Respiratory Complications after Cystectomy with Urinary Diversion: Avoidable Complications or Ineluctable Destiny?
Abstract
1. Introduction
2. Materials and Methods
2.1. ERAS® Protocol
2.2. Patient Selection
2.3. Data Collection
2.4. Outcome Measurement
- Respiratory failure confirmed by hypoxemic (PaO2 < 60 mmHg) or hypercapnic (PaCO2 > 500 mmHg) arterial gasometry.
- Radiologically confirmed pneumonia, lobar atelectasis, or pulmonary embolism by conventional X-ray or by computer tomography (CT) and certified by a radiologist.
- Pleural fluid or pneumothorax needing thorax drainage.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Mungan, N.; Aben, K.K.; Schoenberg, M.P.; Visser, O.; Coebergh, J.-W.W.; Witjes, J.; Kiemeney, L.A. Gender differences in stage-adjusted bladder cancer survival. Urology 2000, 55, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2013 Guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, E.; Sanchez-Salas, R.; Gandaglia, G.; Rocchini, L.; Moschini, M.; Lizee, D.; Carneiro, A.; Sivaraman, A.; Barret, E.; Rozet, F.; et al. A nomogram predicting the cancer-specific mortality in patients eligible for radical cystectomy evaluating clinical data and neoadjuvant cisplatinum-based chemotherapy. World J. Urol. 2016, 34, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.; Becker, M.; Anderson, J. The changing pattern of mortality and morbidity from radical cystectomy. BJU Int. 2000, 85, 427–430. [Google Scholar] [CrossRef]
- Knap, M.M.; Lundbeck, F.; Overgaard, J. Early and late treatment-related morbidity following radical cystectomy. Scand. J. Urol. Nephrol. 2004, 38, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Konety, B.R.; Dhawan, V.; Allareddy, V.; Joslyn, S.A. Impact of Hospital and Surgeon Volume on In-Hospital Mortality from Radical Cystectomy: Data from the Health Care Utilization Project. J. Urol. 2005, 173, 1695–1700. [Google Scholar] [CrossRef]
- Khuri, S.F.; Henderson, W.G.; DePalma, R.G.; Mosca, C.; Healey, N.A.; Kumbhani, D.J. Determinants of Long-Term Survival After Major Surgery and the Adverse Effect of Postoperative Complications. Ann. Surg. 2005, 242, 326–343. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colonic Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef]
