Abstract
Background: The aim of this study was to evaluate the association of oral health status and habits with the occurrence of ankylosing spondylitis (AS) in a nationwide population-based cohort in a longitudinal setting. Methods: A total of 2,415,963 individuals aged 40–79 years who underwent oral health examinations were included from the National Health Insurance Service-National Health Screening (NHIS-HEALS) cohort of Korea between 2003 and 2004. The occurrence of AS was analyzed according to the oral health status and oral hygiene habits. Results: Among 2,271,221 of the participants, AS occurred in 6366 (0.3%) participants over 16.7 years. The likelihood of AS was higher in participants who had periodontitis (hazard ratio [HR]: 1.33, 95% confidence interval [CI]: 1.20–1.46, p < 0.0001) and more missing teeth (HR: 1.68, 95% CI: 1.42–1.99, p < 0.0001). However, better oral hygiene habits such as frequent tooth brushing (HR: 0.77, 95% CI: 0.71–0.83, p < 0.0001) and a history of dental scaling within the last year (HR 0.88, 95% CI 0.82–0.95, p = 0.001) were associated with a lower occurrence of AS. Conclusions: Periodontitis and an increased number of missing teeth could be related to the occurrence of late-onset AS. Improved oral hygiene care may attenuate the likelihood of late-onset AS.
1. Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease characterized by axial and enthesis inflammation leading to new bone formation [1]. AS frequently occurs during the third decade of life, most commonly among young people, with 0.1–1.4% of all people prevalence the disease [2]. Although AS is usually an early-onset disease, and given that the assessment of SpondyloArthritis International Society (ASAS) classification criteria defines inflammatory back pain as starting before the age of 45 years, late-onset AS increases with a higher life expectancy [3,4].
Periodontitis is one of the most common infectious diseases and is characterized by chronic inflammation of the tooth-supporting tissue causing bone loss [5]. Although periodontitis originates from the local inflammatory process associated with the accumulation of oral bacteria in dental plaque, severe damage to soft and hard periodontal tissues occurs as systemic inflammation continues owing to immune system activation [6]. Thus, periodontitis can influence various systemic diseases including diabetes, certain cancers, neurodegenerative diseases, osteoporosis, coronary artery disease, and strokes [7]. Systemic inflammation and elevated pro-inflammatory cytokines in chronic periodontitis can be associated with insulin resistance leading to diabetes and with endothelial dysfunction leading to impaired vasodilation and promoting atherosclerosis [8]. Recent studies have shown that cytokines produced in periodontitis are involved in the regulation of osteoclasts and there is a link between periodontitis and systemic bone loss such as osteoporosis [9]. Periodontitis has also been shown to be associated with some systemic inflammatory diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and inflammatory bowel diseases (IBD) [10,11,12]. Numerous clinical, epidemiologic, and serologic studies have demonstrated a notable association between RA and periodontitis [13,14]. Low-grade inflammation related to SLE is correlated with the dysbiosis of oral microbiota in periodontal diseases [11]. Moreover, interleukin (IL)-17, which has a pivotal role in many immune-mediated inflammatory diseases including psoriasis, inflammatory arthritis, and AS, has been suggested as a potent proinflammatory mediator that can explain the occurrence of comorbid periodontitis [12].
Evidence has been reported that periodontitis can affect and be affected by systemic inflammation and that it can be a modifiable risk factor in the systemic inflammatory burden [15]. Most commonly, two dimensions are used to stage and classify periodontitis: severity, defined by clinical attachment loss, radiographic bone loss, and tooth loss; and complexity, defined by probing depth and marginal bone loss. However, grading that predicts the progression of periodontitis, including age, smoking, diabetes, and inflammatory markers, is also emerging, reflecting the influence of systemic inflammation of periodontitis [16].
Several studies have suggested an association between AS and periodontitis [17,18,19]. A previous study reported that patients with AS have 6.8 times the chance of periodontitis [17] and 1.8 times the chance of having a previous diagnosis of chronic periodontitis [18]. A systemic review on periodontitis and its association with AS showed significantly higher prevalence of periodontitis in patients with AS compared to patients without AS [20]. A study that evaluated periodontal measurements in patients with AS and healthy controls also identified the intercorrelation between periodontitis and AS. Periodontal indicators such as bleeding on probing (BOP) and clinical attachment loss (CAL) showed a significant positive correlation with Bath Ankylosing Spondylitis Metrology Index (BASMI), an indicator of motor limitation in AS, and with Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), which reflects disease activity of AS by the extent of enthesis involvement [21]. Conversely, another study showed that chronic periodontitis was associated with the severity of spinal immobility but not with AS [19]. This inconsistency might arise from small study sample sizes or inaccurate case definition of each disease, such as that resulting from self-reporting. Until now, there have been limited longitudinal studies on the relationship of overall oral health or related habits such as tooth loss, tooth brushing, and dental scaling, with AS in the general population.
