Risk Factors for Developing Metachronous Superficial Gastric Epithelial Neoplasms after Endoscopic Submucosal Dissection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
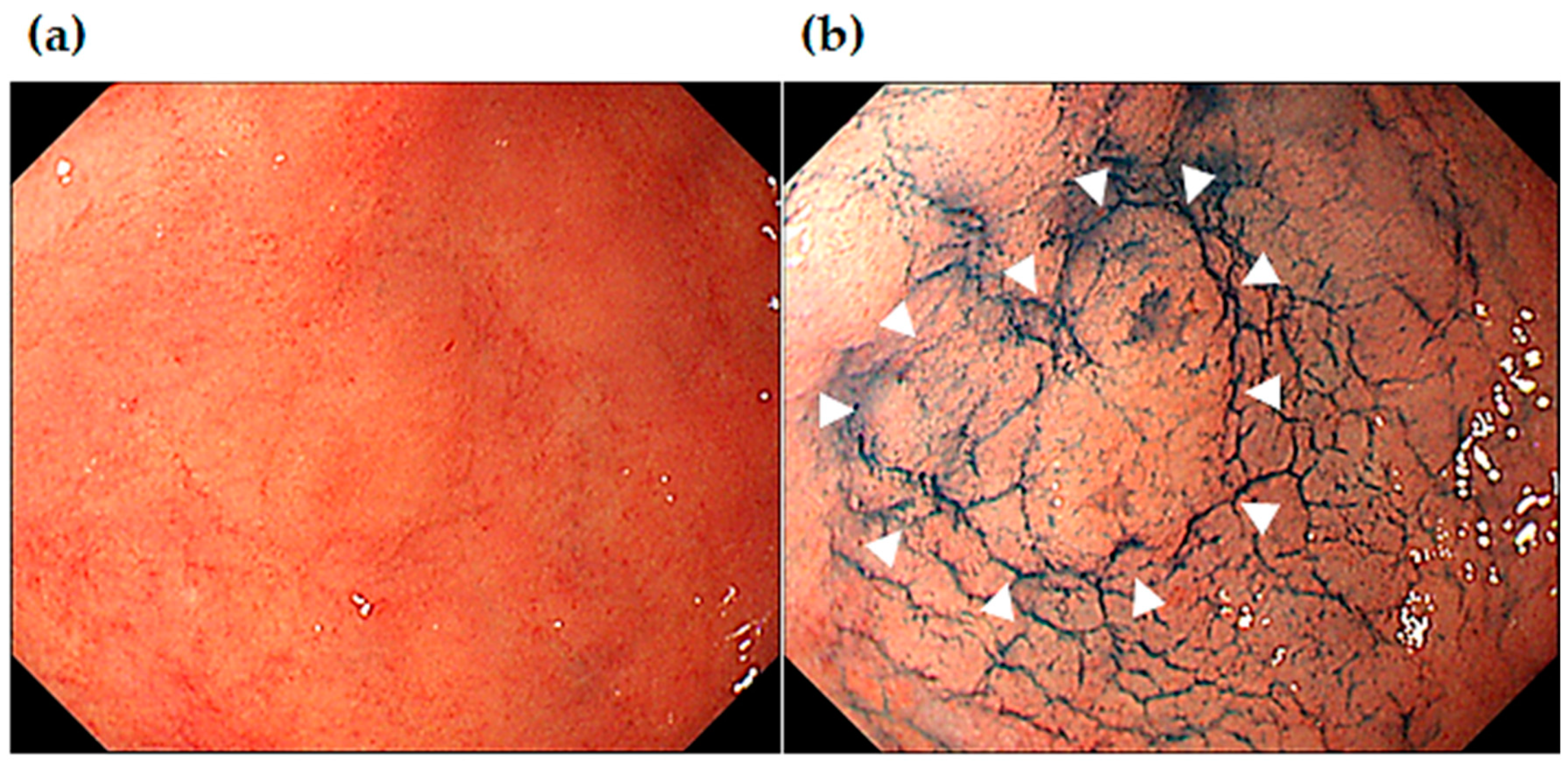
2.3. Confirmation of H. pylori Status
2.4. Histopathologic Analysis
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freddie, B.; Jacques, F.; Isabelle, S.; Rebbeca, L.; Lindsey, A.; Ahmedin, J. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. 2021, 33, 4–20. [Google Scholar] [CrossRef]
- Shiotani, A.; Haruma, K.; Graham, D.Y. Metachronous gastric cancer after successful Helicobacter pylori eradication. World J. Gastroenterol. 2014, 20, 11552–11559. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomized controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef]
- Maehata, Y.; Nakamura, S.; Fujisawa, K.; Esaki, M.; Moriyama, T.; Asano, K.; Fuyuno, Y.; Yamaguchi, K.; Egashira, I.; Kim, H.; et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest. Endosc. 2012, 75, 39–46. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.G.; Yoon, H.; Im, J.P.; Kim, J.S.; Kim, W.H.; Jung, H.C. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin. Gastroenterol. Hepatol. 2014, 12, 793–800. [Google Scholar] [CrossRef]
- Choi, I.J.; Kook, M.C.; Kim, Y.I.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Yoo, H.W.; Hong, S.J.; Kim, S.H. Helicobacter pylori treatment and gastric cancer risk after en-doscopic resection of dysplasia: A nationwide cohort study. Gastroenterology 2023, 166, 313–322. [Google Scholar] [CrossRef]
- Noh, C.K.; Lee, E.; Park, B.; Lim, S.G.; Shin, S.J.; Lee, K.M.; Lee, G.H. Effect of Helicobacter pylori eradication treatment on meta-chronous gastric neoplasm prevention following endoscopic submucosal dissection for gastric adenoma. J. Clin. Med. 2023, 12, 1512. [Google Scholar] [CrossRef]