- Llorente, C.; López, B.; Hernández, V.; Guijarro, A.; Pérez-Fernández, E. Variabilidad en las complicaciones y la mortalidad quirúrgica tras cistectomía radical en España. Actas Urol. Esp. 2017, 41, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Segura Martín, M.; Salinas Sánchez, A.; Lorenzo Romero, J.; Hernández Millán, I.; Giménez Bachs, J.M.; Virseda Rodríguez, J. Complications after radical cystectomy in patients with bladder carcinoma. Arch. Esp. Urol. 2002, 55, 383–393. [Google Scholar] [PubMed]
- Konety, B.R.; Allareddy, V. Influence of Post-Cystectomy Complications on Cost and Subsequent Outcome. J. Urol. 2007, 177, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Crettenand, F.; M’baya, O.; Grilo, N.; Valerio, M.; Dartiguenave, F.B.; Cerantola, Y.; Roth, B.; Rouvé, J.-D.; Blanc, C.; Lucca, I. ERAS® protocol improves survival after radical cystectomy: A single-center cohort study. Medicine 2022, 101, e30258. [Google Scholar] [CrossRef]
- Young, J.; Badgery-Parker, T.; Dobbins, T.; Jorgensen, M.; Gibbs, P.; Faragher, I.; Jones, I.; Currow, D. Comparison of ECOG/WHO Performance Status and ASA Score as a Measure of Functional Status. J. Pain Symptom Manag. 2015, 49, 258–264. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Pisarska, M.; Torbicz, G.; Gajewska, N.; Rubinkiewicz, M.; Wierdak, M.; Major, P.; Budzyński, A.; Ljungqvist, O.; Pędziwiatr, M. Compliance with the ERAS Protocol and 3-Year Survival After Laparoscopic Surgery for Non-metastatic Colorectal Cancer. World J. Surg. 2019, 43, 2552–2560. [Google Scholar] [CrossRef]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of Postoperative Pulmonary Complications in a Population-based Surgical Cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Brunn, J.A. Predictors of Postoperative Pulmonary Complications Following Abdominal Surgery. Chest 1997, 111, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-H.; Shin, B.; Eom, J.S.; Yoo, H.; Song, W.; Han, S.; Lee, K.J.; Jeon, K.; Um, S.-W.; Koh, W.-J.; et al. Development of a Prediction Rule for Estimating Postoperative Pulmonary Complications. PLoS ONE 2014, 9, e113656. [Google Scholar] [CrossRef]