In the current study, we hypothesized that poor oral health status, such as periodontitis, is associated with an increased risk of AS, and that improved oral hygiene habits are related to a lower risk of AS. This study aimed to investigate the association of oral health examination findings and habits with the occurrence of AS using a longitudinal database from a nationwide population-based cohort.
2. Materials and Methods
2.1. Data Source
This research used the National Health Insurance Service-National Health Screening (NHIS-HEALS) cohort database of Korea. The NHIS, as Korea’s exclusive insurance provider, serves nearly 97% of Koreans, while the Medical Aid program, also operated by the NHIS, serves the remaining 3% of the population [22]. The NHIS encourages its subscribers to undergo annual health screening that follows standardized protocols. The NHIS-HEALS cohort database comprises demographic and socioeconomic data, health screening results, and a claims database that encompasses details such as diagnosis, prescription, and treatment methods. In addition to measuring height, weight, blood pressure, and performing laboratory tests and lifestyle questionnaires relating to oral hygiene habits, the health screening process also involves a dental examination conducted by dentists. Participants underwent dental evaluation conducted by dentists to assess the number of teeth lost and other dental issues. The individuals in the NHIS-HEALS dataset did not participate in the planning, analysis, or documentation of this study. The Institutional Review Board of Ewha Woman’s University College of Medicine approved this study (2020-08-018, date of approval: 1 September 2020) and waived the requirement for participant consent.
2.2. Study Population
The NHIS-HEALS cohort comprises representative, randomly selected samples of approximately 2,415,963 individuals aged 40–79 years who participated in health screenings between 2003 and 2004, approximately 40% of the total population who received national health examinations (dataset number: NHIS-2022-01-313) [22,23]. Participants with at least one missing key variable (n = 130,980) were excluded. Then, participants with a previous diagnosis of AS between 2002 and the index date of oral health examination (n = 13,762) were excluded to provide 1 year washout. Finally, 2,271,221 participants were included for the analysis (Figure 1).
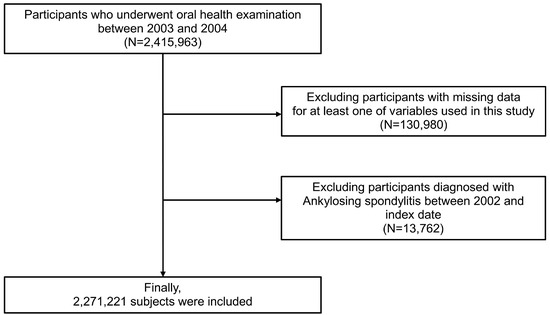
Figure 1.
Flow chart of study population.
2.3. Definition and Variables
The index date was set as the date when the oral health examination was performed. The baseline characteristics of the participants, including age, sex, household income, and body mass index (BMI), were collected at the index date. Data regarding smoking behavior, frequency of alcohol intake (per week), and regularity of physical exercise (measured by frequency per week) were gathered through the questionnaires. Smoking status was categorized as non, former, and current smokers. Periodontitis was defined as meeting one of the two conditions: (1) having at least two claims for periodontitis diagnosis codes (International Classification of Diseases, 10th Revision (ICD)-10 codes: K052-054), with at least one recorded claim under any of following procedure codes: U0010, U1010, U2211, U2221-22, U2231-33, U2240, U4454-55, U1051-52, U1060, U4660, U4662, or M0111); or (2) the presence of a periodontal pocket as detected by a dentist on a dental examination. A probing depth of 4 mm or more was defined as a pathological periodontal pocket on the examination [24]. The dentists also detected the number of teeth missing during the oral health examination. We classified the number of missing teeth into the following four groups: 0, 1–7, 8–14, and more than 15, irrespective of underlying causes such as periodontal disease or other dental problems. The participants’ oral hygiene habits were categorized based on three factors: frequency of tooth brushing (0–1 time, 2 times, and ≥3 times per day), whether they visited a dental clinic for any reason, and whether they underwent dental scaling at least once in the past year. Comorbidities were detected between January 2002 and the index date via the following criteria based on ICD-10 diagnostic codes (Appendix A) [25,26].