- Kato, M.; Nishida, T.; Yamamoto, K.; Hayashi, S.; Kitamura, S.; Yabuta, T.; Yoshio, T.; Nakamura, T.; Komori, M.; Kawai, N.; et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: A multicenter retrospective cohort study by Osaka University ESD study group. Gut 2013, 62, 1425–1432. [Google Scholar] [CrossRef]
- Ono, S.; Kato, M.; Suzuki, M.; Ishigaki, S.; Takahashi, M.; Haneda, M.; Mabe, K.; Shimizu, Y. Frequency of Helicobacter pylori-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion 2012, 86, 59–65. [Google Scholar] [CrossRef]
- Boda, T.; Ito, M.; Yoshihara, M.; Kitamura, Y.; Matsuo, T.; Oka, S.; Tanaka, S.; Chayama, K. Advanced method for evaluating of gastric cancer risk by serum marker: Determination of true low-risk subjects for gastric neoplasia. Helicobacter 2013, 19, 1–8. [Google Scholar] [CrossRef]
- Kishikawa, H.; Ojiro, K.; Nakamura, K.; Katayama, T.; Arahata, K.; Takarabe, S.; Miura, S.; Kanai, T.; Nishida, J. Previous Helicobacter pylori infection–induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter 2020, 25, e12669. [Google Scholar] [CrossRef]
- Ikeda, F.; Shikata, K.; Hata, J.; Fukuhara, M.; Hirakawa, Y.; Ohara, T.; Mukai, N.; Nagata, M.; Yoshida, D.; Yonemoto, K.; et al. Combination of Helicobacter pylori antibody and serum pepsinogen as a good predictive tool of gastric cancer incidence: 20-year prospective data from the Hisayama study. J. Epidemiol. 2016, 26, 629–636. [Google Scholar] [CrossRef]
- Watabe, H.; Mitsushima, T.; Yamaji, Y.; Okamoto, M.; Wada, R.; Kokubo, T.; Doi, H.; Yoshida, H.; Kawabe, T.; Omata, M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: A prospective endoscopic cohort study. Gut 2005, 54, 764–768. [Google Scholar] [CrossRef]
- Yamaji, Y.; Watabe, H.; Yoshida, H.; Kawabe, T.; Wada, R.; Mitsushima, T.; Omata, M. High-risk population for gastric cancer development based on serum pepsinogen status and lifestyle factors. Helicobacter 2009, 14, 81–86. [Google Scholar] [CrossRef]
