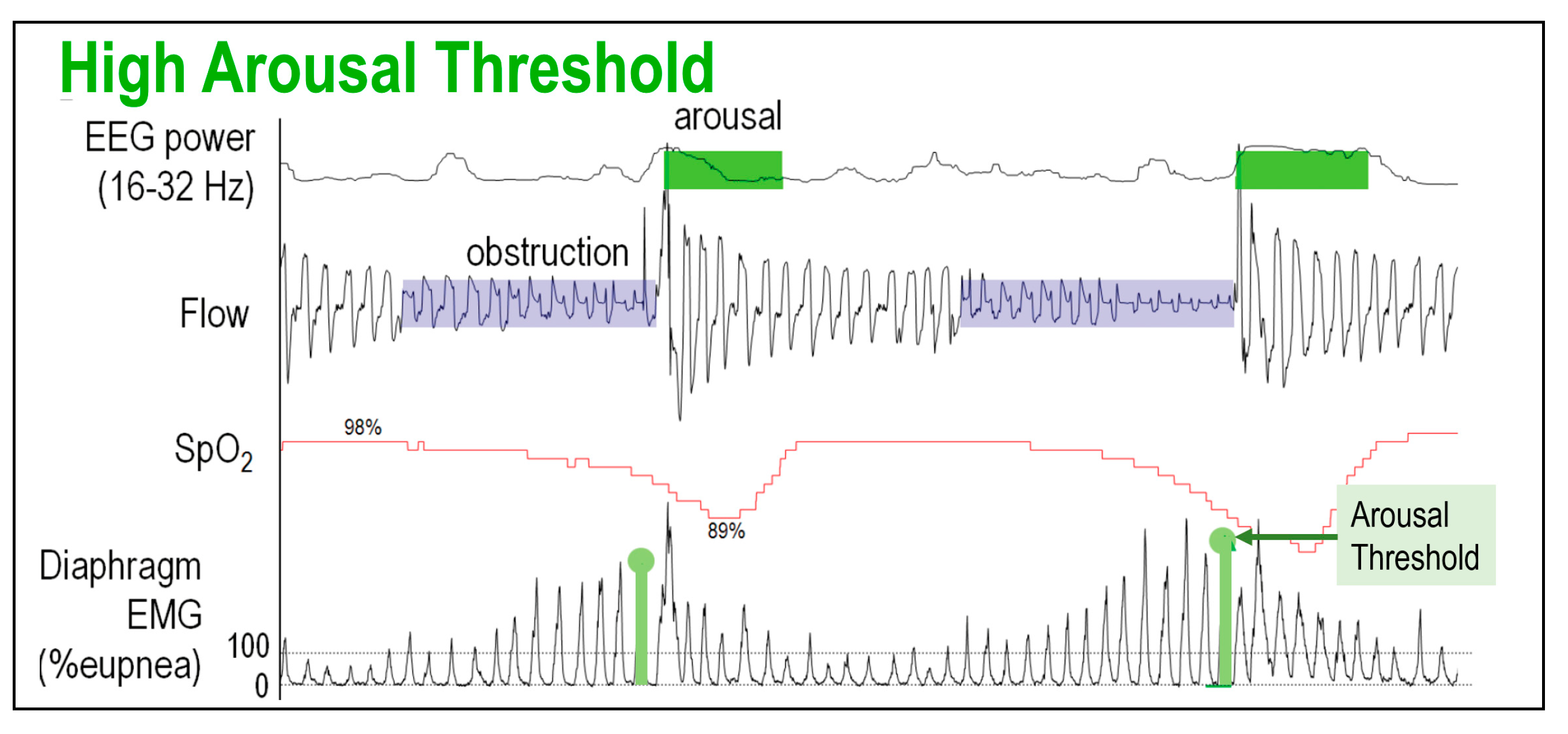
4. Point of Care Surrogate Measures of the Traits
5. Precision Treatment of OSA Using the Traits
5.1. Use of Traits to Predict Responses to OSA Therapy
5.1.1. Conventional PAP therapy
5.1.2. Oral Appliance
5.1.3. Upper Airway Surgery
5.1.4. Hypoglossal Nerve Stimulation
5.1.5. Pharmacotherapy
5.2. Use of Traits to Guide Multi-Modal Therapy for OSA
5.2.1. Targeting Anatomy
5.2.2. Combination Therapy
5.3. Targeting Traits to Select Initial Therapy
6. Current Limitations
7. Future Directions
- (1)
- Standardized, reliable, and reproducible tools must be developed to measure the OSA traits using readily available clinical data (e.g., home respiratory polygraphy, wearables). Such tools should integrate how much each trait, or trait combination, contributes to OSA severity in each individual.
- (2)
- A better understanding is required of how traits relate to clinical phenotypes (e.g., the ArTH and LG in those with OSA and insomnia) and their contributions to OSA in those with common, co-morbid conditions (e.g., post-traumatic stress disorder, chronic obstructive pulmonary disease, opioid dependence).
- (3)
- Studies are needed to determine the effect sizes of interventions targeting the OSA traits (e.g., acetazolamide for high LG) and their impact on OSA severity.
- (4)
- Prospective validation of the traits (or their combinations) is needed to predict treatment responses to established therapies (e.g., randomization to a sedative-hypnotic vs. a placebo with CPAP based on the ArTH to assess the impact on CPAP adherence, daytime function, and quality of life).
- (5)
- Longer-term (at least 3–6 months), randomized clinical trial studies on patient-centered outcomes are needed, for both prediction of outcomes and modification of the OSA traits to improve OSA outcomes. Studies should examine prognostic markers that are more effective than the AHI in assessing OSA alleviation (e.g., hypoxic burden, heart rate response), adherence, patient symptoms, function, and quality of life.
- (6)
- Because little is known about the role of OSA traits in patient outcomes in non-white and non-male individuals, an assessment of traits in pathogenesis, clinical manifestations, and treatment outcomes in non-white and female populations is needed.
8. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Skatrud, J.; Peppard, P.E. Risk factors for obstructive sleep apnea in adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.; Kohler, M.; McNicholas, W.T.; Barbe, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pepin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Simpson, F.C. Obstructive sleep apnea and psychiatric disorders: A systematic review. J. Clin. Sleep Med. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef]
- Sircu, V.; Colesnic, S.I.; Covantsev, S.; Corlateanu, O.; Sukhotko, A.; Popovici, C.; Corlateanu, A. The Burden of Comorbidities in Obstructive Sleep Apnea and the Pathophysiologic Mechanisms and Effects of CPAP. Clocks Sleep 2023, 5, 333–349. [Google Scholar] [CrossRef]
- Cao, M.T.; Sternbach, J.M.; Guilleminault, C. Continuous positive airway pressure therapy in obstuctive sleep apnea: Benefits and alternatives. Expert. Rev. Respir. Med. 2017, 11, 259–272. [Google Scholar] [CrossRef]
- Hidden Health Crisis Costing America Billions. Underdiagnosing and Undertreating Obstructive Sleep Apnea Draining Healthcare System. American Academy of Sleep Medicine. Available online: http://www.aasmnet.org/sleep-apnea-economic-impact.aspx (accessed on 29 February 2024).
- Patel, S.R.; Bakker, J.P.; Stitt, C.J.; Aloia, M.S.; Nouraie, S.M. Age and Sex Disparities in Adherence to CPAP. Chest 2021, 159, 382–389. [Google Scholar] [CrossRef]
- Pandey, A.; Mereddy, S.; Combs, D.; Shetty, S.; Patel, S.I.; Mashaq, S.; Seixas, A.; Littlewood, K.; Jean-Luis, G.; Parthasarathy, S. Socioeconomic Inequities in Adherence to Positive Airway Pressure Therapy in Population-Level Analysis. J. Clin. Med. 2020, 9, 442. [Google Scholar] [CrossRef]
- Bakker, J.P.; Weaver, T.E.; Parthasarathy, S.; Aloia, M.S. Adherence to CPAP: What Should We Be Aiming For, and How Can We Get There? Chest 2019, 155, 1272–1287. [Google Scholar] [CrossRef]
- Crawford, M.R.; Espie, C.A.; Bartlett, D.J.; Grunstein, R.R. Integrating psychology and medicine in CPAP adherence--new concepts? Sleep Med. Rev. 2014, 18, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. Head. Neck Surg. 2016, 45, 43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shukla, G. Polysomnographic determinants of requirement for advanced positive pressure therapeutic options for obstructive sleep apnea. Sleep Breath. 2018, 22, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Mulgrew, A.T.; Lawati, N.A.; Ayas, N.T.; Fox, N.; Hamilton, P.; Cortes, L.; Ryan, C.F. Residual sleep apnea on polysomnography after 3 months of CPAP therapy: Clinical implications, predictors and patterns. Sleep Med. 2010, 11, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.D.; Freedland, K.E.; Carney, R.M.; Duntley, S.P.; Stepanski, E.J. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom. Med. 2007, 69, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Malhotra, A.; Mesarwi, O.; Pepin, J.L.; Owens, R.L. Endotypes and phenotypes in obstructive sleep apnea. Curr. Opin. Pulm. Med. 2020, 26, 609–614. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef]
- Edwards, B.A.; Redline, S.; Sands, S.A.; Owens, R.L. More than the Sum of the Respiratory Events: Personalized Medicine Approaches for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2020, 200, 691–703. [Google Scholar] [CrossRef]
- Zinchuk, A.; Yaggi, H.K. Phenotypic Subtypes of OSA: A Challenge and Opportunity for Precision Medicine. Chest 2020, 157, 403–420. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Barbe, F.; Gozal, D.; Agusti, A. Precision medicine in obstructive sleep apnoea. Lancet Respir. Med. 2019, 7, 456–464. [Google Scholar] [CrossRef]
- Sutherland, K.; Yee, B.J.; Kairaitis, K.; Wheatley, J.; de Chazal, P.; Cistulli, P.A. A Phenotypic Approach for Personalised Management of Obstructive Sleep Apnoea. Curr. Otorhinolaryngol. Rep. 2021, 9, 223–237. [Google Scholar] [CrossRef]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Owens, R.L.; Edwards, B.A.; Eckert, D.J.; Jordan, A.S.; Sands, S.A.; Malhotra, A.; White, D.P.; Loring, S.H.; Butler, J.P.; Wellman, A. An Integrative Model of Physiological Traits Can be Used to Predict Obstructive Sleep Apnea and Response to Non Positive Airway Pressure Therapy. Sleep 2015, 38, 961–970. [Google Scholar] [CrossRef]