- Mazo, V.; Sabaté, S.; Canet, J.; Gallart, L.; de Abreu, M.G.; Belda, J.; Langeron, O.; Hoeft, A.; Pelosi, P. Prospective External Validation of a Predictive Score for Postoperative Pulmonary Complications. Anesthesiology 2014, 121, 219–231. [Google Scholar] [CrossRef]
- Smit, L.C.; Bruins, M.J.; Patijn, G.A.; Ruijs, G.J. Infectious Complications after Major Abdominal Cancer Surgery: In Search of Improvable Risk Factors. Surg. Infect. 2016, 17, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Gupta, P.K.; Fang, X.; Miller, W.J.; Cemaj, S.; Forse, R.A.; Morrow, L.E. Development and Validation of a Risk Calculator Predicting Postoperative Respiratory Failure. Chest 2011, 140, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Baig, M.A.; Brito, V.; Bader, F.; Bergman, M.I.; Alfonso, A. Postoperative Pulmonary Complications after Laparotomy. Respiration 2010, 80, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Vermişli, S.; Çakmak, Ö.; Müezzinoğlu, T.; Aslan, G.; Baydur, H. The Effect of Postoperative Early Mobilization on the Healing Process and Quality of Life Following Radical Cystectomy and Ileal Conduit: A Randomized Prospective Controlled Trial. J. Urol. Surg. 2022, 9, 9–19. [Google Scholar] [CrossRef]
- Jurt, J.; Hübner, M.; Pache, B.; Hahnloser, D.; Demartines, N.; Grass, F. Respiratory Complications After Colorectal Surgery: Avoidable or Fate? World J. Surg. 2018, 42, 2708–2714. [Google Scholar] [CrossRef]
- Gupta, R.; Gan, T.J. Peri-operative fluid management to enhance recovery. Anaesthesia 2016, 71, 40–45. [Google Scholar] [CrossRef]
- Lobo, D.N.; Bostock, K.A.; Neal, K.R.; Perkins, A.C.; Rowlands, B.J.; Allison, S.P. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: A randomised controlled trial. Lancet 2022, 359, 1812–1818. [Google Scholar] [CrossRef]

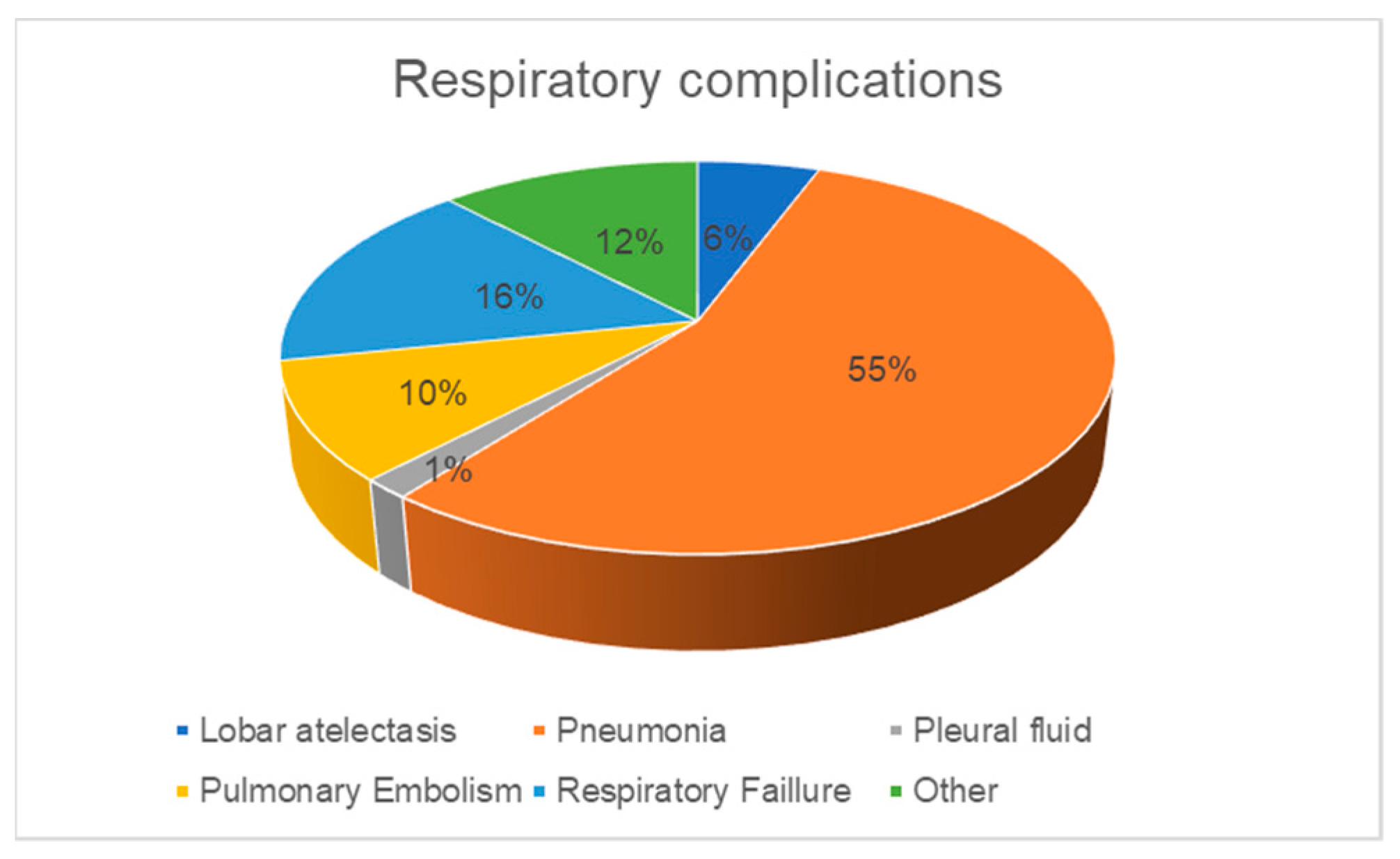
| Variable | All Patients (n = 242) | Respiratory Complications | p | |
|---|---|---|---|---|
| No (n = 201) | Yes (n = 41) | |||
| Age-median (IQR) | 72 (64–77) | 72 (63–77) | 72 (64–76) | 0.72 |
| Gender-n (%) | 0.21 | |||
| Female | 63 (26) | 56 (28) | 7 (17) | |