- Ohata, H.; Kitauchi, S.; Yoshimura, N.; Mugitani, K.; Iwane, M.; Nakamura, H.; Yoshikawa, A.; Yanaoka, K.; Arii, K.; Tamai, H.; et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 2004, 109, 138–143. [Google Scholar] [CrossRef]
- Kaji, K.; Hashiba, A.; Uotani, C.; Yamaguchi, Y.; Ueno, T.; Ohno, K.; Takabatake, I.; Wakabayashi, T.; Doyama, H.; Ninomiya, I.; et al. Grading of atrophic gastritis is useful for risk stratification in endoscopic screening for gastric cancer. Am. J. Gastroenterol. 2019, 114, 71–79. [Google Scholar] [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Yoshida, T.; Ohara, N.; Yokota, K.; Oguma, K.; Okada, H.; Yamamoto, K. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J. Gastrotenterol. 2011, 46, 318–324. [Google Scholar] [CrossRef]
- Kimura, K.; Takemoto, T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–96. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Dixon, M.F. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002, 51, 130–131. [Google Scholar] [CrossRef]
- Kamiya, T.; Morishita, T.; Asakura, H.; Miura, S.; Munakata, Y.; Tsuchiya, M. Long-term follow-up study on gastric adenoma and its relation to gastric protruded carcinoma. Cancer 1982, 50, 2496–2503. [Google Scholar] [CrossRef]
- Rugge, M.; Cassaro, M.; Di Mario, F.; Leo, G.; Leandro, G.; Russo, V.M.; Pennelli, G.; Farinati, F. The long term outcome of gastric non-invasive neoplasia. Gut 2003, 52, 1111–1116. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kanzaki, H.; Tanaka, T.; Sakae, H.; Abe, M.; Iwamuro, M.; Kawano, S.; Kawahara, Y.; Okada, H. Gastric Adenoma: A High Incidence Rate of Developing Carcinoma and Risk of Metachronous Gastric Cancer according to Long-Term Follow-Up. Digestion 2021, 102, 878–886. [Google Scholar] [CrossRef]
- Tamai, N.; Kaise, M.; Nakayoshi, T.; Katoh, M.; Sumiyama, K.; Gohda, K.; Yamasaki, T.; Arakawa, H.; Tajiri, H. Clinical and endoscopic characterization of depressed gastric adenoma. Endoscopy 2006, 38, 391–394. [Google Scholar] [CrossRef]