2.4. Study Outcomes
The study outcome was the occurrence of AS, defined as at least one claim of diagnostic code ICD-10 M45, with an individual copayment beneficiaries program (ICBP) code (V140). Since the Korean government subsidized medical expenses for patients with rare and intractable diseases (RID) through an ICBP, AS was designated as an RID covered by this program. All patients enrolled as having AS were required to have their diagnosis certified by physicians following the modified New York criteria for AS [27] with reasonable test results, which makes the AS diagnostic code reliable. The follow-up period was from the index date until the occurrence of AS, the participant died, or the end of December 2020—whichever appeared first.
2.5. Statistical Analysis
The groups’ baseline characteristics were analyzed using the chi-square test for categorical variables and the independent t-test for continuous variables. Continuous variables are demonstrated as the means ± standard deviation, and categorical variables are presented as numbers (percentages). Propensity score matching (PSM) was applied to equalize the baseline characteristics and reduce potential confounding. PSM was conducted using a greedy nearest-neighbor algorithm with a 1:4 ratio. A standardized mean difference (SMD) < 0.1 indicated suitability. We calculated the AS incidence by dividing the total number of AS cases by the cumulative persons-per-years. Kaplan–Meier survival curves with the log-rank test was employed to assess the association of oral health status and oral hygiene habits with incident AS risk. We used Cox’s proportional hazard regression to determine the risk of oral health status and oral hygiene habits for the occurrence of AS and calculated the hazard ratio (HR) and 95% confidence interval (CI). The multivariable regression model was created with adjusting age, sex, body mass index, income, alcohol consumption, smoking status, frequency of physical activity, and comorbidities (hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, cancer, renal disease, RA, and SLE). All statistical analyses were performed using the Statistical Analysis System software (SAS version 9.2, SAS Institute, Cary, NC, USA). All values with p-values < 0.05 were considered statistically significant.
3. Results
The average age of the 2,271,221 included participants was 42.1 ± 12.8 years, and 66.3% were male. Among all participants, 922,356 (40.6%) brushed their teeth over three times a day, 13,841 (0.6%) had more than 15 missing teeth, and 523,203 (23.0%) had received dental scaling within the last year. The baseline characteristics and comparative analyses based on the presence of periodontitis are demonstrated in Table 1. The results of PSM applied to equalize the baseline characteristics and reduce potential confounding were demonstrated in Table 2.

Table 1.
Baseline characteristics of participants according to periodontitis.

Table 2.
Baseline characteristics of participants according to periodontitis before and after propensity score matching.
A total of 6366 (0.3%) participants developed AS over the median duration of 16.7 [interquartile range 16.2–17.2] years. Figure 2 illustrates Kaplan–Meier survival curves for AS according to the oral health conditions and oral hygiene habits. The curve demonstrated that the participants with periodontitis and increased number of missing teeth exhibited a greater risk for AS (p < 0.0001 for both), and improved oral hygiene habits such as more frequent tooth brushing and having received dental scaling within the past year were also related, with a lower occurrence of AS (p < 0.001).
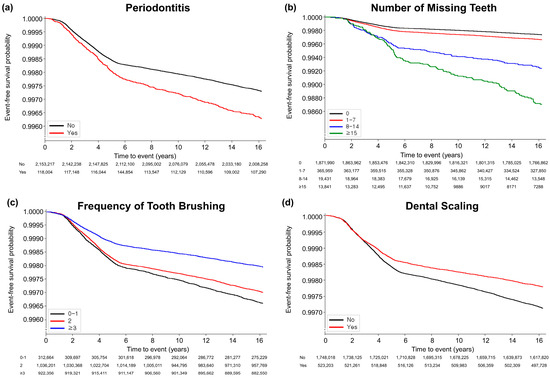
Figure 2.
Kaplan-Meier survival curves for occurrence of ankylosing spondylitis according to oral hygiene status and habits. (a) Periodontitis (p < 0.0001); (b) number of missing teeth (p < 0.0001); (c) frequency of tooth brushing (times/per day) (p < 0.0001); (d) dental scaling within previous year (p = 0.001).