| Male | 179 (74) | 145 (72) | 34 (83) | |
| Smoking-n (%) | 98 (40) | 78 (39) | 20 (49) | 0.31 |
| Alcohol-n (%) | 43 (18) | 35 (17) | 8 (20) | 0.92 |
| BMI-median (IQR) | 26 (23–29) | 26 (23–29) | 26 (22–27) | 0.29 |
| Diabetes-n (%) | 46 (19) | 32 (16) | 9 (22) | 0.76 |
| WHO performance score ≥ 2-n (%) | 16 (7) | 13 (6) | 3 (7) | 0.66 |
| Preoperative chemotherapy-n (%) | 49 (20) | 38 (19) | 11 (27) | 0.35 |
| Previous abdominal surgery-n (%) | 94 (39) | 81 (40) | 13 (32) | 0.39 |
| Underlying disease-n (%) | ||||
| Severe heart disease | 23 (10) | 18 (9) | 5 (12) | 0.72 |
| Severe pulmonary disease | 8 (3) | 5 (2) | 3 (7) | 0.27 |
| ASA class-n (%) | 0.11 | |||
| I | 3 (1) | 3 (1) | 0 (0) | |
| II | 135 (56) | 112 (56) | 23 (56) | |
| III | 101 (42) | 85 (42) | 16 (39) | |
| IV | 3 (1) | 1 (1) | 2 (5) | |
| Urothelial Carcinoma-n (%) | 213 (88) | 172 (86) | 41 (100) | 0.02 |
| pT stage-n (%) | 0.29 | |||
| pT0 | 17 (8) | 12 (6) | 5 (12) | |
| pTis | 24 (11) | 17 (8) | 7 (17) | |
| pTa | 9 (4) | 9 (4) | 0 (0) | |
| pT1 | 17 (8) | 13 (6) | 4 (10) | |
| pT2 | 36 (17) | 32 (16) | 5 (12) | |
| pT3 | 74 (35) | 65 (32) | 11 (27) | |
| pT4 | 36 (17) | 27 (13) | 9 (22) | |
| pN stage-n (%) | 0.19 | |||
| pN0 | 135 (63) | 113 (66) | 22 (54) | |
| pN1 | 22 (10) | 19 (11) | 3 (7) | |
| pN2 | 30 (14) | 21 (12) | 9 (22) | |
| pN3 | 3 (1) | 3 (2) | 0 (0) | |
| pNX | 23 (11) | 16 (9) | 7 (17) | |
| Functional disorder-n (%) | 25 (10) | 25 (12) | 0 (0) | 0.04 |
| Other malignancy-n (%) | 4 (2) | 4 (2) | 0 (0) | 0.81 |
| Variable | All Patients (n = 242) | Respiratory Complications | p | |
|---|---|---|---|---|
| No (n = 201) | Yes (n = 41) | |||
| Surgical procedure | ||||
| Bricker Ileal conduit-n (%) | 189 (78) | 156 (78) | 33 (81) | 0.84 |
| Cutaneous ureterostomy-n (%) | 15 (6) | 14 (7) | 1 (2) | 0.46 |
| Orthotopic neobladder-n (%) | 38 (16) | 31 (15) | 7 (17) | 0.98 |
| Operation duration (min) median-(IQR) | 383 (327–450) | 380 (330–450) | 401 (327–446) | 0.48 |
| Intraoperative blood loss (mL) median-(IQR) | 400 (300–787) | 400 (275–700) | 700 (350–1000) | 0.04 * |
| Intraoperative fluids (mL) median-(IQR) | 3500 (2500–4500) | 3500 (2500–4500) | 3500 (3000–4540) | 0.17 |