- Mori, G.; Nakajima, T.; Asada, K.; Shimazu, T.; Yamamichi, N.; Maekita, T.; Yokoi, C.; Fujishiro, M.; Gotoda, T.; Ichinose, M.; et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: Results of a large-scale, multicenter cohort study in Japan. Gastric Cancer 2016, 19, 911–918. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 15th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2017. [Google Scholar]
- Kodama, M.; Murakami, K.; Okimoto, T.; Abe, H.; Sato, R.; Ogawa, R.; Mizukami, K.; Shiota, S.; Nakagawa, Y.; Soma, W.; et al. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand. J. Gastroenterol. 2013, 48, 1249–1256. [Google Scholar] [CrossRef]
- Lahner, E.; Carabotti, M.; Annibale, B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J. Gastroenterol. 2018, 24, 2373–2380. [Google Scholar] [CrossRef]
- Uedo, N.; Ishihara, R.; Iishi, H.; Yamada, T.; Imanaka, K.; Takeuchi, Y.; Higashino, K.; Ishiguro, S.; Tatsuta, M. A new method of diagnosing gastric intestinal metaplasia: Narrowband imaging with magnifying endoscopy. Endoscopy 2006, 38, 819–824. [Google Scholar] [CrossRef]
- Ono, S.; Kato, M.; Tsuda, M.; Miyamoto, S.; Abiko, S.; Shimizu, Y.; Sakamoto, N. Lavender Color in Linked Color Imaging Enables Noninvasive Detection of Gastric Intestinal Metaplasia. Digestion 2018, 98, 222–230. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Correa, P. Helicobacter pylori and gastric carcinogenesis. Am. J. Surg. Pathol. 1995, 19, S37–S43. [Google Scholar]
- Take, S.; Mizuno, M.; Ishiki, K.; Nagahara, Y.; Yoshida, T.; Yokota, K.; Oguma, K. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J. Gastroenterol. Hepatol. 2007, 42, 21–27. [Google Scholar] [CrossRef]
- Sakitani, K.; Hirata, Y.; Watabe, H.; Yamada, A.; Sugimoto, T.; Yamaji, Y.; Yoshida, H.; Maeda, S.; Omata, M.; Koike, K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J. Gastroenterol. Hepatol. 2011, 26, 1570–1575. [Google Scholar] [CrossRef]