In multivariable analysis, periodontitis was associated with the higher occurrence of AS (adjusted HR: 1.33, 95% CI: 1.20–1.46, p < 0.0001). An increased number of missing teeth was correlated with an increased risk for the occurrence of AS. The adjusted HRs (in reference to the subject without missing teeth) were: 1.68 (95% CI: 1.42–1.99, p < 0.0001, p for trend <0.0001) for subjects with more than 15 missing teeth. Moreover, brushing teeth more frequently showed negative correlation with the occurrence of AS. Compared to the subjects who brushed their teeth less than once a day, those who brushed over three times a day (adjusted HR: 0.77, 95% CI: 0.71–0.83, p < 0.0001) had a decreased risk of AS. Furthermore, those who underwent dental scaling within one year showed a reduced risk for the occurrence of AS (adjusted HR: 0.88, 95% CI: 0.82–0.95, p = 0.001) (Table 3).

Table 3.
Association of oral health status and oral hygiene behaviors with occurrence of ankylosing spondylitis.
According to the cox regression analysis for each variable, the risk of AS was higher in those aged 65–79 years than in those aged 40–64 years (adjusted HR: 3.23, 95% CI: 2.85–3.65, p < 0.0001). Drinking alcohol more than five times a week increased the HR of AS to 1.38 (1.10–1.72) according to the crude model, but did not show significance after adjustment. Moreover, having metabolic comorbidities such as hypertension and diabetes mellitus were associated with increased risk of AS (adjusted HR: 1.21, 95% CI: 1.09–1.33, p = 0.001 and adjusted HR: 1.21, 95% CI: 1.05–1.39, p = 0.009). Overall, the HR of AS occurrence related to oral health status and behaviors maintained a similar pattern even after PSM and variable adjustment (Supplementary Table S1).
4. Discussion
This study demonstrated that periodontitis and increased tooth loss are associated with an increased risk of AS occurrence and that improved oral hygiene through frequent tooth brushing and dental scaling was related to a decreased risk of AS.
Several studies have investigated the association between AS and periodontitis [17,18,19,20,28,29,30]. A German study comparing patients with AS and healthy controls showed that patients with AS had a higher risk of periodontitis than the controls [17]. Additionally, it has been reported that bleeding on probing among the periodontal parameters was significantly higher in patients with AS than in the control group [28]. In a study using Taiwanese administrative claim data, patients with AS had an increased history of having been previously diagnosed with periodontitis than the control group, suggesting a bidirectional association between the two diseases [18]. Moreover, some previous studies have suggested a relationship between immobility and periodontitis in patients with AS [19,29]. Systemic reviews on the association between periodontitis and the disease activity of AS have shown that patients with AS have a high prevalence of periodontitis, which is related to poor oral hygiene [20,30]. However, other studies have failed to show an association between periodontitis and AS. Although bleeding on probing was significantly associated with AS and spondylarthritis, some studies have shown that other periodontal parameters and periodontitis were not associated with AS [19,28,31]. The differences in the results of previous studies could be attributed to the difference in the definition of the disease group, a small sample size, or a short follow-up period. In this research, periodontitis was associated with an increased AS risk. This result is considered to be reliable given that errors due to disease definition were minimized as we identified periodontitis not by questionnaires but according to the result of direct oral examination by a dentist or diagnostic code combined with dental procedures, and AS according to the accurate diagnostic code registered as RID in the NHIS. This finding indirectly suggests that treating and controlling periodontitis may contribute to reducing risk factors for developing AS. However, the mechanism for the high risk of AS occurrence in patients with periodontitis is unclear, and research related to this mechanism is still limited. IL-17, which plays a vital role in the development and ossification of AS, has also been revealed as important in periodontitis. Additionally, periodontitis is associated with spondyloarthropathy other than AS, such as psoriasis and IBD, meaning that additional research will be needed.
In addition, a distinctive aspect of this research is that we mainly identified late-onset AS in patients with periodontitis because the participants who underwent oral health examinations were aged 40–79 years. AS usually occurs in young men aged 20–40, and it seldom occurs as a late-onset disease known to have slightly different epidemiologic and clinical characteristics. Late-onset AS shows lower human leukocyte antigen B27 (HLA-B27) positivity, higher levels of inflammatory markers, more cervical or peripheral joint involvement, and a higher incidence in females [32,33,34]. Therefore, these differential characteristics of late-onset AS might have influenced the result as we mainly detected late-onset AS in the study.