- Kazemeini, E.; Van de Perck, E.; Dieltjens, M.; Willemen, M.; Verbraecken, J.; Op de Beeck, S.; Vanderveken, O.M. Critical to Know Pcrit: A Review on Pharyngeal Critical Closing Pressure in Obstructive Sleep Apnea. Front. Neurol. 2022, 13, 775709. [Google Scholar] [CrossRef]
- Gold, A.R.; Schwartz, A.R. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 1996, 110, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Kirkness, J.P.; Schwartz, A.R.; Schneider, H.; Punjabi, N.M.; Maly, J.J.; Laffan, A.M.; McGinley, B.M.; Magnuson, T.; Schweitzer, M.; Smith, P.L.; et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J. Appl. Physiol. 2008, 104, 1618–1624. [Google Scholar] [CrossRef]
- Wilson, S.L.; Thach, B.T.; Brouillette, R.T.; Abu-Osba, Y.K. Upper airway patency in the human infant: Influence of airway pressure and posture. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.L.; Malhotra, A.; Eckert, D.J.; White, D.P.; Jordan, A.S. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J. Appl. Physiol. 2010, 108, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.A.; Edwards, B.A.; Wellman, A.; Turton, A.; Skuza, E.M.; Berger, P.J.; Hamilton, G.S. The Effect of Body Position on Physiological Factors that Contribute to Obstructive Sleep Apnea. Sleep 2015, 38, 1469–1478. [Google Scholar] [CrossRef]
- Jordan, A.S.; Wellman, A.; Edwards, J.K.; Schory, K.; Dover, L.; MacDonald, M.; Patel, S.R.; Fogel, R.B.; Malhotra, A.; White, D.P. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J. Appl. Physiol. 2005, 99, 2020–2027. [Google Scholar] [CrossRef]
- Malhotra, A.; Huang, Y.; Fogel, R.B.; Pillar, G.; Edwards, J.K.; Kikinis, R.; Loring, S.H.; White, D.P. The male predisposition to pharyngeal collapse: Importance of airway length. Am. J. Respir. Crit. Care Med. 2002, 166, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Haxhiu, M.A.; Mitra, J.; van Lunteren, E.; Prabhakar, N.; Bruce, E.N.; Cherniack, N.S. Responses of hypoglossal and phrenic nerves to decreased respiratory drive in cats. Respiration 1986, 50, 130–138. [Google Scholar] [CrossRef]
- Badr, M.S.; Toiber, F.; Skatrud, J.B.; Dempsey, J. Pharyngeal narrowing/occlusion during central sleep apnea. J. Appl. Physiol. 1995, 78, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, A.; Gentile, F.; Navari, A.; Borrelli, C.; Mirizzi, G.; Catapano, G.; Vergaro, G.; Grotti, F.; Betta, M.; Piepoli, M.F.; et al. Contribution of the Lung to the Genesis of Cheyne-Stokes Respiration in Heart Failure: Plant Gain Beyond Chemoreflex Gain and Circulation Time. J. Am. Heart Assoc. 2019, 8, e012419. [Google Scholar] [CrossRef]
- Carberry, J.C.; Jordan, A.S.; White, D.P.; Wellman, A.; Eckert, D.J. Upper Airway Collapsibility (Pcrit) and Pharyngeal Dilator Muscle Activity are Sleep Stage Dependent. Sleep 2016, 39, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A.; Lo, Y.L.; White, D.P.; Jordan, A.S. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 2009, 135, 957–964. [Google Scholar] [CrossRef]
- Sands, S.A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Owens, R.L.; Butler, J.P.; Schwab, R.J.; Loring, S.H.; Malhotra, A.; White, D.P.; et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 930–937. [Google Scholar] [CrossRef]
- Popovic, R.M.; White, D.P. Upper airway muscle activity in normal women: Influence of hormonal status. J. Appl. Physiol. 1998, 84, 1055–1062. [Google Scholar] [CrossRef]
- Eckert, D.J.; Younes, M.K. Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. 2014, 116, 302–313. [Google Scholar] [CrossRef]
- Remmers, J.E.; de Groot, W.J.; Sauerland, E.K.; Anch, A.M. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 931–938. [Google Scholar] [CrossRef]
- Hoshino, T.; Sasanabe, R.; Murotani, K.; Hori, R.; Mano, M.; Nomura, A.; Konishi, N.; Baku, M.; Nishio, Y.; Kato, C.; et al. Estimated respiratory arousal threshold in patients with rapid eye movement obstructive sleep apnea. Sleep Breath. 2022, 26, 347–353. [Google Scholar] [CrossRef]
- Zinchuk, A.; Edwards, B.A.; Jeon, S.; Koo, B.B.; Concato, J.; Sands, S.; Wellman, A.; Yaggi, H.K. Prevalence, Associated Clinical Features, and Impact on Continuous Positive Airway Pressure Use of a Low Respiratory Arousal Threshold Among Male United States Veterans With Obstructive Sleep Apnea. J. Clin. Sleep Med. 2018, 14, 809–817. [Google Scholar] [CrossRef] [PubMed]