- Parisa, K.; Farhad, I.; Sharmila, A.; Neal, D.F.; Frain, K. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar]
- Asada, K.; Nakajima, T.; Shimazu, T.; Yamamichi, N.; Maekita, T.; Yokoi, C.; Oda, I.; Ando, T.; Yoshida, T.; Nanjo, S.; et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut 2015, 64, 388–396. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagata, Y.; Hiratsuka, R.; Kawase, Y.; Tominaga, T.; Takeuchi, S.; Sakagami, S.; Ishida, S. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels—The ABC method. Digestion 2016, 93, 13–18. [Google Scholar] [CrossRef]
- IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer; IARC Working Group Reports, No. 8; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Nakajima, T.; Oda, I.; Gotoda, T.; Hamanaka, H.; Eguchi, T.; Yokoi, C.; Saito, D. Metachronous gastric cancers after endoscopic resection: How effective is annual endoscopic surveillance? Gastric Cancer 2006, 9, 93–98. [Google Scholar] [CrossRef]
- Carneiro, F.; Fukayama, M.; Grabsch, H.I.; Yasui, W. Gastric adenocarcinoma. In WHO Classification of Digestive System Tumors, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1, pp. 85–95. [Google Scholar]
- Dormuth, I.; Liu, T.; Xu, J.; Yu, M.; Pauly, M.; Ditzhaus, M. Which test for crossing survival curves? A user’s guideline. BMC Med. Res. Methodol. 2022, 30, 34. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, D.; Qiu, J.; Chen, T.; Gamalo, M. A win ratio approach for comparing crossing survival curves in clinical trials. J. Biopharm. Stat. 2023, 33, 488–501. [Google Scholar] [CrossRef]

| Sex, Male: Female; n (%) | 257 (70):112 (30) |
|---|---|
| Age, median (IQR) | 73 (67–79) |
| Adenoma: adenocarcinoma, n (%) | 66 (18): 303 (82) |
| Size of initial SGENs: mm, median (IQR) | 15 (10–23) |
| Location of initial SGENs U:M:L, n (%) | 88 (24):133 (36):148 (40) |
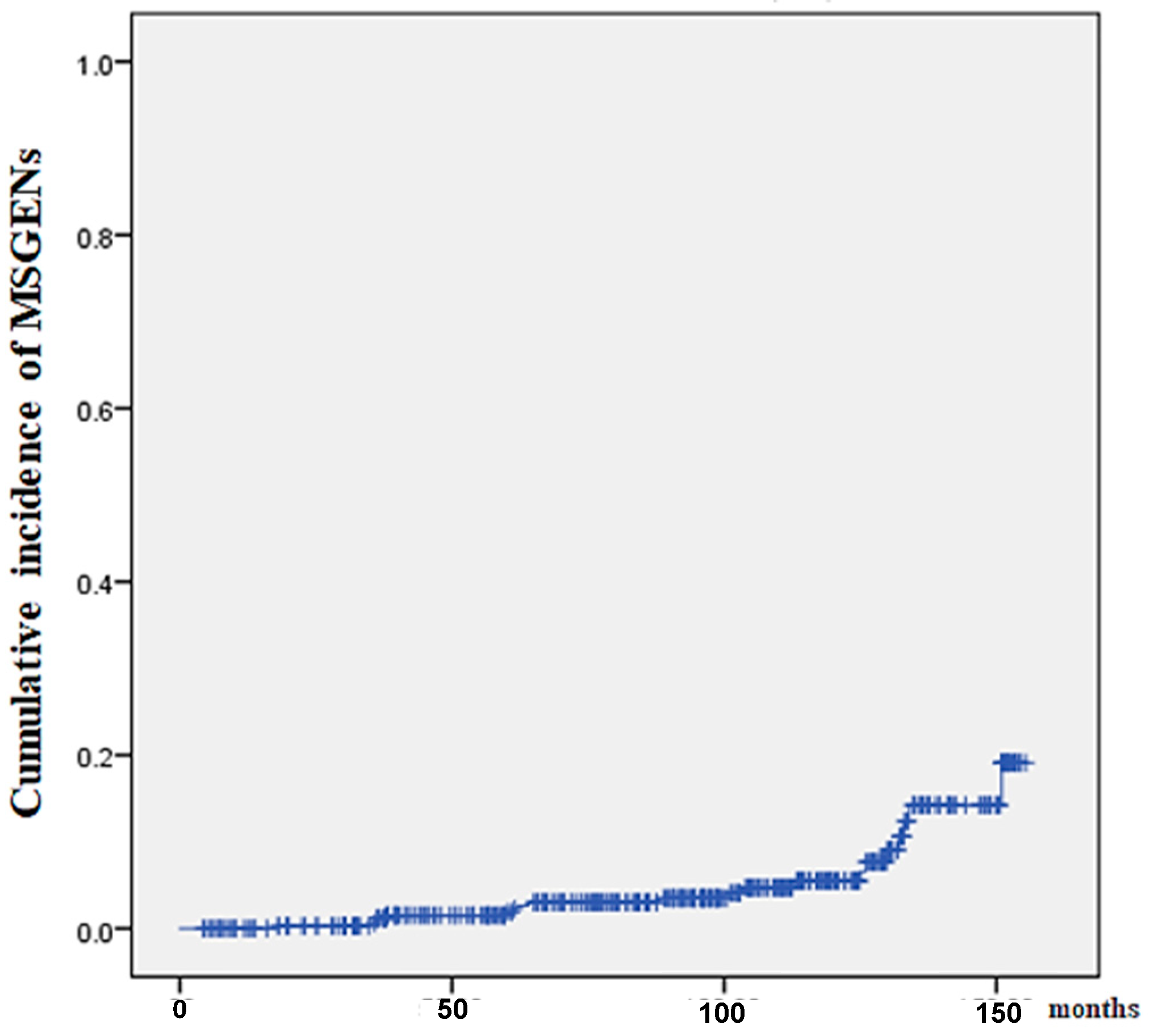
| MSGENs, n (%) | 27 (7.1) |