| Infectious complications-n (%) | 67 (28) | 38 (19) | 29 (71) | 0.02 |
| Cardiovascular complications-n (%) | 66 (27) | 49 (24) | 17 (41) | 0.04 |
| Renal complications-n (%) | 34 (14) | 21 (10) | 13 (32) | <0.001 |
| Ileus-n (%) | 110 (45) | 87 (43) | 23 (56) | <0.001 |
| LOS-median (IQR) | 15 (12–21) | 14 (11–19) | 25 (19–48) | <0.001 |
| POD mobilization-median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 0.01 |
| Weight gain (kg) *-median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–3) | 0.004 |
| Readmission ** rate-n (%) | 37(15) | 33 (89) | 4 (11) | 0.39 |
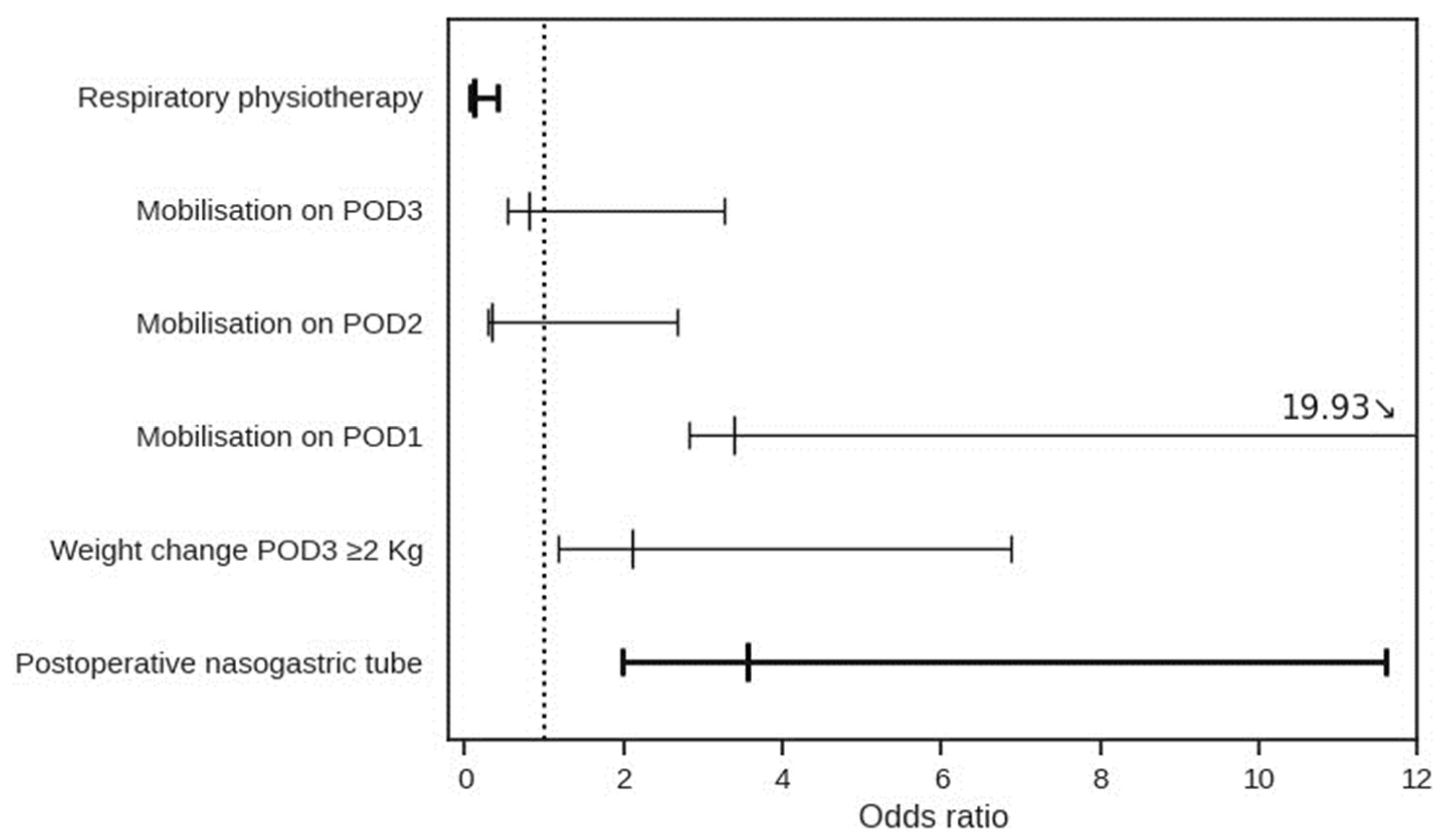
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.01 | 0.97–1.04 | 0.73 | |||
| BMI | 0.96 | 0.13 | ||||
| Smoker | 1.50 | 0.76–2.95 | 0.24 | |||
| Severe pulmonary disease | 3.10 | 0.71–13.50 | 0.13 | |||
| WHO score | 1.22 | 0.77–1.93 | 0.40 | |||
| ASA score | 1.26 | 0.68–2.34 | 0.45 | |||
| Blood loss | 1.01 | 1.00–1.01 | 0.03 | 1.00 | 0.99–1.00 | 0.09 |
| Preoperative chemotherapy | 1.57 | 0.73–3.42 | 0.25 | |||
| Length of operation | 1.00 | 0.99–1.01 | 0.45 | |||
| Tumor stage | 0.93 | 0.74–1.15 | 0.50 | |||
| Time to oral pain control | 1.04 | 1.01–1.08 | 0.03 | 1.02 | 0.98–1.06 | 0.41 |
| Renal complications | 3.98 | 1.79–8.84 | 0.001 | 3.73 | 1.46–9.52 | 0.006 |
| Cardiovascular complications | 2.20 | 1.09–4.42 | 0.03 | 1.55 | 0.69–3.44 | 0.28 |
| Ileus | 5.61 | 2.54–12.38 | <0.001 | 5.72 | 2.45–13.35 | <0.001 |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.02 | 0.94–1.10 | 0.66 | |||
| BMI | 0.98 | 0.83–1.16 | 0.85 | |||
| Smoker | 1.08 | 0.24–4.96 | 0.91 | |||
| Severe pulmonary disease | - | |||||
| WHO score | 0.48 | 0.13–1.85 | 0.29 | |||
| ASA score | 0.60 | 0.14–2.58 | 0.49 | |||
| Blood loss | 1.01 | 0.99–1.00 | 0.09 | |||
| Preoperative chemotherapy | 1.60 | 0.30–8.51 | 0.58 | |||
| Length of operation | 1.01 | 1.01–1.02 | 0.03 | 1.01 | 0.99–1.01 | 0.07 |
| Tumor stage | 0.99 | 0.61–1.61 | 0.97 | |||
| Time to oral pain control | 0.89 | 0.67–1.20 | 0.45 | |||
| Renal complications | 2.47 | 0.46–13.30 | 0.29 | |||
| Infectious complications | 14.92 | 2.77–80.13 | 0.002 | 15.56 | 2.67–90.40 | 0.002 |