- McCall, C.A.; Watson, N.F. A Narrative Review of the Association between Post-Traumatic Stress Disorder and Obstructive Sleep Apnea. J. Clin. Med. 2022, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Alex, R.M.; Mann, D.; Vena, D.; Terrill, P.I.; Gell, L.K.; Zinchuk, A.; Sofer, T.; Patel, S.R.; Taranto-Montemurro, L.; et al. Pathophysiology Underlying Demographic and Obesity Determinants of Sleep Apnea Severity. Ann. Am. Thorac. Soc. 2023, 20, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.; Vasudavan, S.; Hui, D.S.; Prvan, T.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep 2010, 33, 1075–1080. [Google Scholar] [CrossRef]
- Lee, R.W.W.; Sutherland, K.; Sands, S.A.; Edwards, B.A.; Chan, T.O.; Ng, S.S.S.; Hui, D.S.; Cistulli, P.A. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology 2017, 22, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Messineo, L.; Lonni, S.; Magri, R.; Pedroni, L.; Taranto-Montemurro, L.; Corda, L.; Tantucci, C. Lung air trapping lowers respiratory arousal threshold and contributes to sleep apnea pathogenesis in COPD patients with overlap syndrome. Respir. Physiol. Neurobiol. 2020, 271, 103315. [Google Scholar] [CrossRef]
- Messineo, L.; Eckert, D.J.; Taranto-Montemurro, L.; Vena, D.; Azarbarzin, A.; Hess, L.B.; Calianese, N.; White, D.P.; Wellman, A.; Gell, L.; et al. Ventilatory Drive Withdrawal Rather Than Reduced Genioglossus Compensation as a Mechanism of Obstructive Sleep Apnea in REM Sleep. Am. J. Respir. Crit. Care Med. 2022, 205, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Brooker, E.J.; Landry, S.A.; Thomson, L.D.J.; Hamilton, G.S.; Genta, P.; Drummond, S.P.A.; Edwards, B.A. Obstructive Sleep Apnea Is a Distinct Physiological Endotype in Individuals with Comorbid Insomnia and Sleep Apnea (COMISA). Ann. Am. Thorac. Soc. 2023, 10, 1508–1515. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Lawson, Y.; Wilding, G.E. Impact of low arousal threshold on treatment of obstructive sleep apnea in patients with post-traumatic stress disorder. Sleep Breath. 2021, 25, 597–604. [Google Scholar] [CrossRef]
- Eckert, D.J. Phenotypic approaches to obstructive sleep apnoea—New pathways for targeted therapy. Sleep Med. Rev. 2018, 37, 45–59. [Google Scholar] [CrossRef]
- Issa, F.G.; Sullivan, C.E. Upper airway closing pressures in obstructive sleep apnea. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 520–527. [Google Scholar] [CrossRef]
- Wellman, A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Passaglia, C.L.; Jackson, A.C.; Gautam, S.; Owens, R.L.; Malhotra, A.; White, D.P. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J. Appl. Physiol. 2011, 110, 1627–1637. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Lowery, M.M.; Doherty, L.S.; McHugh, M.; O’Muircheartaigh, C.; Cullen, J.; Nolan, P.; McNicholas, W.T.; O’Malley, M.J. Improved surface EMG electrode for measuring genioglossus muscle activity. Respir. Physiol. Neurobiol. 2007, 159, 55–67. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; White, D.P.; Wellman, A. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Terrill, P.I.; Edwards, B.A.; Nemati, S.; Butler, J.P.; Owens, R.L.; Eckert, D.J.; White, D.P.; Malhotra, A.; Wellman, A.; Sands, S.A. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur. Respir. J. 2015, 45, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Terrill, P.I.; Edwards, B.A.; Taranto Montemurro, L.; Azarbarzin, A.; Marques, M.; de Melo, C.M.; Loring, S.H.; Butler, J.P.; White, D.P.; et al. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea. Sleep 2018, 41, zsx183. [Google Scholar] [CrossRef]
- Gell, L.K.; Vena, D.; Alex, R.M.; Azarbarzin, A.; Calianese, N.; Hess, L.B.; Taranto-Montemurro, L.; White, D.P.; Wellman, A.; Sands, S.A. Neural ventilatory drive decline as a predominant mechanism of obstructive sleep apnoea events. Thorax 2022, 77, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.A.; Joosten, S.A.; Eckert, D.J.; Jordan, A.S.; Sands, S.A.; White, D.P.; Malhotra, A.; Wellman, A.; Hamilton, G.S.; Edwards, B.A. Therapeutic CPAP Level Predicts Upper Airway Collapsibility in Patients With Obstructive Sleep Apnea. Sleep 2017, 40, zsx056. [Google Scholar] [CrossRef]
- Schmickl, C.N.; Orr, J.E.; Kim, P.; Nokes, B.; Sands, S.; Manoharan, S.; McGinnis, L.; Parra, G.; DeYoung, P.; Owens, R.L.; et al. Point-of-care prediction model of loop gain in patients with obstructive sleep apnea: Development and validation. BMC Pulm. Med. 2022, 22, 158. [Google Scholar] [CrossRef]