In this study, an increased number of missing teeth was related to a higher risk of AS. In a previous study examining the association between tooth loss and AS disease parameters, older age and increased BASMI were correlated with a lower number of remaining teeth in patients with AS [35]. Additionally, the prevalence of loose teeth or loss of natural teeth was increased in patients with AS compared to the non-AS group [36]. Although tooth loss is a complex problem with various causes, it can be considered as a periodontal disease burden. Hence, our results align with the close relationship between periodontitis and AS. Moreover, as tooth loss is related to nutrient intake, it may contribute to the development of AS by contributing to malnutrition [37] or even dysbiosis of the gut microbiome [38].
Contrary to the association of periodontitis with AS observed in this study, behaviors that improve oral hygiene such as tooth brushing or dental scaling were related to a reduced risk of AS. Until now, no previous study has investigated the effect of oral hygiene improvement on the risk of AS. D’Aluto et al. showed decreased C-reactive protein and inflammatory marker levels after periodontal therapy [39], while other studies revealed that frequent tooth brushing effectively prevented several systemic diseases [25,26,40]. The mechanism by which improvement in oral hygiene reduces the risk of developing AS is unclear. AS develops as a combination of various genetic and environmental factors. Among the known environmental factors, infection is acknowledged to play a contributing role as both germ-free HLA-B27 transgenic rats and SKG mice are disease-free [41,42]. Moreover, a systematic review of the relationship between periodontal pathogens and AS suggested that HLA-B27-specific cytotoxic T-cell mediated autoimmune responses can be initiated when HLA-B27-positive individuals are infected with Porphyromonas gingivalis which has argine- and lysine-specific protease, and peptidylarginine deiminase (PAD) [43]. Therefore, tooth brushing and dental scaling, both of which reduce this bacterial load, may be factors that hinder autoimmune responses, leading to a decreased risk of AS.
This study has some limitations that warrant discussion. First, the degree and severity of periodontitis, including detailed attachment loss, could not be investigated because of the nature of the data. However, it was possible to evaluate the presence of periodontitis relatively accurately given that the experts directly inspected and evaluated the participants’ oral condition. Second, periodontitis on dental examination was defined as having a periodontal pocket with a probing depth of four millimeters or more. Since the American Academy of Periodontology advocates considering both clinical attachment loss and probing depth in periodontitis [44] and the Community Periodontal Index classifies shallow pockets as greater than four millimeters and deep pockets as exceeding six millimeters, milder forms of periodontitis might be included in this study. Third, due to the nature of insurance claim data, data related to the HLA-B27 positivity rate and AS disease activity were not included. Therefore, the possibility that it included the onset at the time and the delayed diagnosis cannot be ruled out. However, habits that improve oral health have also been shown to be associated with a lower incidence risk of AS, raising the possibility of a relationship. Lastly, given this study design, a causational relationship between oral health status, habits, and the risk of AS could not be established.
Nevertheless, this study has some strengths in that it identified the risk of incident patients of AS according to the presence of periodontitis, which might imply a bidirectional effect between periodontitis and AS. Moreover, it is meaningful in that it suggests that changes in oral hygiene habits, modifiable factors, are related to the occurrence of AS. Most importantly, this study has long tracked large-scale, representative national data, which improves the accuracy of our result, showing the association between oral health and the occurrence of late-onset AS.
Further prospective studies are required to uncover the impact of periodontitis and oral hygiene habits on the occurrence of AS. Future research, including the occurrence of AS with serial periodontal status, the effect of local periodontal treatment on the disease activity of AS, the impact of periodontitis on AS at a young age, and experimental studies mediating the correlation, might be needed to elucidate the association between oral health and AS.
5. Conclusions
The presence of periodontitis and an increased number of missing teeth could be associated factors for developing late-onset AS. In contrast, improved oral hygiene care, including frequent tooth brushing and dental scaling, might be related to a reduced risk for late-onset AS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13061606/s1, Supplementary Table S1: Cox regression model result of Ankylosing spondylitis.
Author Contributions
Conceptualization, T.-J.S.; data curation, G.H.L. and T.-J.S.; writing—original draft preparation, M.K.C., Y.C., J.-H.P. and T.-J.S.; writing—review and editing, M.K.C., Y.C., J.-H.P., G.H.L. and T.-J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1I1A1A01059868 to Y.C.). This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-00262087 to T.-J.S.). The funding source had no role in the design, conduct, or reporting of this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Ewha Woman’s University College of Medicine (IRB number: 2020-08-018, date of approval: 1 September 2020).