| Location of MSGENs U:M:L, n (%) | 11 (41):2 (7):14 (52) |
| Findings | Not-MSGEN n = 342 | MSGEN n = 27 | p |
|---|---|---|---|
| Age, years; median (IQR) | 73 (66–78) | 77 (72–81) | 0.20 # |
| Sex, male/female; n | 235/107 | 22/5 | 0.32 † |
| Body mass index, median (IQR) | 24 (21–26) | 24 (20–26) | 0.35 ‡ |
| Alcohol drinking, n (%) | 141 (41) | 12 (44) | 0.84 † |
| Smoking, n (%) | 181 (53) | 13 (48) | 0.29 † |
| Gastric mucosal atrophy (no/closed/open) | 10/29/303 | 0/0/27 | 0.24 † |
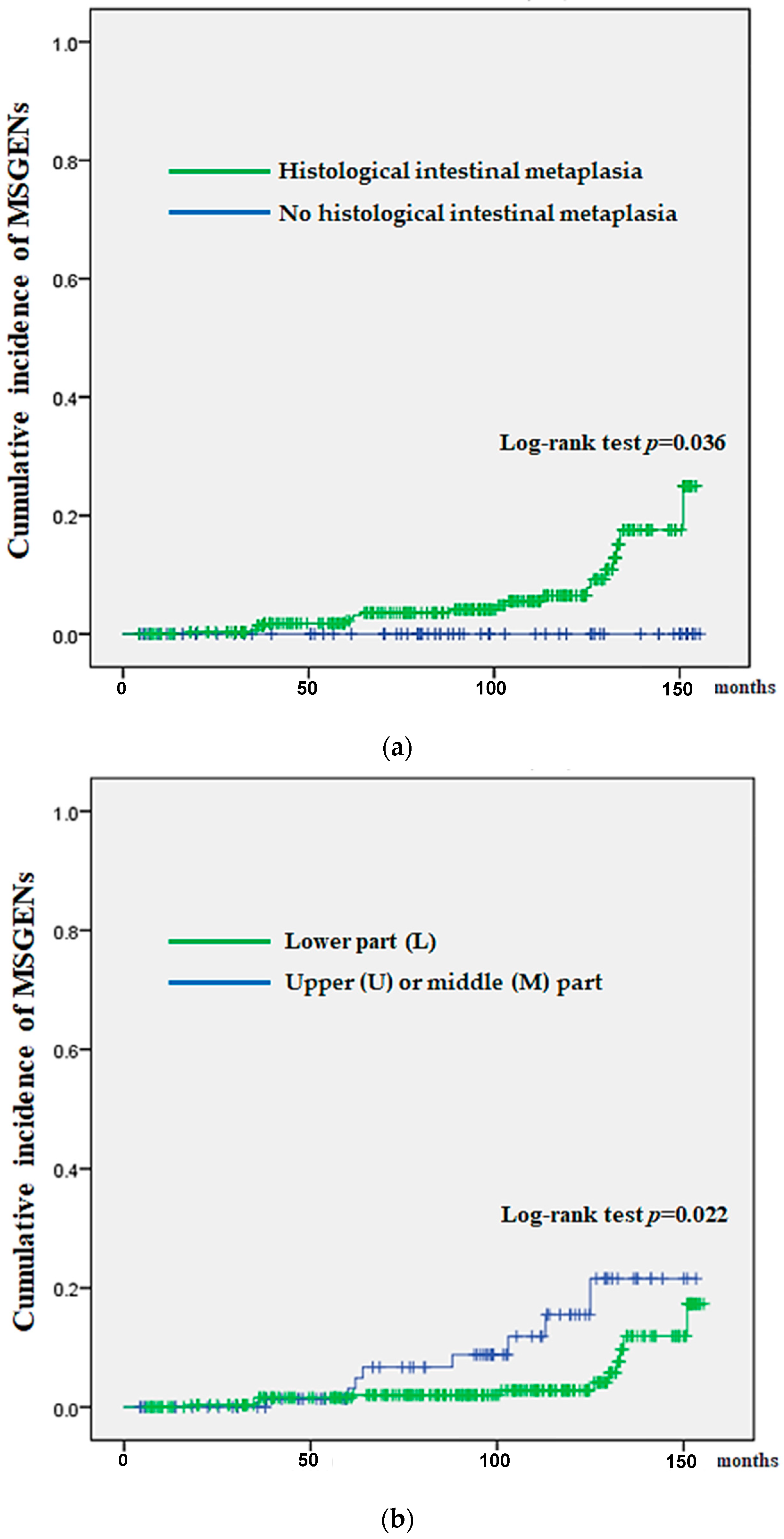
| Histological intestinal metaplasia (HIM), n (%) | 279 (82) | 27 (100) | 0.04 † |
| Hp infection, current/past or negative, n (%) | 213 (62)/102 (30) or 27 (8) | 16 (59)/2 (7) or 9 (34) | 0.05 † |
| Location of initial SGENs (U, M/L) | 78, 120/144 | 10, 13/4 | 0.04 † |
| Histologic type (differentiated/undifferentiated) * | 330/12 | 27/0 | 0.08 † |
| Size, mm; median (IQR) | 15 (10–23) | 12 (10–19) | 0.08 # |
| Depth of initial tumor, M/SM | 305/37 | 25/2 | 0.44 † |
| Ulcer (−/+) | 310/32 | 23/4 | 0.50 † |
| Lymphovascular invasion (−/+) | 342/0 | 27/0 | 0.78 † |
| Vessel invasion (−/+) | 342/0 | 27/0 | 0.97 † |
| Findings | Mnot-MSGEN n = 27 | MSGEN n = 27 | p |
|---|---|---|---|
| Age, years; median (IQR) | 70 (66–78) | 77 (72–81) | 0.29 # |
| Sex, male/female; n | 23/4 | 22/5 | 0.72 † |
| Body mass index, median (IQR) | 23 (17–30) | 24 (20–226) | 0.76 ‡ |
| Alcohol drinking, n (%) | 10 (37) | 12 (44) | 0.31 † |
| Smoking, n (%) | 16 (59) | 13 (48) | 0.26 † |
| Gastric mucosal atrophy (no/closed/open) | 3/1/23 | 0/0/27 | 0.06 † |
| Histological intestinal metaplasia (HIM), n (%) | 21 (78) | 27 (100) | 0.09 † |
| Hp infection, current/past or negative, n (%) | 19 (70)/7 (26) or 1 (4) | 16 (59)/2 (7) or 9 (34) | 0.45 † |
| Location of initial SGENs (U, M/L) | 8, 10/9 | 10, 13/4 | 0.21 † |
| Histologic type (differentiated/undifferentiated) * | 25/2 | 27/0 | 0.49 † |
| Size, mm; median (IQR) | 15 (10–23) | 12 (10–19) | 0.57 # |