- Stanchina, M.; Robinson, K.; Corrao, W.; Donat, W.; Sands, S.; Malhotra, A. Clinical Use of Loop Gain Measures to Determine Continuous Positive Airway Pressure Efficacy in Patients with Complex Sleep Apnea. A Pilot Study. Ann. Am. Thorac. Soc. 2015, 12, 1351–1357. [Google Scholar] [CrossRef]
- Edwards, B.A.; Eckert, D.J.; McSharry, D.G.; Sands, S.A.; Desai, A.; Kehlmann, G.; Bakker, J.P.; Genta, P.R.; Owens, R.L.; White, D.P.; et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 1293–1300. [Google Scholar] [CrossRef]
- Dutta, R.; Delaney, G.; Toson, B.; Jordan, A.S.; White, D.P.; Wellman, A.; Eckert, D.J. A Novel Model to Estimate Key Obstructive Sleep Apnea Endotypes from Standard Polysomnography and Clinical Data and Their Contribution to Obstructive Sleep Apnea Severity. Ann. Am. Thorac. Soc. 2021, 18, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, A.; Yaggi, H.K. Treatment-Emergent Central Sleep Apnea. In Complex Sleep Breathing Disorders; Won, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 92–94. [Google Scholar]
- Joosten, S.A.; Landry, S.A.; Wong, A.M.; Mann, D.L.; Terrill, P.I.; Sands, S.A.; Turton, A.; Beatty, C.; Thomson, L.; Hamilton, G.S.; et al. Assessing the Physiologic Endotypes Responsible for REM- and NREM-Based OSA. Chest 2021, 159, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fang, F.; Wu, C.; Zhan, X.; Wei, Y. Low arousal threshold is associated with unfavorable shift of PAP compliance over time in patients with OSA. Sleep Breath. 2021, 25, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, A.V.; Redeker, N.S.; Chu, J.H.; Liang, J.; Stepnowsky, C.; Brandt, C.A.; Bravata, D.M.; Wellman, A.; Sands, S.A.; Yaggi, H.K. Physiological Traits and Adherence to Obstructive Sleep Apnea Treatment in Patients with Stroke. Am. J. Respir. Crit. Care Med. 2020, 201, 1568–1572. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Chu, J.H.; Liang, J.; Celik, Y.; Op de Beeck, S.; Redeker, N.S.; Wellman, A.; Yaggi, H.K.; Peker, Y.; Sands, S.A. Physiological Traits and Adherence to Sleep Apnea Therapy in Individuals with Coronary Artery Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 703–712. [Google Scholar] [CrossRef]
- Bakker, J.P.; Wang, R.; Weng, J.; Aloia, M.S.; Toth, C.; Morrical, M.G.; Gleason, K.J.; Rueschman, M.; Dorsey, C.; Patel, S.R.; et al. Motivational Enhancement for Increasing Adherence to CPAP: A Randomized Controlled Trial. Chest 2016, 150, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Smith, S.S.; Oei, T.P.; Douglas, J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: A randomized controlled trial. J. Consult. Clin. Psychol. 2012, 80, 151–163. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.; Catcheside, P.G.; Antic, N.A.; Smith, S.; Chai-Coetzer, C.L.; Douglas, J.; O’Grady, A.; Dunn, N.; Robinson, J.; et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: A randomized clinical trial. Sleep 2019, 42, zsz178. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Shah, A.A.; Holley, A.B.; Kelly, W.F.; Chang, A.S.; Roop, S.A.; Promotion, C.; Prognosis-The Army Sleep Apnea Program, T. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: A randomized trial. Ann. Intern. Med. 2009, 151, 696–702. [Google Scholar] [CrossRef]
- Antonaglia, C.; Vidoni, G.; Contardo, L.; Giudici, F.; Salton, F.; Ruaro, B.; Confalonieri, M.; Caneva, M. Low Arousal Threshold Estimation Predicts Failure of Mandibular Advancement Devices in Obstructive Sleep Apnea Syndrome. Diagnostics 2022, 12, 2548. [Google Scholar] [CrossRef]
- Edwards, B.A.; Andara, C.; Landry, S.; Sands, S.A.; Joosten, S.A.; Owens, R.L.; White, D.P.; Hamilton, G.S.; Wellman, A. Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1413–1422. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Cistulli, P.A.; Sutherland, K.; Madronio, M.; Eckert, D.J.; Hess, L.; Edwards, B.A.; Wellman, A.; Sands, S.A. Polysomnographic Endotyping to Select Patients with Obstructive Sleep Apnea for Oral Appliances. Ann. Am. Thorac. Soc. 2019, 16, 1422–1431. [Google Scholar] [CrossRef]