Informed Consent Statement
Patient consent was waived as the data were anonymized and freely accessible by the NHIS for study purposes.
Data Availability Statement
The data used in this study are available from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) database, but restrictions apply to the public availability of these data used under license for the current study. Requests for access to the NHIS data can be made through the National Health Insurance Sharing Service homepage [http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do], accessed on 15 September 2020. For access to the database, a completed application form, research proposal, and application for approval from the institutional review board should be submitted to the inquiry committee of research support in the NHIS for review.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| AS | Ankylosing spondylitis |
| ASAS | Assessment of SpondyloArthritis International Society |
| BASMI | Bath Ankylosing Spondylitis Metrology Index |
| BMI | Body mass index |
| BOP | Bleeding on probing |
| CAL | Clinical attachment loss |
| CI | Confidence interval |
| HEALS | Health screening |
| HLA-B27 | Human Leukocyte Antigen B27 |
| HR | Hazard ratio |
| IBD | Inflammatory bowel disease |
| ICBP | Individual copayment beneficiaries program |
| ICD | International classification of diseases |
| IL | Interleukin |
| MASES | Maastricht Ankylosing Spondylitis Enthesitis Score |
| NHIS | National Health Insurance Service |
| PAD | PeptidylArginine Deiminase |
| PSM | Propensity score matching |
| RA | Rheumatoid arthritis |
| RID | Rare and intractable diseases |
| SLE | Systemic lupus erythematosus |
| SMD | Standardized mean difference |
Appendix A
Hypertension was defined as satisfying one of the following criteria: (1) at least one claim of diagnostic codes ICD-10 I10–15, with the prescription of an antihypertensive agent; (2) two or more claims of diagnostic codes ICD-10 E11–14; (3) systolic/diastolic blood pressure ≥140/90 mmHg; or (4) self-reported hypertension in the questionnaire. Diabetes mellitus was defined as satisfying one of the following criteria: (1) at least one claim of diagnostic codes ICD-10 E11–14 with the prescription of an antidiabetic agent; (2) two or more claims of diagnostic codes ICD-10 E11–14; (3) fasting serum glucose level ≥7.0 mmol/L; or (4) self-reported diabetes mellitus in the questionnaire. Dyslipidemia was defined as satisfying one of following criteria: (1) at least one claim of diagnostic codes ICD-10 E78 with the prescription of a dyslipidemia-related agent; (2) two or more claims of diagnostic codes ICD-10 E78; or (3) total cholesterol ≥240 mg/dL. Atrial fibrillation was defined as two or more claims of diagnostic code ICD-10 I48. Cancer was defined as having one admission or at least three outpatient claims of diagnostic code ICD-10 C00–97, with a specific registration code of ‘V027’ or ‘V193–4’. Renal disease was defined as two or more claims of diagnostic codes ICD-10 N17-19, I12-13, E082, E102, E112, and E132, or an estimated glomerular filtration rate <60 mL/min/1.73 m2. Rheumatoid arthritis was defined as two or more claims of diagnostic codes ICD-10 M05 pr specific registration code of ‘V223’. Systemic lupus erythematosus was defined as two or more claims of diagnostic codes ICD-10 M32 or specific registration code of ‘V136’ [25,26].