| Depth of initial tumor, M/SM | 26/1 | 25/2 | 0.09 † |
| Ulcer (−/+) | 24/3 | 23/4 | 0.69 † |
| Lyphovascular invasion (−/+) | 27/0 | 27/0 | - |
| Vessel invasion (−/+) | 27/0 | 27/0 | - |
| Synchronous SGEN, n (%) | 0 (0.0) | 2 (7.4) | 0.07 † |
| Age of Hp therapy, years (IQR) | 73 (63–82) | 70 (63–77) | 0.261 ‡ |
| Spontaneous disappearance of Hp, n (%) ** | 1 (4) | 9 (33) | 0.003 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Goda, K.; Ishikawa, M.; Yamaguchi, S.; Yoshinaga, T.; Kondo, M.; Kanazawa, M.; Kunogi, Y.; Tanaka, T.; Kanamori, A.; et al. Risk Factors for Developing Metachronous Superficial Gastric Epithelial Neoplasms after Endoscopic Submucosal Dissection. J. Clin. Med. 2024, 13, 1587. https://doi.org/10.3390/jcm13061587
Suzuki T, Goda K, Ishikawa M, Yamaguchi S, Yoshinaga T, Kondo M, Kanazawa M, Kunogi Y, Tanaka T, Kanamori A, et al. Risk Factors for Developing Metachronous Superficial Gastric Epithelial Neoplasms after Endoscopic Submucosal Dissection. Journal of Clinical Medicine. 2024; 13(6):1587. https://doi.org/10.3390/jcm13061587
Chicago/Turabian StyleSuzuki, Tsunehiro, Kenichi Goda, Manabu Ishikawa, Shintaro Yamaguchi, Tomonori Yoshinaga, Masayuki Kondo, Mimari Kanazawa, Yasuhito Kunogi, Takanao Tanaka, Akira Kanamori, and et al. 2024. "Risk Factors for Developing Metachronous Superficial Gastric Epithelial Neoplasms after Endoscopic Submucosal Dissection" Journal of Clinical Medicine 13, no. 6: 1587. https://doi.org/10.3390/jcm13061587
APA StyleSuzuki, T., Goda, K., Ishikawa, M., Yamaguchi, S., Yoshinaga, T., Kondo, M., Kanazawa, M., Kunogi, Y., Tanaka, T., Kanamori, A., Abe, K., Yamamiya, A., Sugaya, T., Tominaga, K., Yamagishi, H., Masuyama, H., & Irisawa, A. (2024). Risk Factors for Developing Metachronous Superficial Gastric Epithelial Neoplasms after Endoscopic Submucosal Dissection. Journal of Clinical Medicine, 13(6), 1587. https://doi.org/10.3390/jcm13061587