- Joosten, S.A.; Leong, P.; Landry, S.A.; Sands, S.A.; Terrill, P.I.; Mann, D.; Turton, A.; Rangaswamy, J.; Andara, C.; Burgess, G.; et al. Loop Gain Predicts the Response to Upper Airway Surgery in Patients With Obstructive Sleep Apnea. Sleep 2017, 40, zsx094. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, J.; Han, D.; Cao, X.; Ding, X.; Zhang, Y.; Xu, W.; Orr, J.; Jen, R.; Sands, S.; et al. Physiology-Based Modeling May Predict Surgical Treatment Outcome for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J., Jr.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-airway stimulation for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Op de Beeck, S.; Wellman, A.; Dieltjens, M.; Strohl, K.P.; Willemen, M.; Van de Heyning, P.H.; Verbraecken, J.A.; Vanderveken, O.M.; Sands, S.A.; Investigators, S.T. Endotypic Mechanisms of Successful Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2021, 203, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Perger, E.; Bertoli, S.; Lombardi, C. Pharmacotherapy for obstructive sleep apnea: Targeting specific pathophysiological traits. Expert. Rev. Respir. Med. 2023, 17, 663–673. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Wellman, A. Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature. J. Clin. Med. 2019, 8, 1846. [Google Scholar] [CrossRef]
- Wellman, A.; Malhotra, A.; Jordan, A.S.; Stevenson, K.E.; Gautam, S.; White, D.P. Effect of oxygen in obstructive sleep apnea: Role of loop gain. Respir. Physiol. Neurobiol. 2008, 162, 144–151. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Butler, J.P.; Owens, R.L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; Smales, E.T.; et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur. Respir. J. 2018, 52, 1800674. [Google Scholar] [CrossRef]
- Messineo, L.; Eckert, D.J.; Lim, R.; Chiang, A.; Azarbarzin, A.; Carter, S.G.; Carberry, J.C. Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. J. Physiol. 2020, 598, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Owens, R.L.; Kehlmann, G.B.; Wellman, A.; Rahangdale, S.; Yim-Yeh, S.; White, D.P.; Malhotra, A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin. Sci. 2011, 120, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.G.; Carberry, J.C.; Cho, G.; Fisher, L.P.; Rollo, C.M.; Stevens, D.J.; D’Rozario, A.L.; McKenzie, D.K.; Grunstein, R.R.; Eckert, D.J. Effect of 1 month of zopiclone on obstructive sleep apnoea severity and symptoms: A randomised controlled trial. Eur. Respir. J. 2018, 52, 1800149. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, C.N.; Landry, S.; Orr, J.E.; Nokes, B.; Edwards, B.A.; Malhotra, A.; Owens, R.L. Effects of acetazolamide on control of breathing in sleep apnea patients: Mechanistic insights using meta-analyses and physiological model simulations. Physiol. Rep. 2021, 9, e15071. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, C.N.; Landry, S.A.; Orr, J.E.; Chin, K.; Murase, K.; Verbraecken, J.; Javaheri, S.; Edwards, B.A.; Owens, R.L.; Malhotra, A. Acetazolamide for OSA and Central Sleep Apnea: A Comprehensive Systematic Review and Meta-Analysis. Chest 2020, 158, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Sands, S.A.; Eckert, D.J.; White, D.P.; Butler, J.P.; Owens, R.L.; Malhotra, A.; Wellman, A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J. Physiol. 2012, 590, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Connolly, J.G.; Campana, L.M.; Sands, S.A.; Trinder, J.A.; White, D.P.; Wellman, A.; Malhotra, A. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep 2013, 36, 281–285. [Google Scholar] [CrossRef]
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef]
- Grace, K.P.; Hughes, S.W.; Horner, R.L. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am. J. Respir. Crit. Care Med. 2013, 187, 311–319. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. The Combination of Atomoxetine and Oxybutynin Greatly Reduces Obstructive Sleep Apnea Severity. A Randomized, Placebo-controlled, Double-Blind Crossover Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1267–1276. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Azarbarzin, A.; Vena, D.; Hess, L.B.; Calianese, N.A.; White, D.P.; Wellman, A.; Sands, S.A. Effects of the Combination of Atomoxetine and Oxybutynin on OSA Endotypic Traits. Chest 2020, 157, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, P.K.; Taranto-Montemurro, L.; Ojile, J.M.; Thein, S.G.; Drake, C.L.; Rosenberg, R.; Corser, B.; Abaluck, B.; Sangal, R.B.; Maynard, J. The Combination of Aroxybutynin and Atomoxetine in the Treatment of Obstructive Sleep Apnea (MARIPOSA): A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2023, 208, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Perger, E.; Taranto Montemurro, L.; Rosa, D.; Vicini, S.; Marconi, M.; Zanotti, L.; Meriggi, P.; Azarbarzin, A.; Sands, S.A.; Wellman, A.; et al. Reboxetine Plus Oxybutynin for OSA Treatment: A 1-Week, Randomized, Placebo-Controlled, Double-Blind Crossover Trial. Chest 2022, 161, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Corser, B.; Eves, E.; Warren-McCormick, J.; Rucosky, G. Effects of atomoxetine plus a hypnotic on obstructive sleep apnea severity in patients with a moderately collapsible pharyngeal airway. J. Clin. Sleep Med. 2023, 19, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Allain, H.; Bentue-Ferrer, D.; Polard, E.; Akwa, Y.; Patat, A. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: A comparative review. Drugs Aging 2005, 22, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Sedative Hypnotics and the Risk of Falls and Fractures in the Elderly. J. Clin. Psychiatry 2018, 79, 18f12340. [Google Scholar] [CrossRef]
- Amari, D.T.; Juday, T.; Frech, F.H.; Wang, W.; Wu, Z.; Atkins, N., Jr.; Wickwire, E.M. Falls, healthcare resources and costs in older adults with insomnia treated with zolpidem, trazodone, or benzodiazepines. BMC Geriatr. 2022, 22, 484. [Google Scholar] [CrossRef]
- Heller, I.; Halevy, J.; Cohen, S.; Theodor, E. Significant metabolic acidosis induced by acetazolamide. Not a rare complication. Arch. Intern. Med. 1985, 145, 1815–1817. [Google Scholar] [CrossRef]
- Nishtala, P.S.; Chyou, T.Y. Risk of delirium associated with antimuscarinics in older adults: A case-time-control study. Pharmacoepidemiol. Drug Saf. 2022, 31, 883–891. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Gold, A.R.; Schubert, N.; Stryzak, A.; Wise, R.A.; Permutt, S.; Smith, P.L. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 1991, 144, 494–498. [Google Scholar] [CrossRef]
- Landry, S.A.; Mann, D.L.; Beare, R.; McIntyre, R.; Beatty, C.; Thomson, L.D.J.; Collet, J.; Joosten, S.A.; Hamilton, G.S.; Edwards, B.A. Oronasal vs Nasal Masks: The Impact of Mask Type on CPAP Requirement, Pharyngeal Critical Closing Pressure (P(crit)), and Upper Airway Cross-Sectional Areas in Patients With OSA. Chest 2023, 164, 747–756. [Google Scholar] [CrossRef]
- Choi, J.K.; Hur, Y.K.; Lee, J.M.; Clark, G.T. Effects of mandibular advancement on upper airway dimension and collapsibility in patients with obstructive sleep apnea using dynamic upper airway imaging during sleep. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2010, 109, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.T.; Gotsopoulos, H.; Qian, J.; Cistulli, P.A. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2003, 168, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Touyz, G.; Tanner, S.; Hillman, D.R.; Eastwood, P.R.; Walsh, J.H. Variability of human upper airway collapsibility during sleep and the influence of body posture and sleep stage. J. Sleep Res. 2011, 20, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Penzel, T.; Moller, M.; Becker, H.F.; Knaack, L.; Peter, J.H. Effect of sleep position and sleep stage on the collapsibility of the upper airways in patients with sleep apnea. Sleep 2001, 24, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.A.; Beatty, C.; Thomson, L.D.J.; Wong, A.M.; Edwards, B.A.; Hamilton, G.S.; Joosten, S.A. A review of supine position related obstructive sleep apnea: Classification, epidemiology, pathogenesis and treatment. Sleep Med. Rev. 2023, 72, 101847. [Google Scholar] [CrossRef]
- Szollosi, I.; Roebuck, T.; Thompson, B.; Naughton, M.T. Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Stokes respiration. Sleep 2006, 29, 1045–1051. [Google Scholar] [CrossRef]
- Pinna, G.D.; Dacosto, E.; Maestri, R.; Crotti, P.; Montemartini, S.; Caporotondi, A.; Guazzotti, G.; Bruschi, C. Postural changes in lung volumes in patients with heart failure and Cheyne-Stokes respiration: Relationship with sleep apnea severity. Sleep Med. 2023, 101, 154–161. [Google Scholar] [CrossRef]