References
- Ranganathan, V.; Gracey, E.; Brown, M.A.; Inman, R.D.; Haroon, N. Pathogenesis of ankylosing spondylitis—Recent advances and future directions. Nat. Rev. Rheumatol. 2017, 13, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.E.; Jones, G.T.; MacDonald, A.G.; Downham, C.; Sturrock, R.D.; Macfarlane, G.J. Global prevalence of ankylosing spondylitis. Rheumatology 2014, 53, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, E. Late-onset ankylosing spondylitis and spondylarthritis: An update on clinical manifestations, differential diagnosis and pharmacological therapies. Drugs Aging 2010, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and chemokines in periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Ray, R.R. Periodontitis: An oral disease with severe consequences. Appl. Biochem. Biotechnol. 2023, 195, 17–32. [Google Scholar] [CrossRef]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, low-grade inflammation and systemic health: A scoping review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef]
- Penoni, D.C.; Vettore, M.V.; Torres, S.R.; Farias, M.L.F.; Leão, A.T.T. An investigation of the bidirectional link between osteoporosis and periodontitis. Arch. Osteoporos. 2019, 14, 94. [Google Scholar] [CrossRef]
- Krutyhołowa, A.; Strzelec, K.; Dziedzic, A.; Bereta, G.P.; Łazarz-Bartyzel, K.; Potempa, J.; Gawron, K. Host and bacterial factors linking periodontitis and rheumatoid arthritis. Front. Immunol. 2022, 13, 980805. [Google Scholar] [CrossRef]
- Pessoa, L.; Aleti, G.; Choudhury, S.; Nguyen, D.; Yaskell, T.; Zhang, Y.; Li, W.; Nelson, K.E.; Neto, L.L.S.; Sant’Ana, A.C.P.; et al. Host-microbial interactions in systemic lupus erythematosus and periodontitis. Front. Immunol. 2019, 10, 2602. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 cells and the il-23/il-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Huang, N.; Chen, Y.M.; Chen, T.J.; Chou, P.; Lee, Y.L.; Chou, Y.J.; Lan, J.L.; Lai, K.L.; Lin, C.H.; et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: A nationwide, population-based, case-control study. Ann. Rheum. Dis. 2013, 72, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Karlson, E.W.; Costenbader, K.H. A prospective study of periodontal disease and risk of rheumatoid arthritis. J. Rheumatol. 2010, 37, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Van Dyke, T.E. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the joint efp/aapworkshop on periodontitis and systemic diseases. J. Periodontol. 2013, 84 (Suppl. S4), S24–S29. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Pischon, N.; Pischon, T.; Gülmez, E.; Kröger, J.; Purucker, P.; Kleber, B.M.; Landau, H.; Jost-Brinkmann, P.G.; Schlattmann, P.; Zernicke, J.; et al. Periodontal disease in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2010, 69, 34–38. [Google Scholar] [CrossRef]
- Keller, J.J.; Kang, J.H.; Lin, H.C. Association between ankylosing spondylitis and chronic periodontitis: A population-based study. Arthritis Rheum. 2013, 65, 167–173. [Google Scholar] [CrossRef]
- Kang, E.H.; Lee, J.T.; Lee, H.J.; Lee, J.Y.; Chang, S.H.; Cho, H.J.; Choi, B.Y.; Ha, Y.J.; Park, K.U.; Song, Y.W.; et al. Chronic periodontitis is associated with spinal dysmobility in patients with ankylosing spondylitis. J. Periodontol. 2015, 86, 1303–1313. [Google Scholar] [CrossRef]
- Pandey, A.; Rajak, R.; Pandey, M. Periodontal diseases and its association with disease activity in ankylosing spondylitis/spa: A systematic review. Eur. J. Rheumatol. 2021, 8, 168–179. [Google Scholar] [CrossRef]
- Daltaban, Ö.; Enginar, A.; Üstün, K.; Hatipoğlu, M.; Kaçar, C.; Tuncer, T. Evaluating the relationship between ankylosing spondylitis and periodontal disease: A case-control study. Clin. Oral. Investig. 2023, 27, 411–420. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The national health insurance service-national health screening cohort (nhis-heals) in korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Park, M.S.; Jeon, J.; Song, T.J.; Kim, J. Association of periodontitis with microvascular complications of diabetes mellitus: A nationwide cohort study. J. Diabetes Complicat. 2022, 36, 108107. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.H.; Kim, S.J.; Kim, J.W. Recovery from chronic periodontal disease is associated with lower risk for incident diabetes. J. Clin. Periodontol. 2022, 49, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Woo, H.G.; Lee, J.S.; Song, T.J. Better oral hygiene is associated with lower risk of stroke. J. Periodontol. 2021, 92, 87–94. [Google Scholar] [CrossRef]