| Cardiovascular complications | 7.08 | 1.33–37.45 | 0.02 | 6.10 | 1.03–36.32 | 0.05 |
| Ileus | 0.19 | 0.02–1.59 | 0.12 | |||
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 0.99 | 0.60 | ||||
| BMI | 0.98 | 0.59 | ||||
| Smoker | 1.68 | 0.19 | ||||
| Severe pulmonary disease | 2.55 | 0.26 | ||||
| WHO score | 1.04 | 0.87 | ||||
| ASA score | 1.07 | 0.84 | ||||
| Blood loss | 1.00 | 0.32 | ||||
| Preoperative chemotherapy | 1.29 | 0.58 | ||||
| Length of operation | 1.01 | 0.78 | ||||
| Tumor stage | 0.98 | 0.91 | ||||
| Time to oral pain control | 1.05 | 1.01–1.09 | 0.01 | 1.03 | 0.98–1.08 | 0.17 |
| Renal complications | 2.74 | 1.10–6.82 | 0.03 | 2.54 | 0.92–7.03 | 0.07 |
| Cardiovascular complications | 1.76 | 0.78–3.95 | 0.17 | |||
| Ileus | 7.09 | 2.60–19.30 | <0.001 | 6.51 | 2.35–18.10 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Carrique, S.; Crettenand, F.; Stritt, K.; Bohner, P.; Grilo, N.; Rodrigues-Dias, S.; Roth, B.; Lucca, I. Respiratory Complications after Cystectomy with Urinary Diversion: Avoidable Complications or Ineluctable Destiny? J. Clin. Med. 2024, 13, 1585. https://doi.org/10.3390/jcm13061585
Martinez Carrique S, Crettenand F, Stritt K, Bohner P, Grilo N, Rodrigues-Dias S, Roth B, Lucca I. Respiratory Complications after Cystectomy with Urinary Diversion: Avoidable Complications or Ineluctable Destiny? Journal of Clinical Medicine. 2024; 13(6):1585. https://doi.org/10.3390/jcm13061585
Chicago/Turabian StyleMartinez Carrique, Silvia, François Crettenand, Kevin Stritt, Perrine Bohner, Nuno Grilo, Sonia Rodrigues-Dias, Beat Roth, and Ilaria Lucca. 2024. "Respiratory Complications after Cystectomy with Urinary Diversion: Avoidable Complications or Ineluctable Destiny?" Journal of Clinical Medicine 13, no. 6: 1585. https://doi.org/10.3390/jcm13061585
APA StyleMartinez Carrique, S., Crettenand, F., Stritt, K., Bohner, P., Grilo, N., Rodrigues-Dias, S., Roth, B., & Lucca, I. (2024). Respiratory Complications after Cystectomy with Urinary Diversion: Avoidable Complications or Ineluctable Destiny? Journal of Clinical Medicine, 13(6), 1585. https://doi.org/10.3390/jcm13061585