- Javaheri, S.; Javaheri, S. Update on Persistent Excessive Daytime Sleepiness in OSA. Chest 2020, 158, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, M.; Vroegop, A.V.; Verbruggen, A.E.; Wouters, K.; Willemen, M.; De Backer, W.A.; Verbraecken, J.A.; Van de Heyning, P.H.; Braem, M.J.; de Vries, N.; et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015, 19, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chen, Y.J.; Lai, Y.C.; Huang, C.Y.; Huang, Y.L.; Lin, M.T.; Han, S.Y.; Chen, C.L.; Yu, C.J.; Lee, P.L. Combining MAD and CPAP as an effective strategy for treating patients with severe sleep apnea intolerant to high-pressure PAP and unresponsive to MAD. PLoS ONE 2017, 12, e0187032. [Google Scholar] [CrossRef]
- Wang, D.; Tang, Y.; Chen, Y.; Zhang, S.; Ma, D.; Luo, Y.; Li, S.; Su, X.; Wang, X.; Liu, C.; et al. The effect of non-benzodiazepine sedative hypnotics on CPAP adherence in patients with OSA: A systematic review and meta-analysis. Sleep 2021, 44, zsab077. [Google Scholar] [CrossRef]
- Schmickl, C.N.; Lettieri, C.J.; Orr, J.E.; DeYoung, P.; Edwards, B.A.; Owens, R.L.; Malhotra, A. The Arousal Threshold as a Drug Target to Improve Continuous Positive Airway Pressure Adherence: Secondary Analysis of a Randomized Trial. Am. J. Respir. Crit. Care Med. 2020, 202, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.N.; Holzer, R.C.; Thomas, R.J. Acute and long-term effects of acetazolamide in presumed high loop gain sleep apnea. Sleep Med. 2023, 107, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, D.; Zou, D.; Grote, L.; Hoff, E.; Hedner, J. Acetazolamide Reduces Blood Pressure and Sleep-Disordered Breathing in Patients With Hypertension and Obstructive Sleep Apnea: A Randomized Controlled Trial. J. Clin. Sleep Med. 2018, 14, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Aishah, A.; Tong, B.K.Y.; Osman, A.M.; Pitcher, G.; Donegan, M.; Kwan, B.C.H.; Brown, E.; Altree, T.J.; Adams, R.; Mukherjee, S.; et al. Stepwise Add-on and Endotype-informed Targeted Combination Therapy to Treat Obstructive Sleep Apnea: A Proof-of-Concept Study. Ann. Am. Thorac. Soc. 2023, 20, 1316–1325. [Google Scholar] [CrossRef]
- Edwards, B.A.; Sands, S.A.; Owens, R.L.; Eckert, D.J.; Landry, S.; White, D.P.; Malhotra, A.; Wellman, A. The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility. Sleep 2016, 39, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
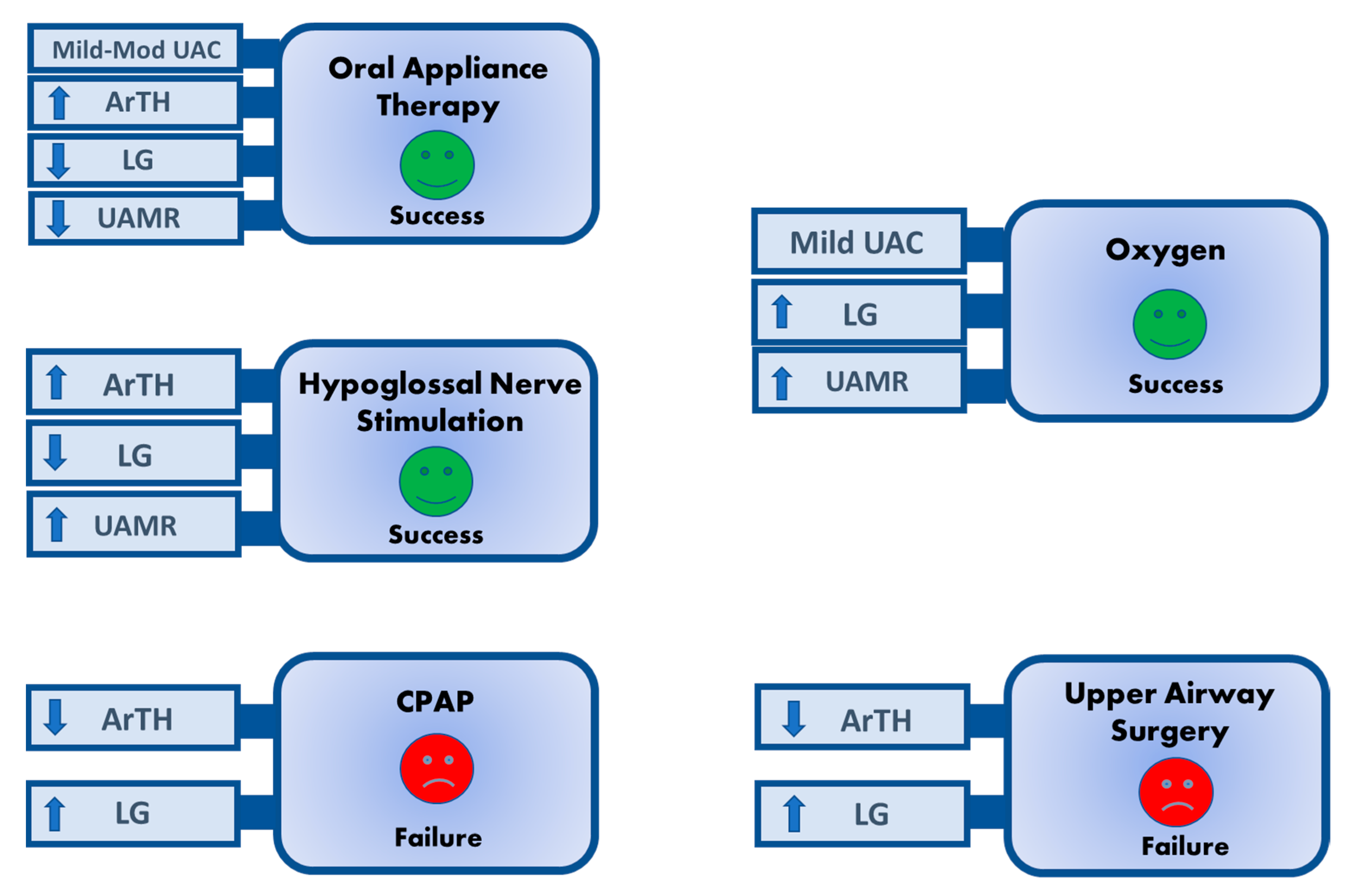
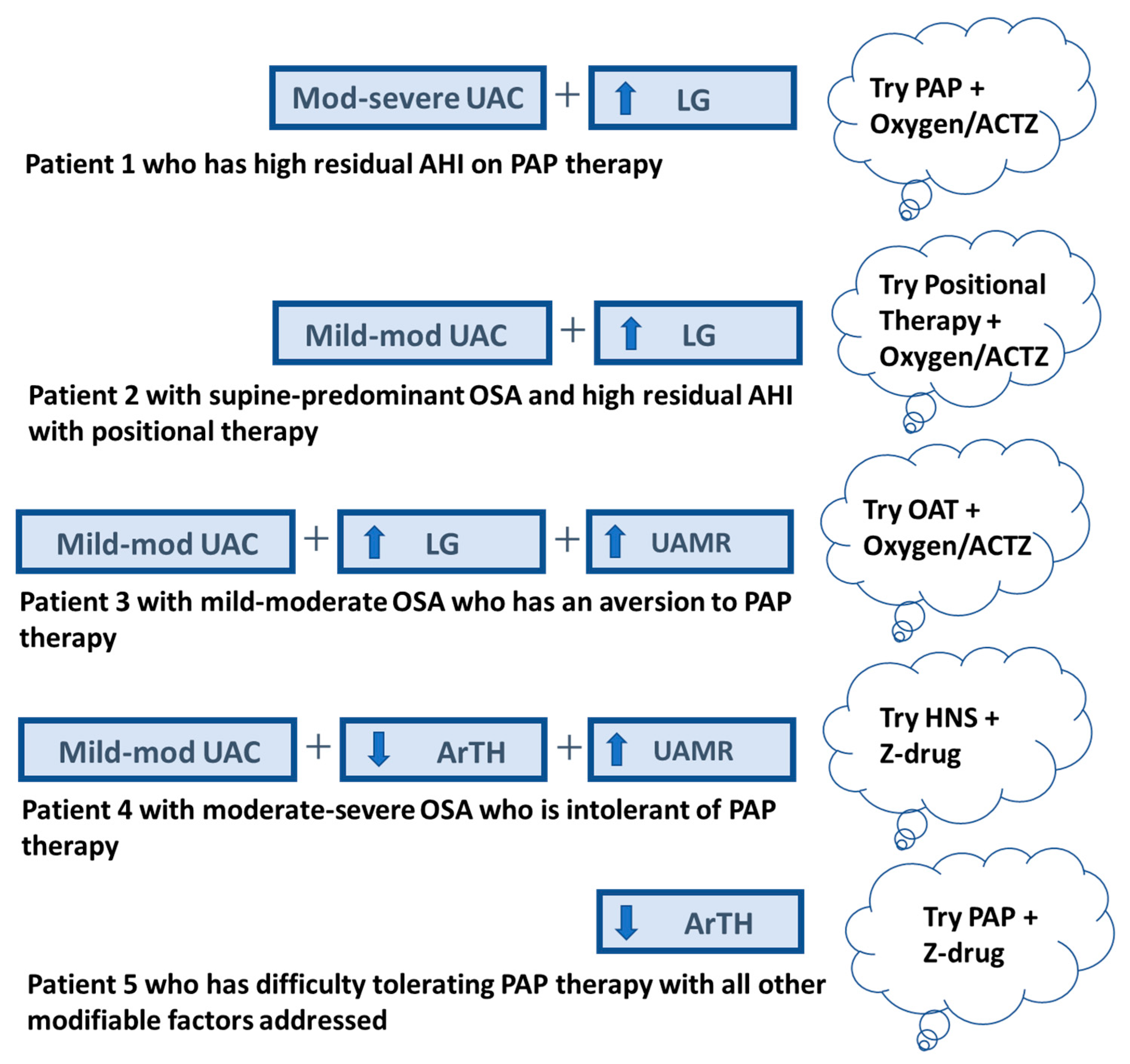
|
|
|
|
|
|
| Upper Airway Collapsibility: A CPAP requirement of 8 cm H2O on titration PSG predicts a mildly collapsible airway (89% sensitive and 84% specific) [62]. |
| Arousal Threshold: A point is given for each of the following: AHI < 30 events per hour, nadir oxygen saturation > 82.5%, and the fraction of the AHI that are hypopneas (Fhypopneas) > 58.3%. A score of 2 or more predicts a low ArTH (80% sensitive and 88% specific) [65]. |
| Loop Gain: (1) LG = 2π/[2πDR − sin(2πDR)] on titration PSG with the presence of treatment-emergent central sleep apnea (TE-CSA) where DR is the ratio of the duration of the ventilatory phase (time from the end of one apnea to the start of the next) to the total cycle duration (time from the end of one apnea to the end of the next) [64]. (2) A “zipper-like” pattern of oxygen desaturation on hypnograms in NREM-predominant OSA suggest high LG [67,68]. |
| Upper Airway Muscle Responsiveness: No published POC tool exist. A tool developed by Sands et al. [58] can be used to estimate UAMR and other traits from clinical PSG. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Zinchuk, A. The Present and Future of the Clinical Use of Physiological Traits for the Treatment of Patients with OSA: A Narrative Review. J. Clin. Med. 2024, 13, 1636. https://doi.org/10.3390/jcm13061636
Chu Y, Zinchuk A. The Present and Future of the Clinical Use of Physiological Traits for the Treatment of Patients with OSA: A Narrative Review. Journal of Clinical Medicine. 2024; 13(6):1636. https://doi.org/10.3390/jcm13061636
Chicago/Turabian StyleChu, Yvonne, and Andrey Zinchuk. 2024. "The Present and Future of the Clinical Use of Physiological Traits for the Treatment of Patients with OSA: A Narrative Review" Journal of Clinical Medicine 13, no. 6: 1636. https://doi.org/10.3390/jcm13061636
APA StyleChu, Y., & Zinchuk, A. (2024). The Present and Future of the Clinical Use of Physiological Traits for the Treatment of Patients with OSA: A Narrative Review. Journal of Clinical Medicine, 13(6), 1636. https://doi.org/10.3390/jcm13061636