- Song, T.J.; Chang, Y.; Jeon, J.; Kim, J. Oral health and longitudinal changes in fasting glucose levels: A nationwide cohort study. PLoS ONE 2021, 16, e0253769. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the new york criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Sezer, U.; Erciyas, K.; Pehlivan, Y.; Ustün, K.; Tarakçioğlu, M.; Senyurt, S.Z.; Onat, A.M. Serum cytokine levels and periodontal parameters in ankylosing spondylitis. J. Periodontal Res. 2012, 47, 396–401. [Google Scholar] [CrossRef]
- Ziebolz, D.; Douglas, D.; Douglas, D.; Schmickler, J.; Patschan, D.; Müller, G.A.; Haak, R.; Schmidt, J.; Schmalz, G.; Patschan, S. Periodontal condition is associated with disease duration and motoric disabilities in patients with ankylosing spondylitis: Results of a cross-sectional study. Rheumatol. Int. 2018, 38, 855–863. [Google Scholar] [CrossRef]
- Ratz, T.; Dean, L.E.; Atzeni, F.; Reeks, C.; Macfarlane, G.J.; Macfarlane, T.V. A possible link between ankylosing spondylitis and periodontitis: A systematic review and meta-analysis. Rheumatology 2015, 54, 500–510. [Google Scholar] [CrossRef]
- Białowąs, K.; Radwan-Oczko, M.; Duś-Ilnicka, I.; Korman, L.; Świerkot, J. Periodontal disease and influence of periodontal treatment on disease activity in patients with rheumatoid arthritis and spondyloarthritis. Rheumatol. Int. 2020, 40, 455–463. [Google Scholar] [CrossRef]
- Montilla, C.; Del Pino-Montes, J.; Collantes-Estevez, E.; Font, P.; Zarco, P.; Mulero, J.; Gratacós, J.; Rodríguez, C.; Juanola, X.; Fernández-Sueiro, J.L.; et al. Clinical features of late-onset ankylosing spondylitis: Comparison with early-onset disease. J. Rheumatol. 2012, 39, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, A.; Yilmaz, H.; Aycan, H.; Orman, M.; Kobak, S. Demographic, clinical, and laboratory features of turkish patients with late onset ankylosing spondylitis. Bosn. J. Basic. Med. Sci. 2015, 15, 64–67. [Google Scholar] [CrossRef]
- Endo, Y.; Fujikawa, K.; Koga, T.; Mizokami, A.; Mine, M.; Tsukada, T.; Uetani, M.; Kawakami, A. Characteristics of late-onset spondyloarthritis in japan: A retrospective cohort study. Medicine 2019, 98, e14431. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Bartl, M.; Schmickler, J.; Patschan, S.; Patschan, D.; Ziebolz, D. Tooth loss is associated with disease-related parameters in patients with rheumatoid arthritis and ankylosing spondylitis-a cross-sectional study. J. Clin. Med. 2021, 10, 3052. [Google Scholar] [CrossRef] [PubMed]
- Abbood, H.M.; Pathan, E.; Cherukara, G.P. The link between ankylosing spondylitis and oral health conditions: Two nested case-control studies using data of the uk biobank. J. Appl. Oral. Sci. 2018, 27, e20180207. [Google Scholar] [CrossRef] [PubMed]
- Zelig, R.; Goldstein, S.; Touger-Decker, R.; Firestone, E.; Golden, A.; Johnson, Z.; Kaseta, A.; Sackey, J.; Tomesko, J.; Parrott, J.S. Tooth loss and nutritional status in older adults: A systematic review and meta-analysis. JDR Clin. Trans. Res. 2022, 7, 4–15. [Google Scholar] [CrossRef]
- Chawla, M.; Gupta, R.; Das, B. Gut microbiome dysbiosis in malnutrition. Prog. Mol. Biol. Transl. Sci. 2022, 192, 205–229. [Google Scholar]
- D’Aiuto, F.; Nibali, L.; Parkar, M.; Suvan, J.; Tonetti, M.S. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J. Dent. Res. 2005, 84, 269–273. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.S.; Kim, J.; Lee, H.; Chang, Y.; Woo, H.G.; Kim, J.W.; Song, T.J. Oral health and gastrointestinal cancer: A nationwide cohort study. J. Clin. Periodontol. 2020, 47, 796–808. [Google Scholar] [CrossRef]
- Taurog, J.D.; Richardson, J.A.; Croft, J.T.; Simmons, W.A.; Zhou, M.; Fernández-Sueiro, J.L.; Balish, E.; Hammer, R.E. The germfree state prevents development of gut and joint inflammatory disease in hla-b27 transgenic rats. J. Exp. Med. 1994, 180, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Rehaume, L.M.; Mondot, S.; Aguirre de Cárcer, D.; Velasco, J.; Benham, H.; Hasnain, S.Z.; Bowman, J.; Ruutu, M.; Hansbro, P.M.; McGuckin, M.A.; et al. Zap-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in skg mice. Arthritis Rheumatol. 2014, 66, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Ogrendik, M. Periodontal pathogens are likely to be responsible for the development of ankylosing spondylitis. Curr. Rheumatol. Rev. 2015, 11, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).